 Found 165 hits with Last Name = 'mckeown' and Initial = 's'
Found 165 hits with Last Name = 'mckeown' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099960
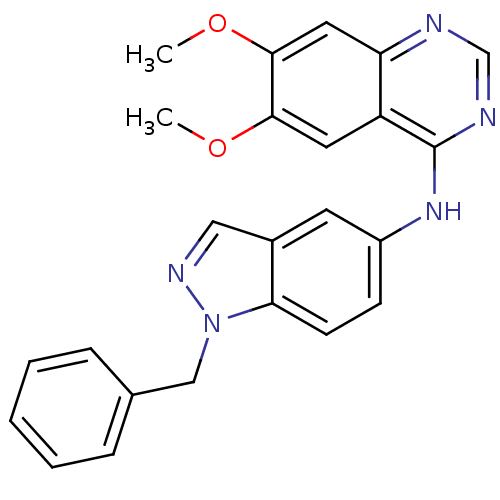
((1-Benzyl-1H-indazol-5-yl)-(6,7-dimethoxy-quinazol...)Show SMILES COc1cc2ncnc(Nc3ccc4n(Cc5ccccc5)ncc4c3)c2cc1OC Show InChI InChI=1S/C24H21N5O2/c1-30-22-11-19-20(12-23(22)31-2)25-15-26-24(19)28-18-8-9-21-17(10-18)13-27-29(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of EGF receptor |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099969
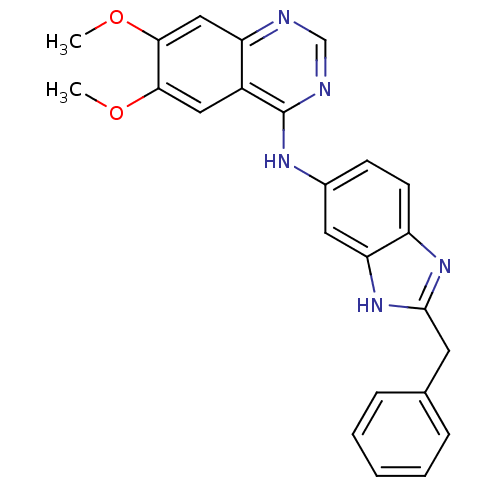
((2-Benzyl-1H-benzoimidazol-5-yl)-(6,7-dimethoxy-qu...)Show SMILES COc1cc2ncnc(Nc3ccc4nc(Cc5ccccc5)[nH]c4c3)c2cc1OC Show InChI InChI=1S/C24H21N5O2/c1-30-21-12-17-19(13-22(21)31-2)25-14-26-24(17)27-16-8-9-18-20(11-16)29-23(28-18)10-15-6-4-3-5-7-15/h3-9,11-14H,10H2,1-2H3,(H,28,29)(H,25,26,27) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099965
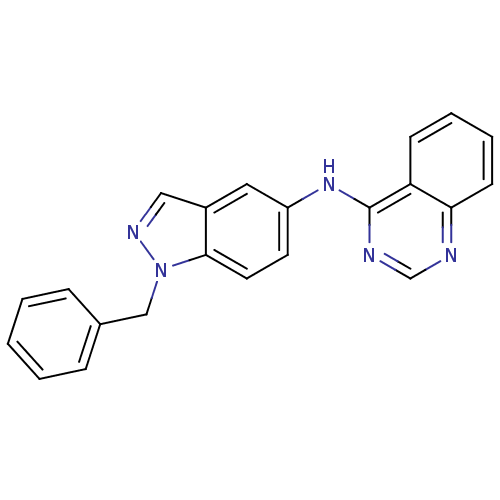
((1-Benzyl-1H-indazol-5-yl)-quinazolin-4-yl-amine |...)Show InChI InChI=1S/C22H17N5/c1-2-6-16(7-3-1)14-27-21-11-10-18(12-17(21)13-25-27)26-22-19-8-4-5-9-20(19)23-15-24-22/h1-13,15H,14H2,(H,23,24,26) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of erbB4 receptor |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099971
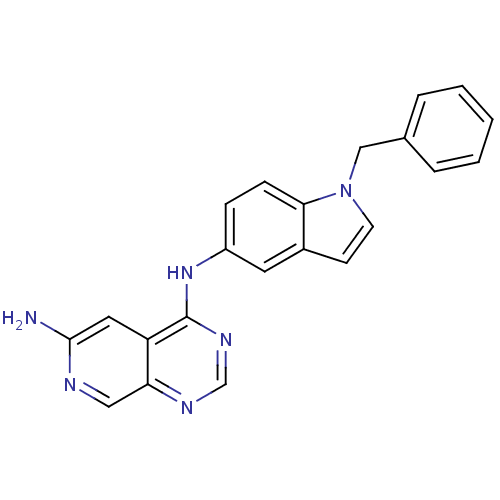
(CHEMBL288557 | N*4*-(1-Benzyl-1H-indol-5-yl)-pyrid...)Show InChI InChI=1S/C22H18N6/c23-21-11-18-19(12-24-21)25-14-26-22(18)27-17-6-7-20-16(10-17)8-9-28(20)13-15-4-2-1-3-5-15/h1-12,14H,13H2,(H2,23,24)(H,25,26,27) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099966
