 Found 186 hits with Last Name = 'menard' and Initial = 'ca'
Found 186 hits with Last Name = 'menard' and Initial = 'ca' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466891
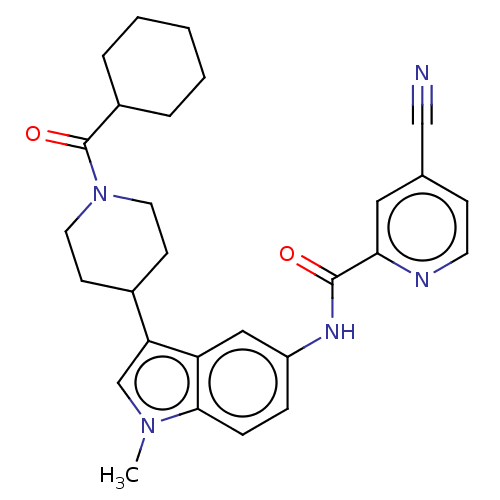
(CHEMBL4281109)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C28H31N5O2/c1-32-18-24(20-10-13-33(14-11-20)28(35)21-5-3-2-4-6-21)23-16-22(7-8-26(23)32)31-27(34)25-15-19(17-29)9-12-30-25/h7-9,12,15-16,18,20-21H,2-6,10-11,13-14H2,1H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466897
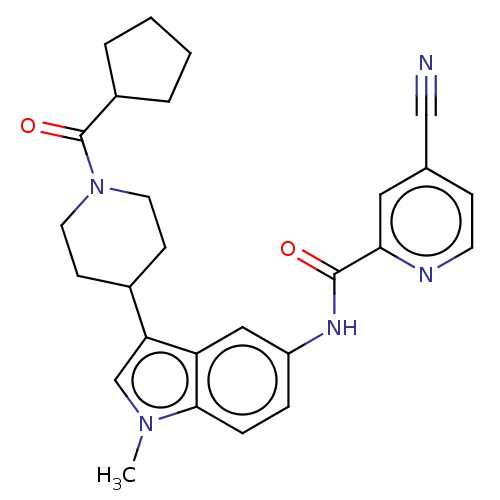
(CHEMBL4287715)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C27H29N5O2/c1-31-17-23(19-9-12-32(13-10-19)27(34)20-4-2-3-5-20)22-15-21(6-7-25(22)31)30-26(33)24-14-18(16-28)8-11-29-24/h6-8,11,14-15,17,19-20H,2-5,9-10,12-13H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466893
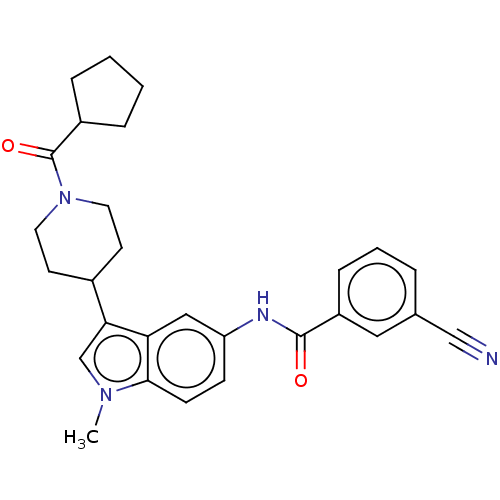
(CHEMBL4291727)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cccc(c3)C#N)ccc12 Show InChI InChI=1S/C28H30N4O2/c1-31-18-25(20-11-13-32(14-12-20)28(34)21-6-2-3-7-21)24-16-23(9-10-26(24)31)30-27(33)22-8-4-5-19(15-22)17-29/h4-5,8-10,15-16,18,20-21H,2-3,6-7,11-14H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human norepinephrine transporter |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466892
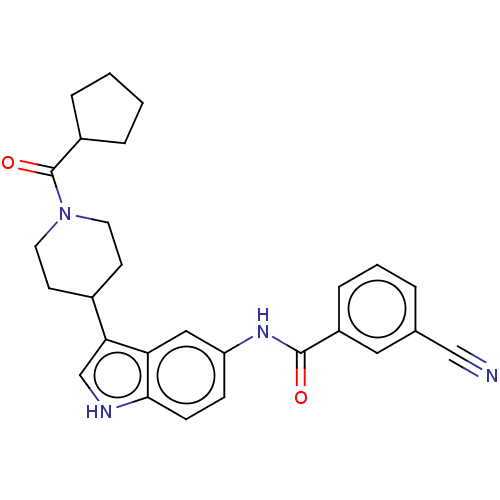
(CHEMBL4283871)Show SMILES O=C(Nc1ccc2[nH]cc(C3CCN(CC3)C(=O)C3CCCC3)c2c1)c1cccc(c1)C#N Show InChI InChI=1S/C27H28N4O2/c28-16-18-4-3-7-21(14-18)26(32)30-22-8-9-25-23(15-22)24(17-29-25)19-10-12-31(13-11-19)27(33)20-5-1-2-6-20/h3-4,7-9,14-15,17,19-20,29H,1-2,5-6,10-13H2,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-20
