

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50399537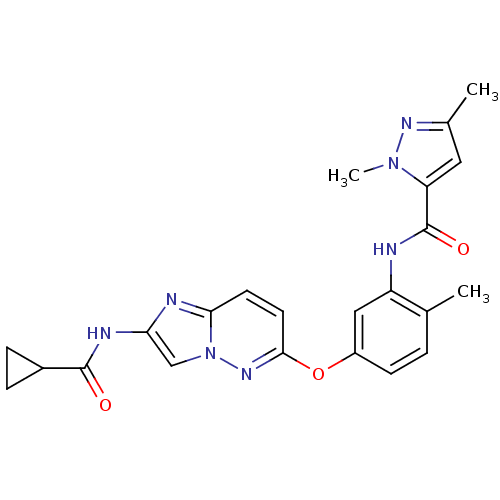 (CHEMBL2180604) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 60 mins by AlphaScreen assay in presence of 1000 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828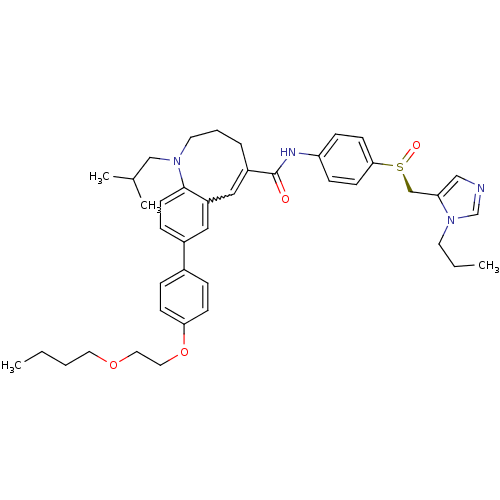 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432398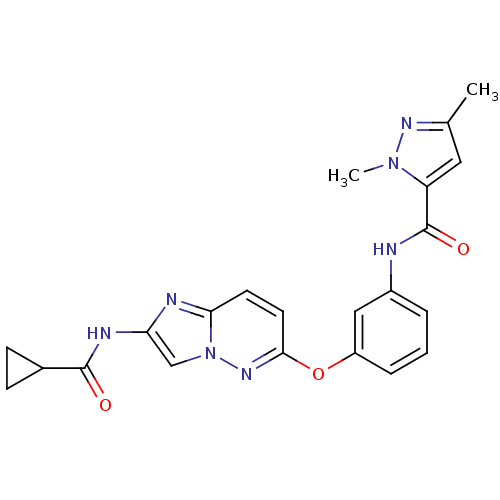 (CHEMBL2348996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 60 mins by AlphaScreen assay in presence of 1000 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184396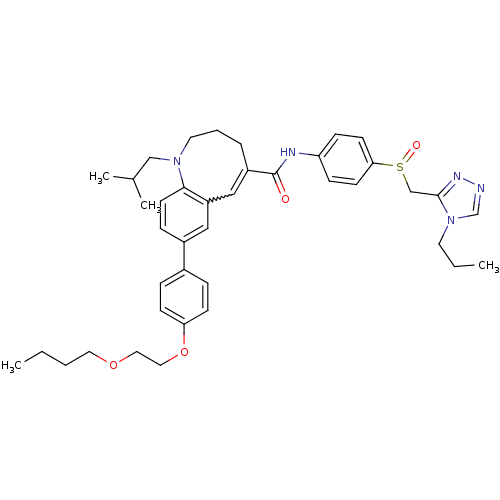 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399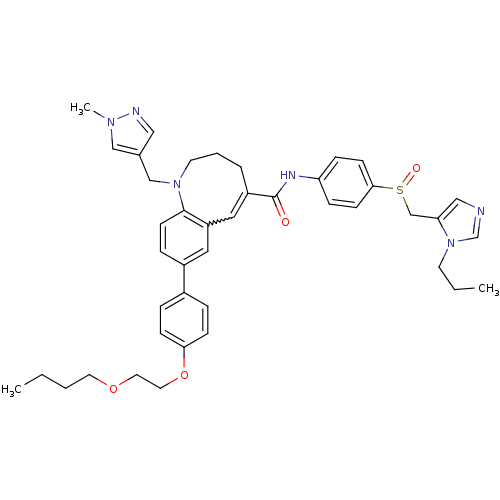 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398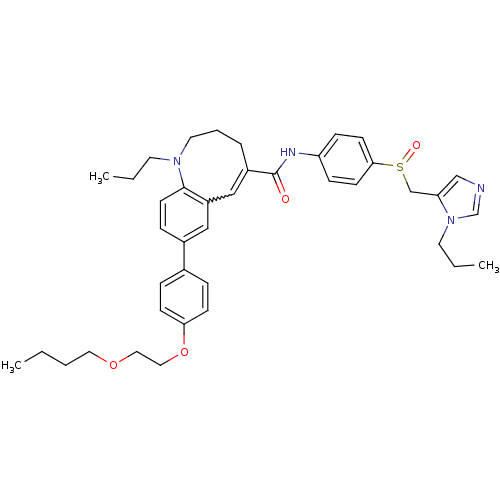 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184400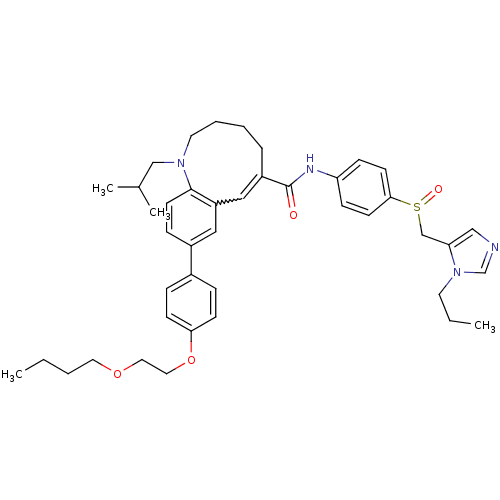 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444208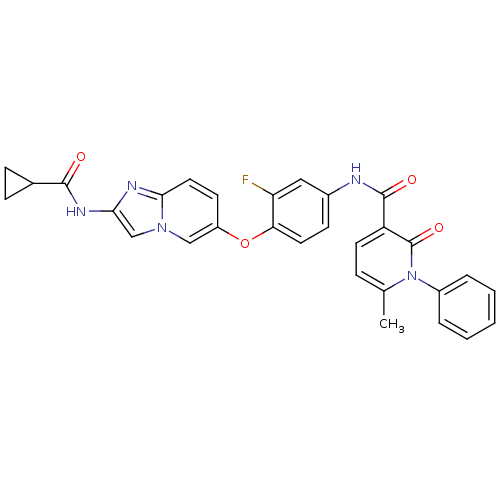 (CHEMBL3093579 | D3RKN_15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 60 mins followed by 1000 uM of ATP addition m... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444209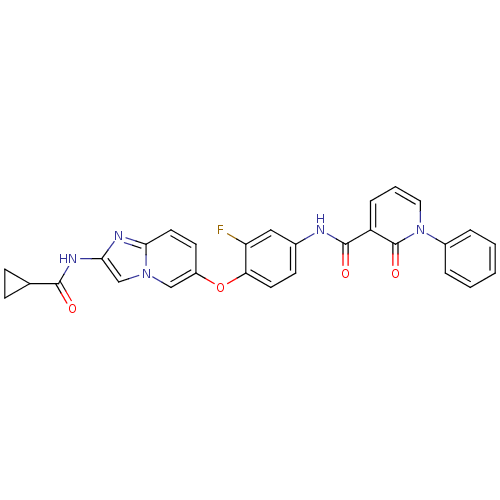 (CHEMBL3093581) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 60 mins followed by 1000 uM of ATP addition m... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444210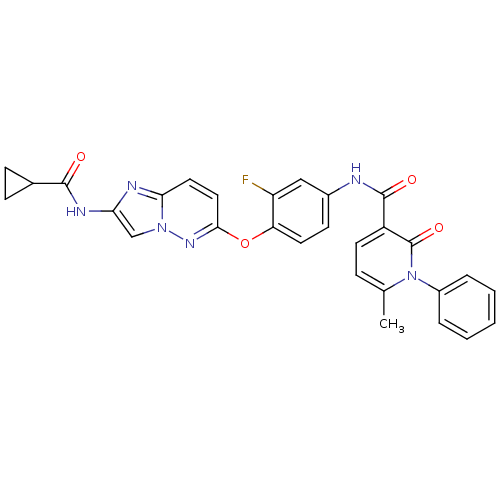 (CHEMBL3093582) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 60 mins followed by 1000 uM of ATP addition m... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184402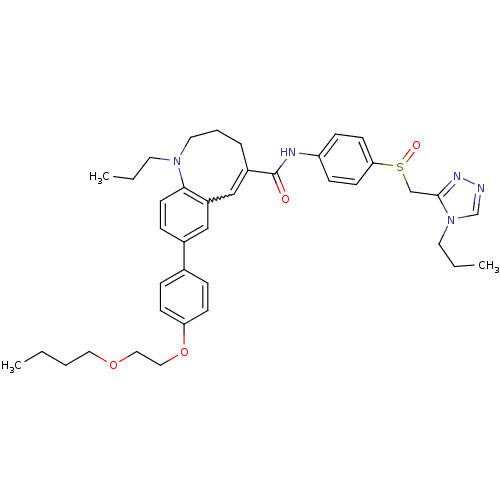 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50399537 (CHEMBL2180604) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432408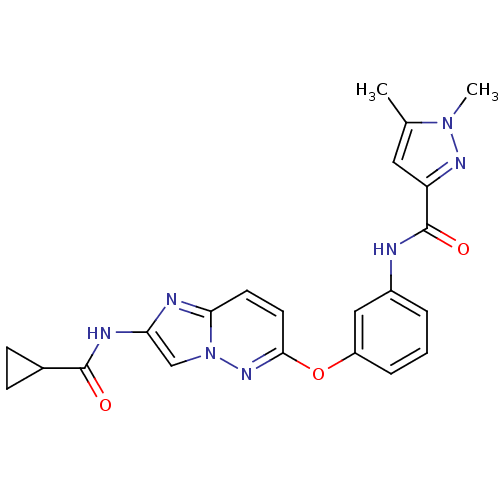 (CHEMBL2348998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432399 (CHEMBL2349006) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184403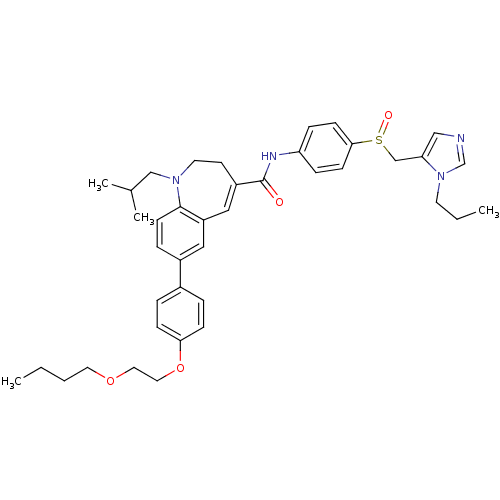 (7-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-((1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432415 (CHEMBL2349005) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50399537 (CHEMBL2180604) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR3 (unknown origin) by