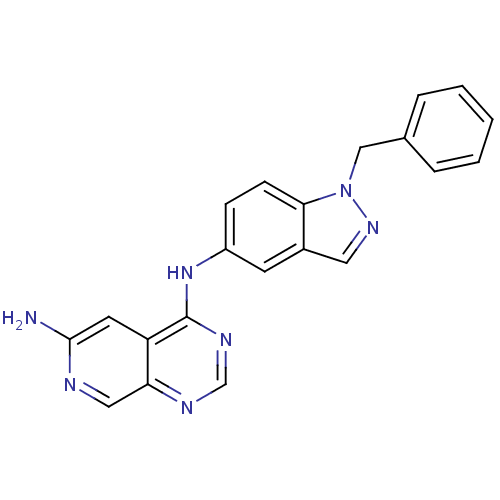
(CHEMBL281006 | N*4*-(1-Benzyl-1H-indazol-5-yl)-pyr...)Show InChI InChI=1S/C21H17N7/c22-20-9-17-18(11-23-20)24-13-25-21(17)27-16-6-7-19-15(8-16)10-26-28(19)12-14-4-2-1-3-5-14/h1-11,13H,12H2,(H2,22,23)(H,24,25,27) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099970
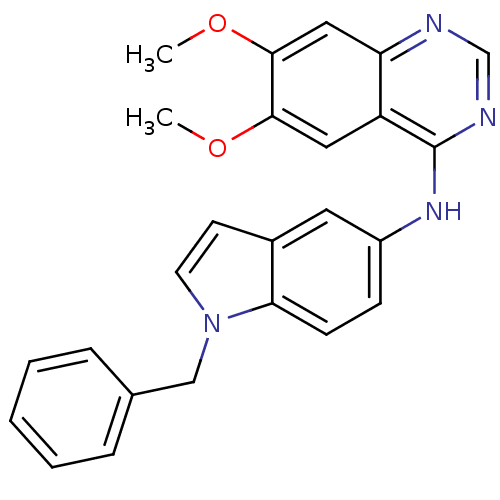
((1-Benzyl-1H-indol-5-yl)-(6,7-dimethoxy-quinazolin...)Show SMILES COc1cc2ncnc(Nc3ccc4n(Cc5ccccc5)ccc4c3)c2cc1OC Show InChI InChI=1S/C25H22N4O2/c1-30-23-13-20-21(14-24(23)31-2)26-16-27-25(20)28-19-8-9-22-18(12-19)10-11-29(22)15-17-6-4-3-5-7-17/h3-14,16H,15H2,1-2H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099968
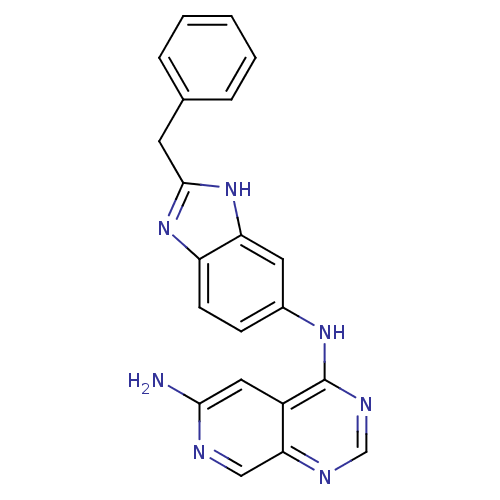
(CHEMBL31653 | N*4*-(2-Benzyl-1H-benzoimidazol-5-yl...)Show SMILES Nc1cc2c(Nc3ccc4nc(Cc5ccccc5)[nH]c4c3)ncnc2cn1 Show InChI InChI=1S/C21H17N7/c22-19-10-15-18(11-23-19)24-12-25-21(15)26-14-6-7-16-17(9-14)28-20(27-16)8-13-4-2-1-3-5-13/h1-7,9-12H,8H2,(H2,22,23)(H,27,28)(H,24,25,26) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 overexpressing BT 474 cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099967
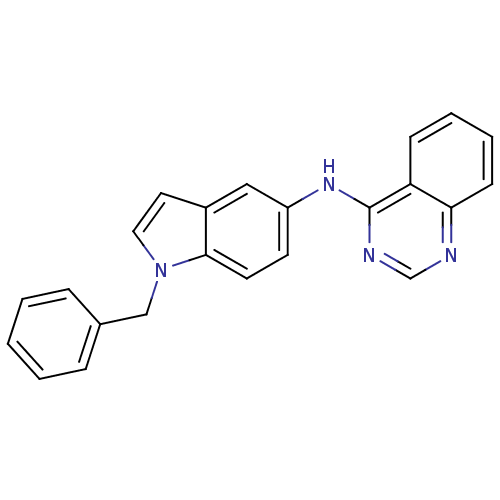
((1-Benzyl-1H-indol-5-yl)-quinazolin-4-yl-amine | C...)Show InChI InChI=1S/C23H18N4/c1-2-6-17(7-3-1)15-27-13-12-18-14-19(10-11-22(18)27)26-23-20-8-4-5-9-21(20)24-16-25-23/h1-14,16H,15H2,(H,24,25,26) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099966