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP20 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-24
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP24 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-25
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP25 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-26
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP26 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP1 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP3 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP7 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP16 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-15
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP15 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Stromelysin-2
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP10 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TACE |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP14 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466913

(CHEMBL4289304)Show SMILES CCn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C28H31N5O2/c1-2-32-18-24(20-10-13-33(14-11-20)28(35)21-5-3-4-6-21)23-16-22(7-8-26(23)32)31-27(34)25-15-19(17-29)9-12-30-25/h7-9,12,15-16,18,20-21H,2-6,10-11,13-14H2,1H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395921
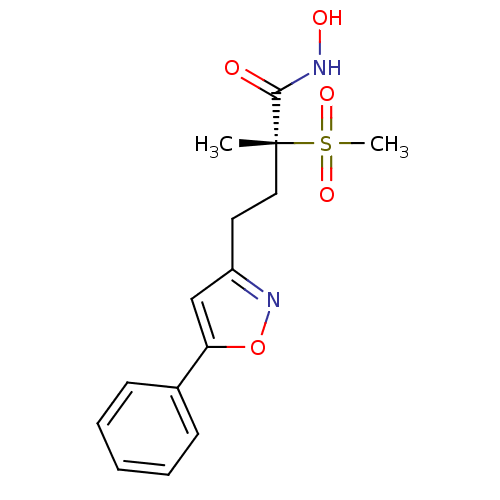