AlphaScreen assay | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432397 (CHEMBL2348989) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 60 mins by AlphaScreen assay in presence of 1000 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50399537 (CHEMBL2180604) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 1000 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432401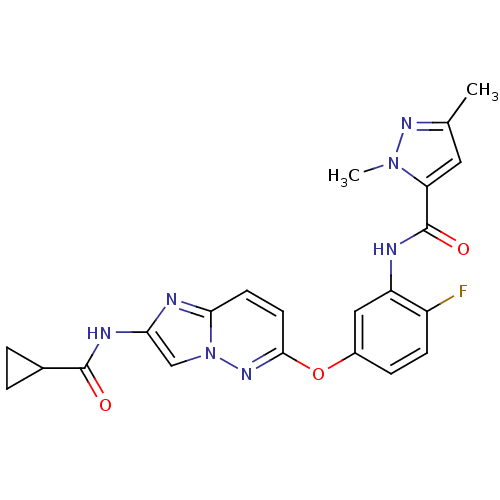 (CHEMBL2348991) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50182869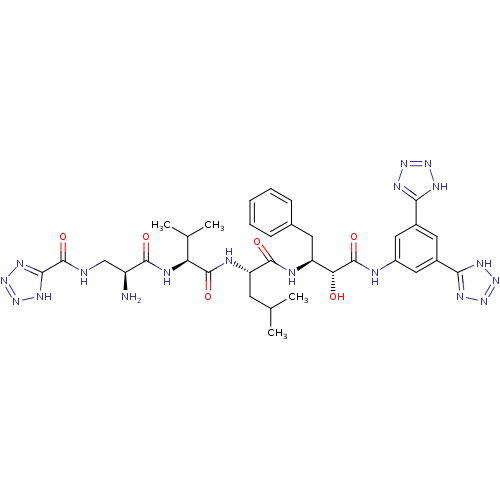 (CHEMBL439521 | N-((S)-2-amino-3-((S)-1-((S)-1-((2S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by FRET assay | Bioorg Med Chem Lett 18: 1643-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.056 BindingDB Entry DOI: 10.7270/Q2J38S96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432406 (CHEMBL2348995) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432400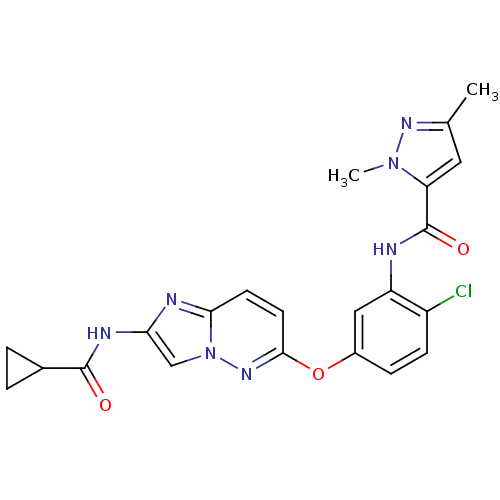 (CHEMBL2348990) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425719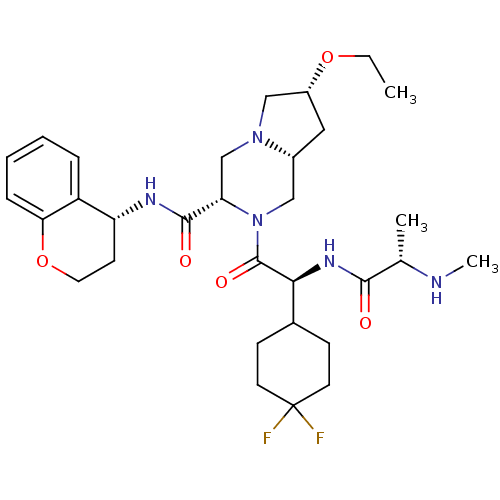 (CHEMBL2316217) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432412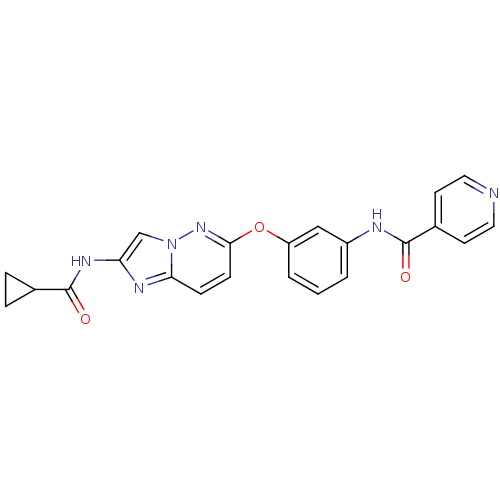 (CHEMBL2349002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432411 (CHEMBL2349001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432409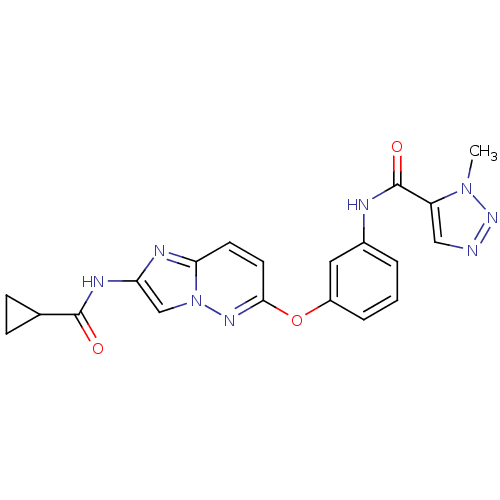 (CHEMBL2348999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432410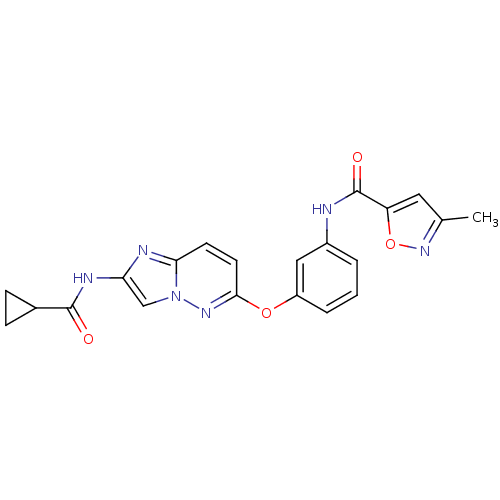 (CHEMBL2349000) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432398 (CHEMBL2348996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425721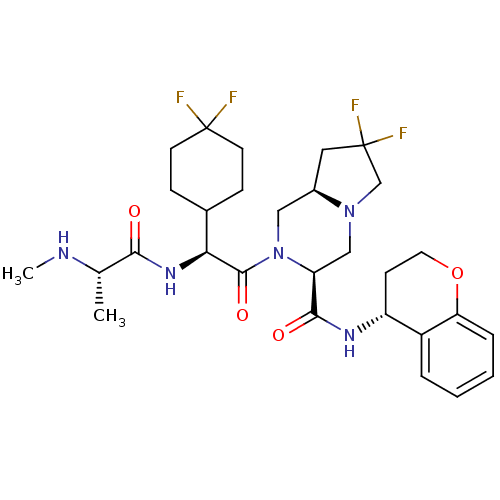 (CHEMBL2311586) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444212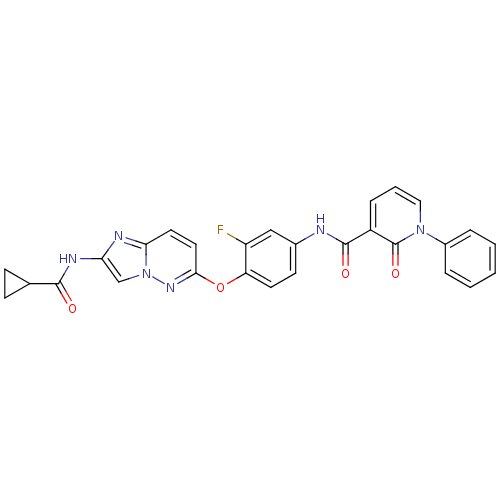 (CHEMBL3093583 | D3RKN_14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 5 mins followed by 2 uM of ATP addition measu... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444209 (CHEMBL3093581) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 5 mins followed by 2 uM of ATP addition measu... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432398 (CHEMBL2348996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 1000 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432404 (CHEMBL2348994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444210 (CHEMBL3093582) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 5 mins followed by 2 uM of ATP addition measu... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184403 (7-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-((1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444208 (CHEMBL3093579 | D3RKN_15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 5 mins followed by 2 uM of ATP addition measu... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425728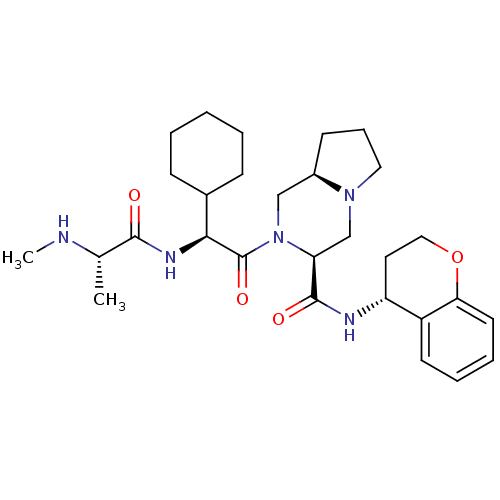 (CHEMBL2365533) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184400 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184402 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444213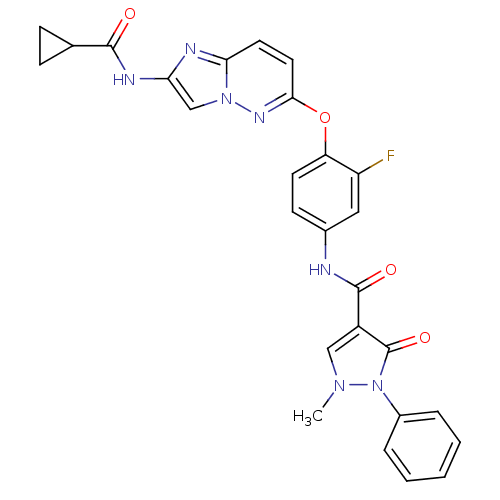 (CHEMBL3093585) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 5 mins followed by 2 uM of ATP addition measu... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184396 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425722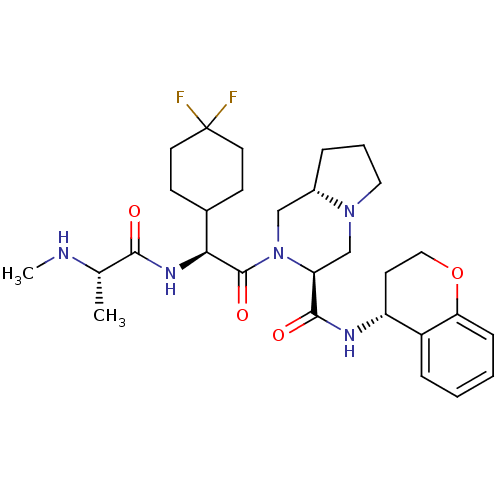 (CHEMBL2316215) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 306 total ) | Next | Last >> |