(CHEMBL281006 | N*4*-(1-Benzyl-1H-indazol-5-yl)-pyr...)Show InChI InChI=1S/C21H17N7/c22-20-9-17-18(11-23-20)24-13-25-21(17)27-16-6-7-19-15(8-16)10-26-28(19)12-14-4-2-1-3-5-14/h1-11,13H,12H2,(H2,22,23)(H,24,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of c-erbB-2 overexpressing HB4a cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 overexpressing HB4a.e5.2 cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of EGF receptor overexpressing HN5 cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099960

((1-Benzyl-1H-indazol-5-yl)-(6,7-dimethoxy-quinazol...)Show SMILES COc1cc2ncnc(Nc3ccc4n(Cc5ccccc5)ncc4c3)c2cc1OC Show InChI InChI=1S/C24H21N5O2/c1-30-22-11-19-20(12-23(22)31-2)25-15-26-24(19)28-18-8-9-21-17(10-18)13-27-29(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of c-erbB-2 overexpressing HB4a cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099961

((2-Benzyl-2H-indazol-5-yl)-(6,7-dimethoxy-quinazol...)Show SMILES COc1cc2ncnc(Nc3ccc4nn(Cc5ccccc5)cc4c3)c2cc1OC Show InChI InChI=1S/C24H21N5O2/c1-30-22-11-19-21(12-23(22)31-2)25-15-26-24(19)27-18-8-9-20-17(10-18)14-29(28-20)13-16-6-4-3-5-7-16/h3-12,14-15H,13H2,1-2H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099970

((1-Benzyl-1H-indol-5-yl)-(6,7-dimethoxy-quinazolin...)Show SMILES COc1cc2ncnc(Nc3ccc4n(Cc5ccccc5)ccc4c3)c2cc1OC Show InChI InChI=1S/C25H22N4O2/c1-30-23-13-20-21(14-24(23)31-2)26-16-27-25(20)28-19-8-9-22-18(12-19)10-11-29(22)15-17-6-4-3-5-7-17/h3-14,16H,15H2,1-2H3,(H,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of c-erbB-2 overexpressing HB4a cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 overexpressing Calu3 cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 overexpressing Calu3 cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099959
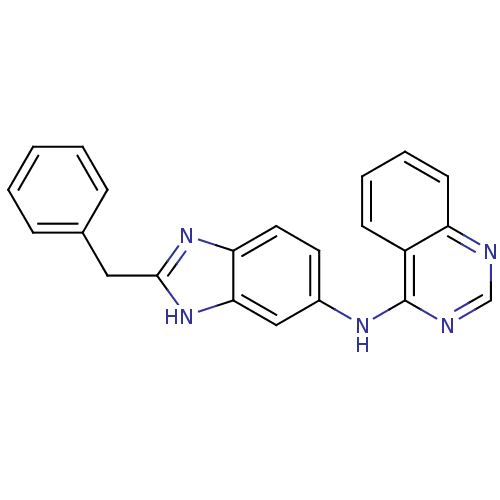
((2-Benzyl-1H-benzoimidazol-5-yl)-quinazolin-4-yl-a...)Show SMILES C(c1nc2ccc(Nc3ncnc4ccccc34)cc2[nH]1)c1ccccc1 Show InChI InChI=1S/C22H17N5/c1-2-6-15(7-3-1)12-21-26-19-11-10-16(13-20(19)27-21)25-22-17-8-4-5-9-18(17)23-14-24-22/h1-11,13-14H,12H2,(H,26,27)(H,23,24,25) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099969