(CHEMBL2164511)Show SMILES C[C@@](CCc1cc(on1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C15H18N2O5S/c1-15(14(18)16-19,23(2,20)21)9-8-12-10-13(22-17-12)11-6-4-3-5-7-11/h3-7,10,19H,8-9H2,1-2H3,(H,16,18)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.511 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485077
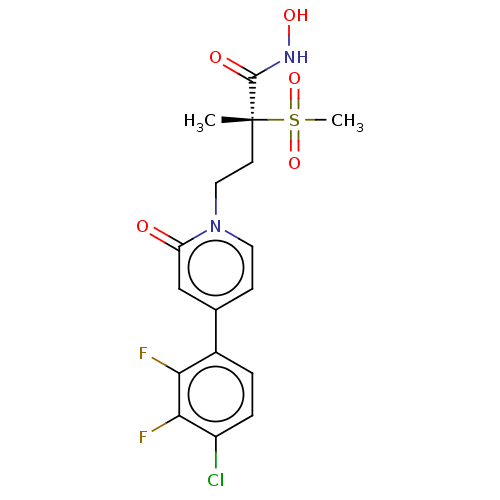
(CHEMBL2023517)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(Cl)c(F)c1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C17H17ClF2N2O5S/c1-17(16(24)21-25,28(2,26)27)6-8-22-7-5-10(9-13(22)23)11-3-4-12(18)15(20)14(11)19/h3-5,7,9,25H,6,8H2,1-2H3,(H,21,24)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485061

(CHEMBL2023524)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-n1nccn1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C19H21N5O5S/c1-19(18(26)22-27,30(2,28)29)8-12-23-11-7-15(13-17(23)25)14-3-5-16(6-4-14)24-20-9-10-21-24/h3-7,9-11,13,27H,8,12H2,1-2H3,(H,22,26)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395911
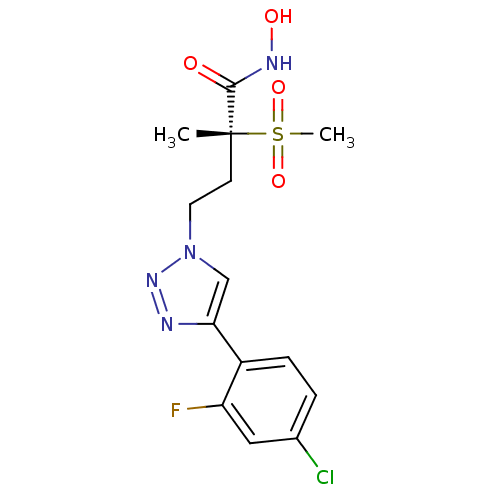
(CHEMBL2164521)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)cc1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16ClFN4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)10-4-3-9(15)7-11(10)16/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.657 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395920
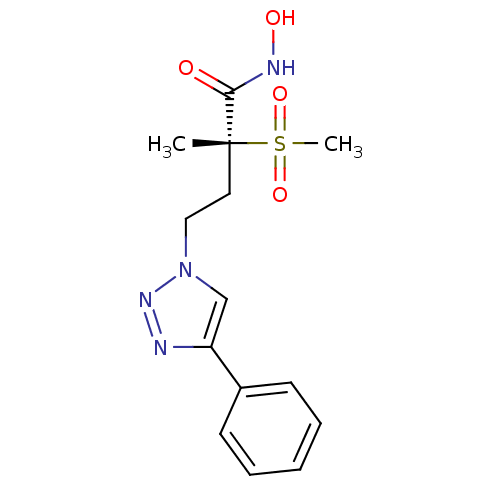