((2-Benzyl-1H-benzoimidazol-5-yl)-(6,7-dimethoxy-qu...)Show SMILES COc1cc2ncnc(Nc3ccc4nc(Cc5ccccc5)[nH]c4c3)c2cc1OC Show InChI InChI=1S/C24H21N5O2/c1-30-21-12-17-19(13-22(21)31-2)25-14-26-24(17)27-16-8-9-18-20(11-16)29-23(28-18)10-15-6-4-3-5-7-15/h3-9,11-14H,10H2,1-2H3,(H,28,29)(H,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of c-erbB-2 overexpressing HB4a cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099967

((1-Benzyl-1H-indol-5-yl)-quinazolin-4-yl-amine | C...)Show InChI InChI=1S/C23H18N4/c1-2-6-17(7-3-1)15-27-13-12-18-14-19(10-11-22(18)27)26-23-20-8-4-5-9-21(20)24-16-25-23/h1-14,16H,15H2,(H,24,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of c-erbB-2 overexpressing HB4a cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099959

((2-Benzyl-1H-benzoimidazol-5-yl)-quinazolin-4-yl-a...)Show SMILES C(c1nc2ccc(Nc3ncnc4ccccc34)cc2[nH]1)c1ccccc1 Show InChI InChI=1S/C22H17N5/c1-2-6-15(7-3-1)12-21-26-19-11-10-16(13-20(19)27-21)25-22-17-8-4-5-9-18(17)23-14-24-22/h1-11,13-14H,12H2,(H,26,27)(H,23,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of c-erbB-2 overexpressing HB4a cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099968

(CHEMBL31653 | N*4*-(2-Benzyl-1H-benzoimidazol-5-yl...)Show SMILES Nc1cc2c(Nc3ccc4nc(Cc5ccccc5)[nH]c4c3)ncnc2cn1 Show InChI InChI=1S/C21H17N7/c22-19-10-15-18(11-23-19)24-12-25-21(15)26-14-6-7-16-17(9-14)28-20(27-16)8-13-4-2-1-3-5-13/h1-7,9-12H,8H2,(H2,22,23)(H,27,28)(H,24,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of c-erbB-2 overexpressing HB4a cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50411560

(CHEMBL240436)Show SMILES CCc1ccccc1N(CC(=O)NCc1ccc(Cl)cc1)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C24H25ClN2O3S/c1-3-20-6-4-5-7-23(20)27(31(29,30)22-14-8-18(2)9-15-22)17-24(28)26-16-19-10-12-21(25)13-11-19/h4-15H,3,16-17H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 17: 1750-4 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.060
BindingDB Entry DOI: 10.7270/Q2SB470M |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099961

((2-Benzyl-2H-indazol-5-yl)-(6,7-dimethoxy-quinazol...)Show SMILES COc1cc2ncnc(Nc3ccc4nn(Cc5ccccc5)cc4c3)c2cc1OC Show InChI InChI=1S/C24H21N5O2/c1-30-22-11-19-21(12-23(22)31-2)25-15-26-24(19)27-18-8-9-20-17(10-18)14-29(28-20)13-16-6-4-3-5-7-16/h3-12,14-15H,13H2,1-2H3,(H,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of c-erbB-2 overexpressing HB4a cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50411560

(CHEMBL240436)Show SMILES CCc1ccccc1N(CC(=O)NCc1ccc(Cl)cc1)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C24H25ClN2O3S/c1-3-20-6-4-5-7-23(20)27(31(29,30)22-14-8-18(2)9-15-22)17-24(28)26-16-19-10-12-21(25)13-11-19/h4-15H,3,16-17H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of FP receptor |
Bioorg Med Chem Lett 17: 1750-4 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.060
BindingDB Entry DOI: 10.7270/Q2SB470M |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099962
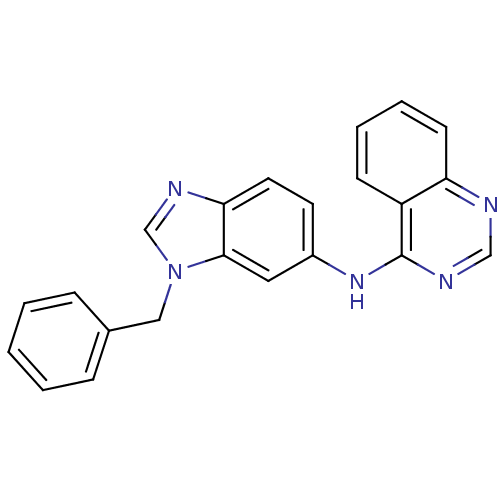
((3-Benzyl-3H-benzoimidazol-5-yl)-quinazolin-4-yl-a...)Show InChI InChI=1S/C22H17N5/c1-2-6-16(7-3-1)13-27-15-25-20-11-10-17(12-21(20)27)26-22-18-8-4-5-9-19(18)23-14-24-22/h1-12,14-15H,13H2,(H,23,24,26) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50411560

(CHEMBL240436)Show SMILES CCc1ccccc1N(CC(=O)NCc1ccc(Cl)cc1)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C24H25ClN2O3S/c1-3-20-6-4-5-7-23(20)27(31(29,30)22-14-8-18(2)9-15-22)17-24(28)26-16-19-10-12-21(25)13-11-19/h4-15H,3,16-17H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 17: 1750-4 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.060
BindingDB Entry DOI: 10.7270/Q2SB470M |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Rattus norvegicus) | BDBM50099964

((1-Benzyl-1H-benzoimidazol-5-yl)-quinazolin-4-yl-a...)Show InChI InChI=1S/C22H17N5/c1-2-6-16(7-3-1)13-27-15-25-20-12-17(10-11-21(20)27)26-22-18-8-4-5-9-19(18)23-14-24-22/h1-12,14-15H,13H2,(H,23,24,26) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of rat Receptor protein-tyrosine kinase erbB2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 2 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 1 |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 overexpressing BT 474 cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50191163

(1-(2-(benzyloxy)-5-bromobenzyl)-5-methyl-1H-pyrazo...)Show InChI InChI=1S/C19H17BrN2O3/c1-13-9-17(19(23)24)21-22(13)11-15-10-16(20)7-8-18(15)25-12-14-5-3-2-4-6-14/h2-10H,11-12H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 1A2 |
Bioorg Med Chem Lett 16: 4767-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.086
BindingDB Entry DOI: 10.7270/Q2MC8ZN4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099962

((3-Benzyl-3H-benzoimidazol-5-yl)-quinazolin-4-yl-a...)Show InChI InChI=1S/C22H17N5/c1-2-6-16(7-3-1)13-27-15-25-20-11-10-17(12-21(20)27)26-22-18-8-4-5-9-19(18)23-14-24-22/h1-12,14-15H,13H2,(H,23,24,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of c-erbB-2 overexpressing HB4a cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50099964

((1-Benzyl-1H-benzoimidazol-5-yl)-quinazolin-4-yl-a...)Show InChI InChI=1S/C22H17N5/c1-2-6-16(7-3-1)13-27-15-25-20-12-17(10-11-21(20)27)26-22-18-8-4-5-9-19(18)23-14-24-22/h1-12,14-15H,13H2,(H,23,24,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of c-erbB-2 overexpressing HB4a cell proliferation |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50027852
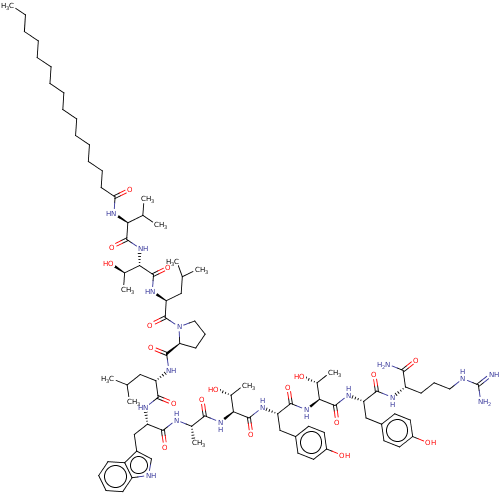
(CHEMBL3338677)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C88H137N17O18/c1-12-13-14-15-16-17-18-19-20-21-22-23-24-33-71(111)101-72(52(6)7)83(119)104-75(56(11)108)86(122)100-69(45-51(4)5)87(123)105-43-28-32-70(105)82(118)97-65(44-50(2)3)79(115)96-68(48-59-49-93-63-30-26-25-29-62(59)63)78(114)94-53(8)77(113)102-73(54(9)106)84(120)99-67(47-58-36-40-61(110)41-37-58)81(117)103-74(55(10)107)85(121)98-66(46-57-34-38-60(109)39-35-57)80(116)95-64(76(89)112)31-27-42-92-88(90)91/h25-26,29-30,34-41,49-56,64-70,72-75,93,106-110H,12-24,27-28,31-33,42-48H2,1-11H3,(H2,89,112)(H,94,114)(H,95,116)(H,96,115)(H,97,118)(H,98,121)(H,99,120)(H,100,122)(H,101,111)(H,102,113)(H,103,117)(H,104,119)(H4,90,91,92)/t53-,54+,55+,56+,64-,65-,66-,67-,68-,69-,70-,72-,73-,74-,75-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at D1A receptor (unknown origin) expressed in CHOK1 cells after 5 mins by HTRF cAMP immunoassay in presence of cAMP/SKF38393 |
Bioorg Med Chem Lett 24: 4871-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.045
BindingDB Entry DOI: 10.7270/Q2D50PH2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50027852