(CHEMBL2164512)Show SMILES C[C@@](CCn1cc(nn1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H18N4O4S/c1-14(13(19)16-20,23(2,21)22)8-9-18-10-12(15-17-18)11-6-4-3-5-7-11/h3-7,10,20H,8-9H2,1-2H3,(H,16,19)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395910
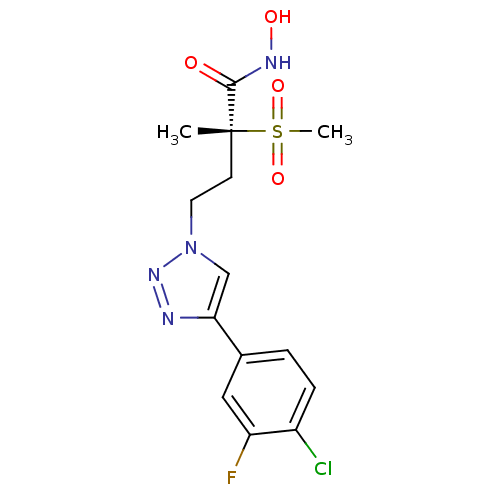
(CHEMBL2164522)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)c(F)c1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16ClFN4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)9-3-4-10(15)11(16)7-9/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485056
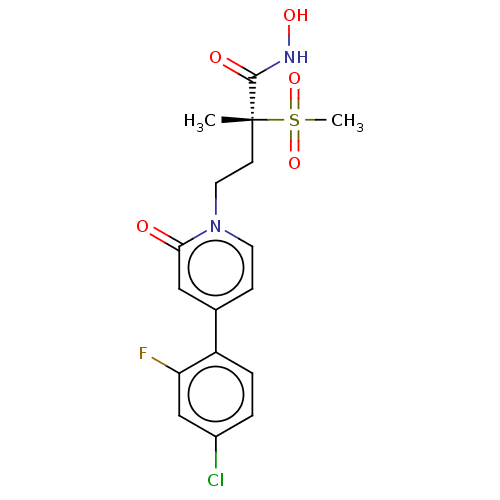
(CHEMBL2023515)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(Cl)cc1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C17H18ClFN2O5S/c1-17(16(23)20-24,27(2,25)26)6-8-21-7-5-11(9-15(21)22)13-4-3-12(18)10-14(13)19/h3-5,7,9-10,24H,6,8H2,1-2H3,(H,20,23)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485076
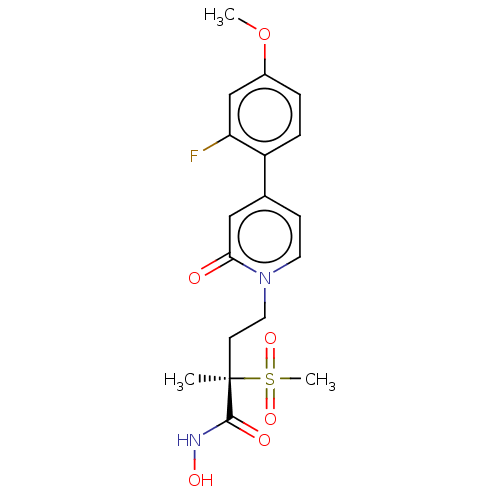
(CHEMBL2023402)Show SMILES COc1ccc(c(F)c1)-c1ccn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)c(=O)c1 |r| Show InChI InChI=1S/C18H21FN2O6S/c1-18(17(23)20-24,28(3,25)26)7-9-21-8-6-12(10-16(21)22)14-5-4-13(27-2)11-15(14)19/h4-6,8,10-11,24H,7,9H2,1-3H3,(H,20,23)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485083
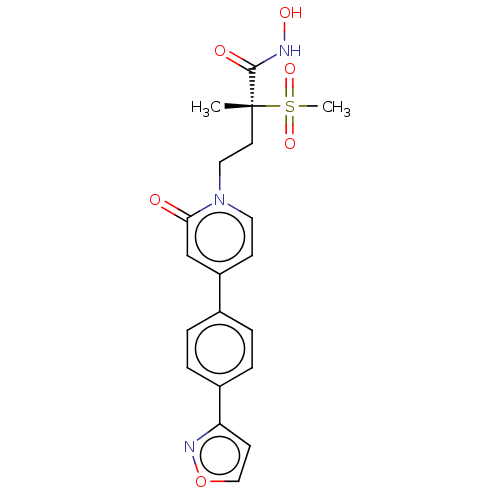
(CHEMBL2023523)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-c1ccon1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C20H21N3O6S/c1-20(19(25)21-26,30(2,27)28)9-11-23-10-7-16(13-18(23)24)14-3-5-15(6-4-14)17-8-12-29-22-17/h3-8,10,12-13,26H,9,11H2,1-2H3,(H,21,25)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485060