(CHEMBL3338677)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C88H137N17O18/c1-12-13-14-15-16-17-18-19-20-21-22-23-24-33-71(111)101-72(52(6)7)83(119)104-75(56(11)108)86(122)100-69(45-51(4)5)87(123)105-43-28-32-70(105)82(118)97-65(44-50(2)3)79(115)96-68(48-59-49-93-63-30-26-25-29-62(59)63)78(114)94-53(8)77(113)102-73(54(9)106)84(120)99-67(47-58-36-40-61(110)41-37-58)81(117)103-74(55(10)107)85(121)98-66(46-57-34-38-60(109)39-35-57)80(116)95-64(76(89)112)31-27-42-92-88(90)91/h25-26,29-30,34-41,49-56,64-70,72-75,93,106-110H,12-24,27-28,31-33,42-48H2,1-11H3,(H2,89,112)(H,94,114)(H,95,116)(H,96,115)(H,97,118)(H,98,121)(H,99,120)(H,100,122)(H,101,111)(H,102,113)(H,103,117)(H,104,119)(H4,90,91,92)/t53-,54+,55+,56+,64-,65-,66-,67-,68-,69-,70-,72-,73-,74-,75-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic acetylcholine receptor M2 (unknown origin) expressed in CHOK1 cells after 15 mins by calcium flux/FLIPR assay in pr... |
Bioorg Med Chem Lett 24: 4871-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.045
BindingDB Entry DOI: 10.7270/Q2D50PH2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50027852

(CHEMBL3338677)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C88H137N17O18/c1-12-13-14-15-16-17-18-19-20-21-22-23-24-33-71(111)101-72(52(6)7)83(119)104-75(56(11)108)86(122)100-69(45-51(4)5)87(123)105-43-28-32-70(105)82(118)97-65(44-50(2)3)79(115)96-68(48-59-49-93-63-30-26-25-29-62(59)63)78(114)94-53(8)77(113)102-73(54(9)106)84(120)99-67(47-58-36-40-61(110)41-37-58)81(117)103-74(55(10)107)85(121)98-66(46-57-34-38-60(109)39-35-57)80(116)95-64(76(89)112)31-27-42-92-88(90)91/h25-26,29-30,34-41,49-56,64-70,72-75,93,106-110H,12-24,27-28,31-33,42-48H2,1-11H3,(H2,89,112)(H,94,114)(H,95,116)(H,96,115)(H,97,118)(H,98,121)(H,99,120)(H,100,122)(H,101,111)(H,102,113)(H,103,117)(H,104,119)(H4,90,91,92)/t53-,54+,55+,56+,64-,65-,66-,67-,68-,69-,70-,72-,73-,74-,75-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT2A receptor (unknown origin) expressed in CHOK1 cells after 15 mins by calcium flux/FLIPR assay in presence of 5HT |
Bioorg Med Chem Lett 24: 4871-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.045
BindingDB Entry DOI: 10.7270/Q2D50PH2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027852

(CHEMBL3338677)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C88H137N17O18/c1-12-13-14-15-16-17-18-19-20-21-22-23-24-33-71(111)101-72(52(6)7)83(119)104-75(56(11)108)86(122)100-69(45-51(4)5)87(123)105-43-28-32-70(105)82(118)97-65(44-50(2)3)79(115)96-68(48-59-49-93-63-30-26-25-29-62(59)63)78(114)94-53(8)77(113)102-73(54(9)106)84(120)99-67(47-58-36-40-61(110)41-37-58)81(117)103-74(55(10)107)85(121)98-66(46-57-34-38-60(109)39-35-57)80(116)95-64(76(89)112)31-27-42-92-88(90)91/h25-26,29-30,34-41,49-56,64-70,72-75,93,106-110H,12-24,27-28,31-33,42-48H2,1-11H3,(H2,89,112)(H,94,114)(H,95,116)(H,96,115)(H,97,118)(H,98,121)(H,99,120)(H,100,122)(H,101,111)(H,102,113)(H,103,117)(H,104,119)(H4,90,91,92)/t53-,54+,55+,56+,64-,65-,66-,67-,68-,69-,70-,72-,73-,74-,75-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT1A receptor (unknown origin) expressed in HeLa cells after 15 mins by calcium flux/FLIPR assay in presence of R-(+)-8-OH-DP... |
Bioorg Med Chem Lett 24: 4871-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.045
BindingDB Entry DOI: 10.7270/Q2D50PH2 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50027852