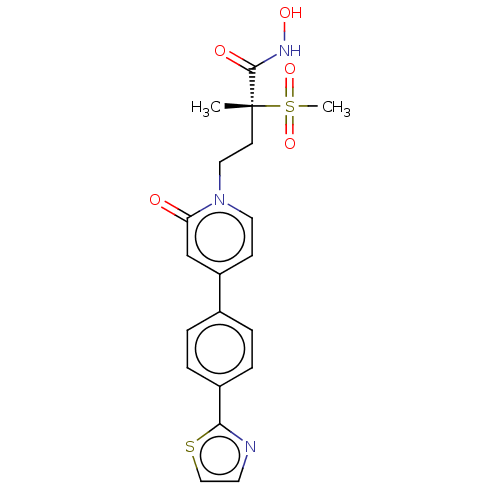
(CHEMBL2023522)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-c1nccs1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C20H21N3O5S2/c1-20(19(25)22-26,30(2,27)28)8-11-23-10-7-16(13-17(23)24)14-3-5-15(6-4-14)18-21-9-12-29-18/h3-7,9-10,12-13,26H,8,11H2,1-2H3,(H,22,25)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485078
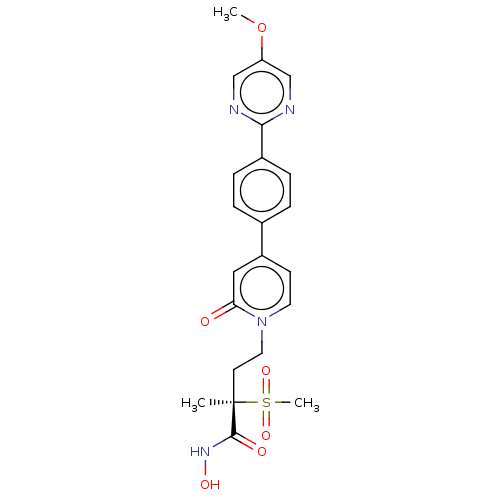
(CHEMBL2023521)Show SMILES COc1cnc(nc1)-c1ccc(cc1)-c1ccn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)c(=O)c1 |r| Show InChI InChI=1S/C22H24N4O6S/c1-22(21(28)25-29,33(3,30)31)9-11-26-10-8-17(12-19(26)27)15-4-6-16(7-5-15)20-23-13-18(32-2)14-24-20/h4-8,10,12-14,29H,9,11H2,1-3H3,(H,25,28)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395909

(CHEMBL2164523)Show SMILES COc1ccc(-c2cn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)nn2)c(F)c1 |r| Show InChI InChI=1S/C15H19FN4O5S/c1-15(14(21)18-22,26(3,23)24)6-7-20-9-13(17-19-20)11-5-4-10(25-2)8-12(11)16/h4-5,8-9,22H,6-7H2,1-3H3,(H,18,21)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485084
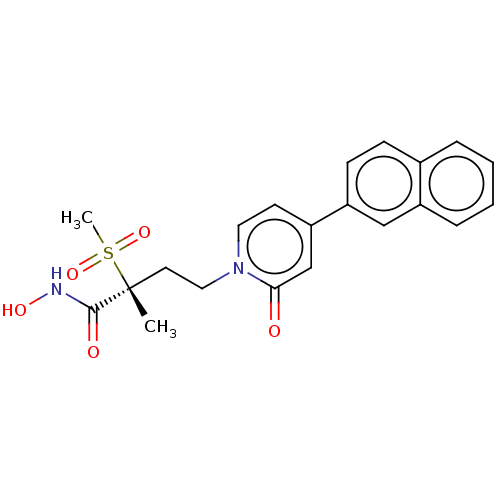
(CHEMBL2023518)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc2ccccc2c1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C21H22N2O5S/c1-21(20(25)22-26,29(2,27)28)10-12-23-11-9-18(14-19(23)24)17-8-7-15-5-3-4-6-16(15)13-17/h3-9,11,13-14,26H,10,12H2,1-2H3,(H,22,25)/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485059