(CHEMBL3338677)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C88H137N17O18/c1-12-13-14-15-16-17-18-19-20-21-22-23-24-33-71(111)101-72(52(6)7)83(119)104-75(56(11)108)86(122)100-69(45-51(4)5)87(123)105-43-28-32-70(105)82(118)97-65(44-50(2)3)79(115)96-68(48-59-49-93-63-30-26-25-29-62(59)63)78(114)94-53(8)77(113)102-73(54(9)106)84(120)99-67(47-58-36-40-61(110)41-37-58)81(117)103-74(55(10)107)85(121)98-66(46-57-34-38-60(109)39-35-57)80(116)95-64(76(89)112)31-27-42-92-88(90)91/h25-26,29-30,34-41,49-56,64-70,72-75,93,106-110H,12-24,27-28,31-33,42-48H2,1-11H3,(H2,89,112)(H,94,114)(H,95,116)(H,96,115)(H,97,118)(H,98,121)(H,99,120)(H,100,122)(H,101,111)(H,102,113)(H,103,117)(H,104,119)(H4,90,91,92)/t53-,54+,55+,56+,64-,65-,66-,67-,68-,69-,70-,72-,73-,74-,75-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha-2A adrenergic receptor (unknown origin) expressed in CHOK1 cells after 15 mins by calcium flux/FLIPR assay in presence o... |
Bioorg Med Chem Lett 24: 4871-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.045
BindingDB Entry DOI: 10.7270/Q2D50PH2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50027852

(CHEMBL3338677)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C88H137N17O18/c1-12-13-14-15-16-17-18-19-20-21-22-23-24-33-71(111)101-72(52(6)7)83(119)104-75(56(11)108)86(122)100-69(45-51(4)5)87(123)105-43-28-32-70(105)82(118)97-65(44-50(2)3)79(115)96-68(48-59-49-93-63-30-26-25-29-62(59)63)78(114)94-53(8)77(113)102-73(54(9)106)84(120)99-67(47-58-36-40-61(110)41-37-58)81(117)103-74(55(10)107)85(121)98-66(46-57-34-38-60(109)39-35-57)80(116)95-64(76(89)112)31-27-42-92-88(90)91/h25-26,29-30,34-41,49-56,64-70,72-75,93,106-110H,12-24,27-28,31-33,42-48H2,1-11H3,(H2,89,112)(H,94,114)(H,95,116)(H,96,115)(H,97,118)(H,98,121)(H,99,120)(H,100,122)(H,101,111)(H,102,113)(H,103,117)(H,104,119)(H4,90,91,92)/t53-,54+,55+,56+,64-,65-,66-,67-,68-,69-,70-,72-,73-,74-,75-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha-1A adrenergic receptor (unknown origin) expressed in CHOK1 cells after 15 mins by calcium flux/FLIPR assay in presence o... |
Bioorg Med Chem Lett 24: 4871-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.045
BindingDB Entry DOI: 10.7270/Q2D50PH2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50027852

(CHEMBL3338677)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C88H137N17O18/c1-12-13-14-15-16-17-18-19-20-21-22-23-24-33-71(111)101-72(52(6)7)83(119)104-75(56(11)108)86(122)100-69(45-51(4)5)87(123)105-43-28-32-70(105)82(118)97-65(44-50(2)3)79(115)96-68(48-59-49-93-63-30-26-25-29-62(59)63)78(114)94-53(8)77(113)102-73(54(9)106)84(120)99-67(47-58-36-40-61(110)41-37-58)81(117)103-74(55(10)107)85(121)98-66(46-57-34-38-60(109)39-35-57)80(116)95-64(76(89)112)31-27-42-92-88(90)91/h25-26,29-30,34-41,49-56,64-70,72-75,93,106-110H,12-24,27-28,31-33,42-48H2,1-11H3,(H2,89,112)(H,94,114)(H,95,116)(H,96,115)(H,97,118)(H,98,121)(H,99,120)(H,100,122)(H,101,111)(H,102,113)(H,103,117)(H,104,119)(H4,90,91,92)/t53-,54+,55+,56+,64-,65-,66-,67-,68-,69-,70-,72-,73-,74-,75-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at CB1 receptor (unknown origin) expressed in rat CHEM-1 cells after 15 mins by calcium flux/FLIPR assay in presence of CP55940 |
Bioorg Med Chem Lett 24: 4871-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.045
BindingDB Entry DOI: 10.7270/Q2D50PH2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50027852

(CHEMBL3338677)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C88H137N17O18/c1-12-13-14-15-16-17-18-19-20-21-22-23-24-33-71(111)101-72(52(6)7)83(119)104-75(56(11)108)86(122)100-69(45-51(4)5)87(123)105-43-28-32-70(105)82(118)97-65(44-50(2)3)79(115)96-68(48-59-49-93-63-30-26-25-29-62(59)63)78(114)94-53(8)77(113)102-73(54(9)106)84(120)99-67(47-58-36-40-61(110)41-37-58)81(117)103-74(55(10)107)85(121)98-66(46-57-34-38-60(109)39-35-57)80(116)95-64(76(89)112)31-27-42-92-88(90)91/h25-26,29-30,34-41,49-56,64-70,72-75,93,106-110H,12-24,27-28,31-33,42-48H2,1-11H3,(H2,89,112)(H,94,114)(H,95,116)(H,96,115)(H,97,118)(H,98,121)(H,99,120)(H,100,122)(H,101,111)(H,102,113)(H,103,117)(H,104,119)(H4,90,91,92)/t53-,54+,55+,56+,64-,65-,66-,67-,68-,69-,70-,72-,73-,74-,75-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT2B receptor (unknown origin) expressed in CHOK1 cells after 15 mins by calcium flux/FLIPR assay in presence of BW723C86 |
Bioorg Med Chem Lett 24: 4871-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.045
BindingDB Entry DOI: 10.7270/Q2D50PH2 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50027852

(CHEMBL3338677)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C88H137N17O18/c1-12-13-14-15-16-17-18-19-20-21-22-23-24-33-71(111)101-72(52(6)7)83(119)104-75(56(11)108)86(122)100-69(45-51(4)5)87(123)105-43-28-32-70(105)82(118)97-65(44-50(2)3)79(115)96-68(48-59-49-93-63-30-26-25-29-62(59)63)78(114)94-53(8)77(113)102-73(54(9)106)84(120)99-67(47-58-36-40-61(110)41-37-58)81(117)103-74(55(10)107)85(121)98-66(46-57-34-38-60(109)39-35-57)80(116)95-64(76(89)112)31-27-42-92-88(90)91/h25-26,29-30,34-41,49-56,64-70,72-75,93,106-110H,12-24,27-28,31-33,42-48H2,1-11H3,(H2,89,112)(H,94,114)(H,95,116)(H,96,115)(H,97,118)(H,98,121)(H,99,120)(H,100,122)(H,101,111)(H,102,113)(H,103,117)(H,104,119)(H4,90,91,92)/t53-,54+,55+,56+,64-,65-,66-,67-,68-,69-,70-,72-,73-,74-,75-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at thromboxane A2 receptor (unknown origin) expressed in HEK293-EBNA cells after 15 mins by calcium flux/FLIPR assay in presence ... |
Bioorg Med Chem Lett 24: 4871-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.045
BindingDB Entry DOI: 10.7270/Q2D50PH2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50411560

(CHEMBL240436)Show SMILES CCc1ccccc1N(CC(=O)NCc1ccc(Cl)cc1)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C24H25ClN2O3S/c1-3-20-6-4-5-7-23(20)27(31(29,30)22-14-8-18(2)9-15-22)17-24(28)26-16-19-10-12-21(25)13-11-19/h4-15H,3,16-17H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 17: 1750-4 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.060
BindingDB Entry DOI: 10.7270/Q2SB470M |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Raf kinase |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50099963

(CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...)Show SMILES CN(C)c1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C23H21N7/c1-29(2)22-11-19-20(13-24-22)25-15-26-23(19)28-18-8-9-21-17(10-18)12-27-30(21)14-16-6-4-3-5-7-16/h3-13,15H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Src kinase |
Bioorg Med Chem Lett 11: 1401-5 (2001)
BindingDB Entry DOI: 10.7270/Q2W37VKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50191163

(1-(2-(benzyloxy)-5-bromobenzyl)-5-methyl-1H-pyrazo...)Show InChI InChI=1S/C19H17BrN2O3/c1-13-9-17(19(23)24)21-22(13)11-15-10-16(20)7-8-18(15)25-12-14-5-3-2-4-6-14/h2-10H,11-12H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 2D6 |
Bioorg Med Chem Lett 16: 4767-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.086
BindingDB Entry DOI: 10.7270/Q2MC8ZN4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50191163

(1-(2-(benzyloxy)-5-bromobenzyl)-5-methyl-1H-pyrazo...)Show InChI InChI=1S/C19H17BrN2O3/c1-13-9-17(19(23)24)21-22(13)11-15-10-16(20)7-8-18(15)25-12-14-5-3-2-4-6-14/h2-10H,11-12H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 2C9 |
Bioorg Med Chem Lett 16: 4767-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.086
BindingDB Entry DOI: 10.7270/Q2MC8ZN4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data