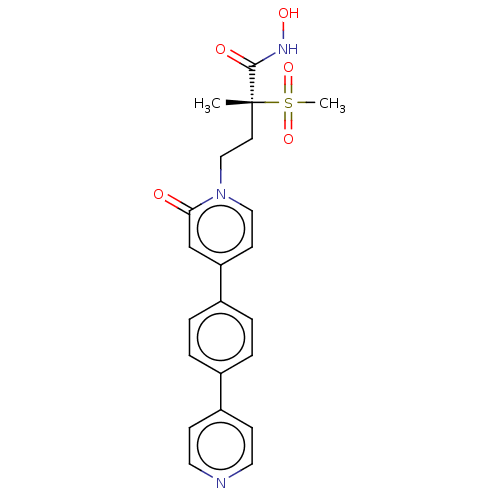
(CHEMBL2023520)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-c1ccncc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C22H23N3O5S/c1-22(21(27)24-28,31(2,29)30)10-14-25-13-9-19(15-20(25)26)17-5-3-16(4-6-17)18-7-11-23-12-8-18/h3-9,11-13,15,28H,10,14H2,1-2H3,(H,24,27)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485058
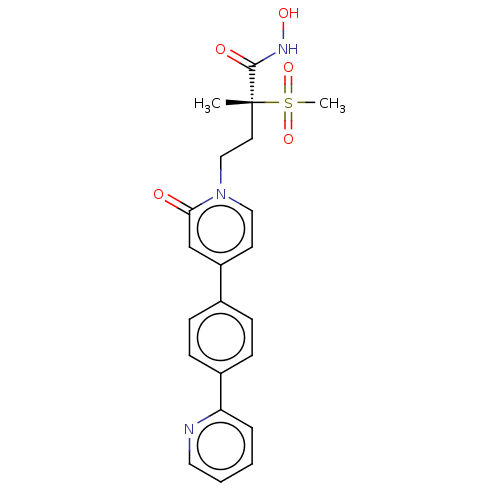
(CHEMBL2023519)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-c1ccccn1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C22H23N3O5S/c1-22(21(27)24-28,31(2,29)30)11-14-25-13-10-18(15-20(25)26)16-6-8-17(9-7-16)19-5-3-4-12-23-19/h3-10,12-13,15,28H,11,14H2,1-2H3,(H,24,27)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395908
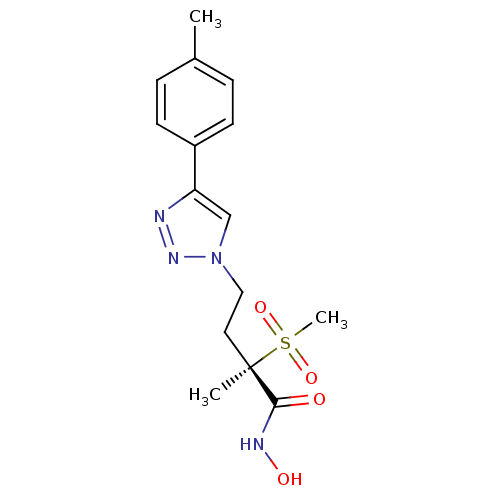
(CHEMBL2164524)Show SMILES Cc1ccc(cc1)-c1cn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)nn1 |r| Show InChI InChI=1S/C15H20N4O4S/c1-11-4-6-12(7-5-11)13-10-19(18-16-13)9-8-15(2,14(20)17-21)24(3,22)23/h4-7,10,21H,8-9H2,1-3H3,(H,17,20)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485057
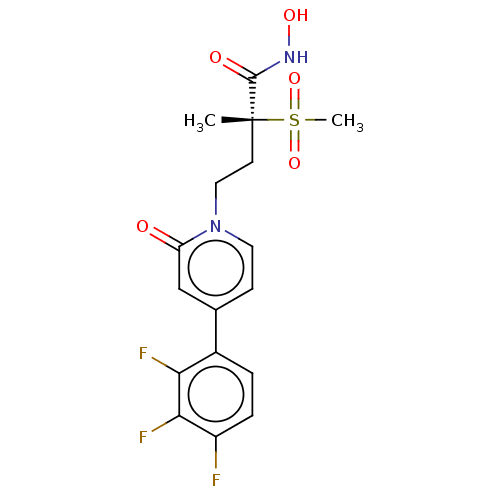
(CHEMBL2023516)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(F)c(F)c1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C17H17F3N2O5S/c1-17(16(24)21-25,28(2,26)27)6-8-22-7-5-10(9-13(22)23)11-3-4-12(18)15(20)14(11)19/h3-5,7,9,25H,6,8H2,1-2H3,(H,21,24)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395919
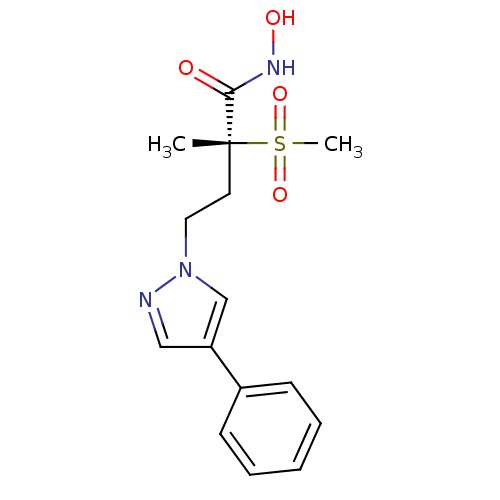
(CHEMBL2164513)Show SMILES C[C@@](CCn1cc(cn1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C15H19N3O4S/c1-15(14(19)17-20,23(2,21)22)8-9-18-11-13(10-16-18)12-6-4-3-5-7-12/h3-7,10-11,20H,8-9H2,1-2H3,(H,17,19)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395907
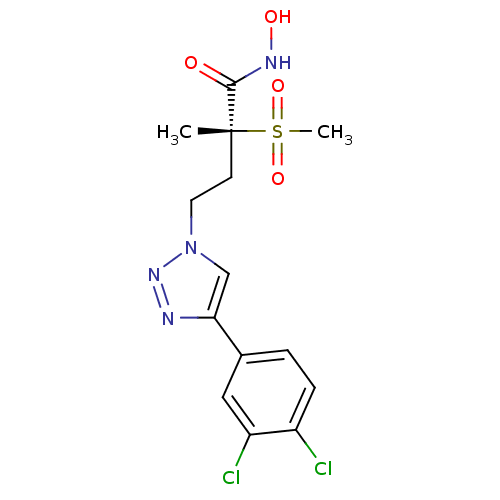
(CHEMBL2164525)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)c(Cl)c1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16Cl2N4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)9-3-4-10(15)11(16)7-9/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485054
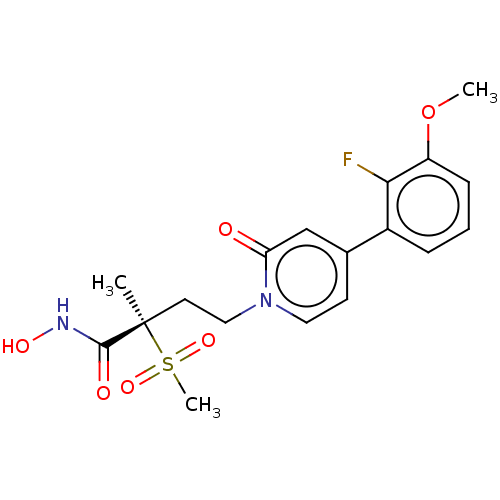
(CHEMBL2023401)Show SMILES COc1cccc(c1F)-c1ccn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)c(=O)c1 |r| Show InChI InChI=1S/C18H21FN2O6S/c1-18(17(23)20-24,28(3,25)26)8-10-21-9-7-12(11-15(21)22)13-5-4-6-14(27-2)16(13)19/h4-7,9,11,24H,8,10H2,1-3H3,(H,20,23)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485047
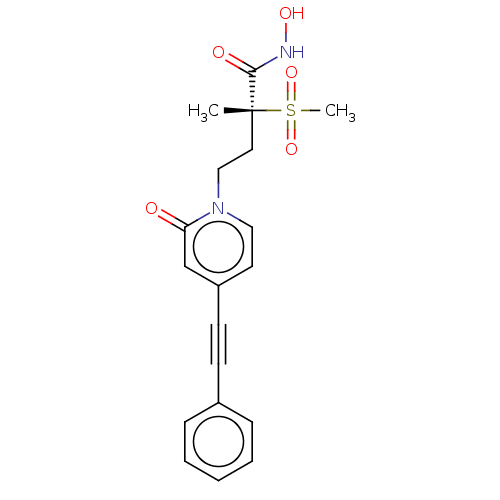
(CHEMBL2023387)Show SMILES C[C@@](CCn1ccc(cc1=O)C#Cc1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C19H20N2O5S/c1-19(18(23)20-24,27(2,25)26)11-13-21-12-10-16(14-17(21)22)9-8-15-6-4-3-5-7-15/h3-7,10,12,14,24H,11,13H2,1-2H3,(H,20,23)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395906
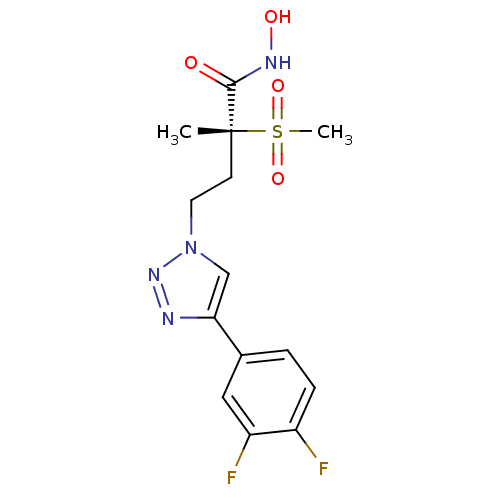
(CHEMBL2164526)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(F)c(F)c1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16F2N4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)9-3-4-10(15)11(16)7-9/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395905
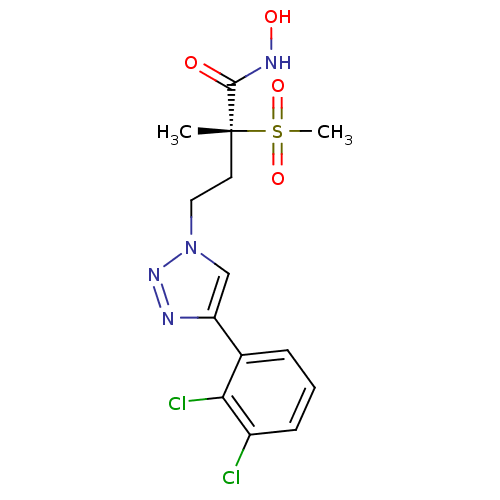
(CHEMBL2164184)Show SMILES C[C@@](CCn1cc(nn1)-c1cccc(Cl)c1Cl)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16Cl2N4O4S/c1-14(13(21)18-22,25(2,23)24)6-7-20-8-11(17-19-20)9-4-3-5-10(15)12(9)16/h3-5,8,22H,6-7H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485055
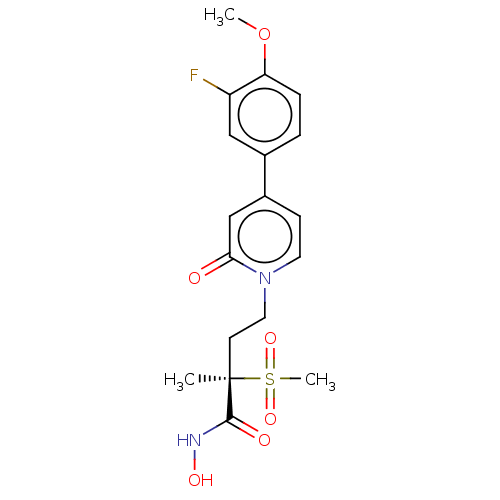
(CHEMBL2023403)Show SMILES COc1ccc(cc1F)-c1ccn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)c(=O)c1 |r| Show InChI InChI=1S/C18H21FN2O6S/c1-18(17(23)20-24,28(3,25)26)7-9-21-8-6-13(11-16(21)22)12-4-5-15(27-2)14(19)10-12/h4-6,8,10-11,24H,7,9H2,1-3H3,(H,20,23)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485079
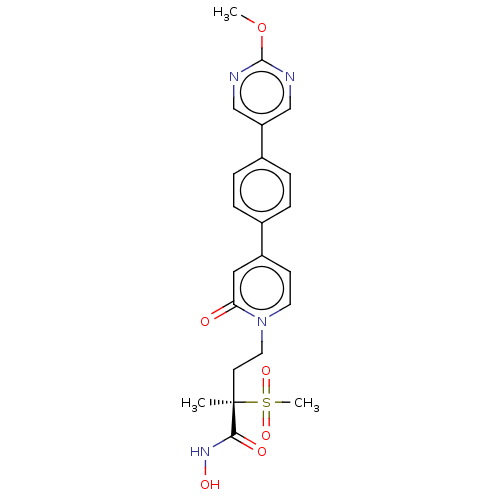
(CHEMBL2021944)Show SMILES COc1ncc(cn1)-c1ccc(cc1)-c1ccn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)c(=O)c1 |r| Show InChI InChI=1S/C22H24N4O6S/c1-22(20(28)25-29,33(3,30)31)9-11-26-10-8-17(12-19(26)27)15-4-6-16(7-5-15)18-13-23-21(32-2)24-14-18/h4-8,10,12-14,29H,9,11H2,1-3H3,(H,25,28)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485073
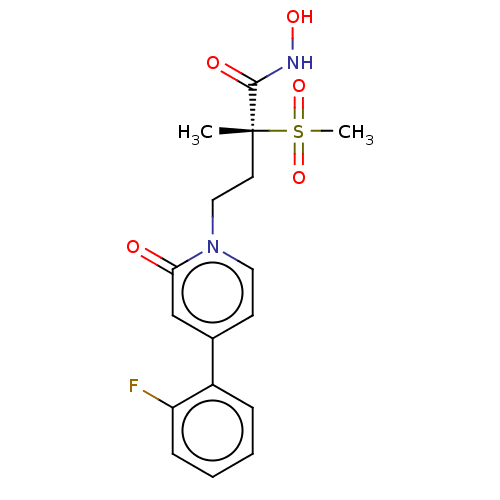
(CHEMBL2023390)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccccc1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C17H19FN2O5S/c1-17(16(22)19-23,26(2,24)25)8-10-20-9-7-12(11-15(20)21)13-5-3-4-6-14(13)18/h3-7,9,11,23H,8,10H2,1-2H3,(H,19,22)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data