

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286344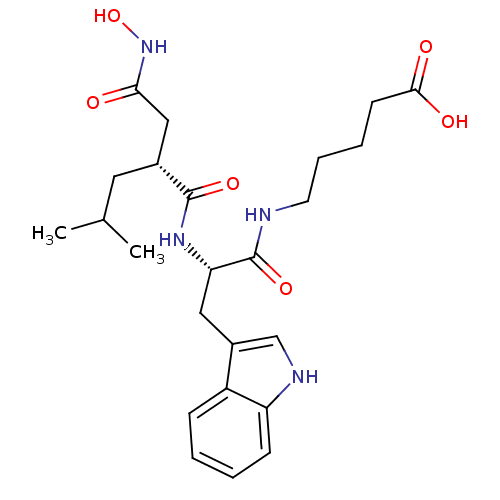 (5-[(S)-2-((R)-2-Hydroxycarbamoylmethyl-4-methyl-pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286335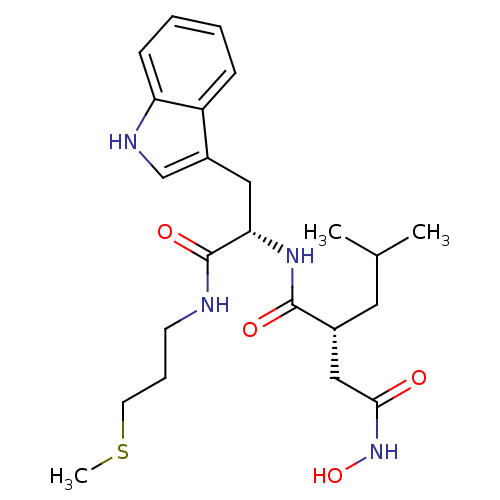 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286337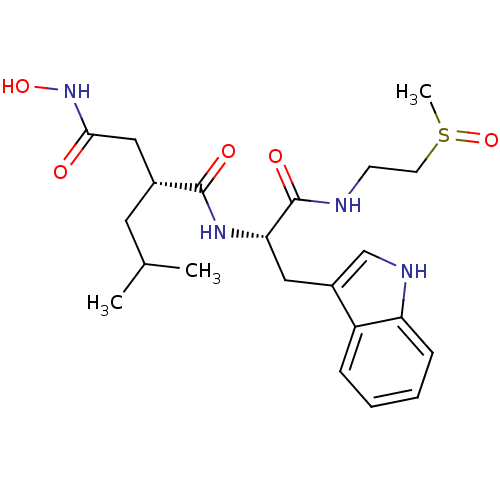 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286340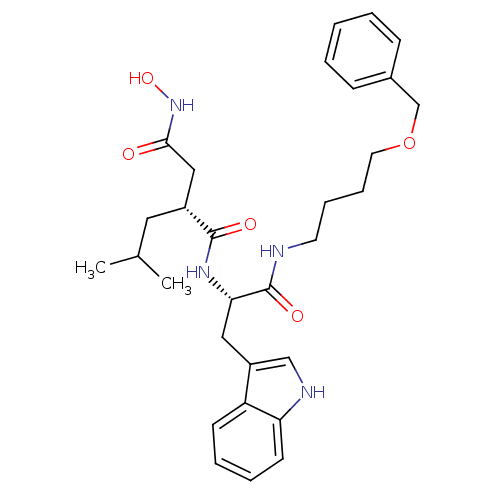 ((R)-N*1*-[(S)-1-(4-Benzyloxy-butylcarbamoyl)-2-(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286334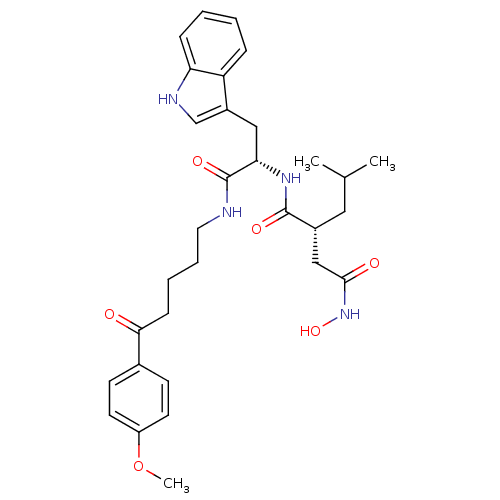 ((R)-N*4*-Hydroxy-N*1*-{(S)-2-(1H-indol-3-yl)-1-[5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50065451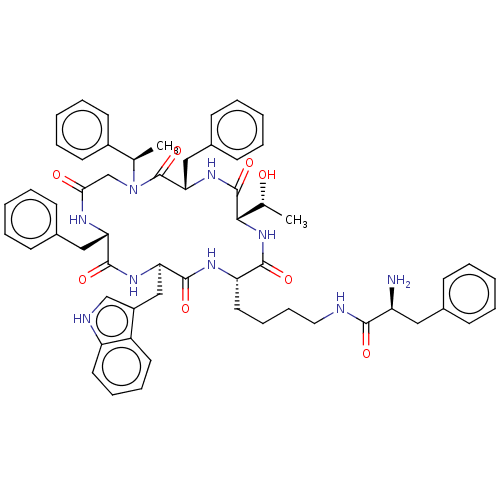 (2-Amino-N-{4-[(2S,5S,8S,14S,17R)-8,14-dibenzyl-5-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst2) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286349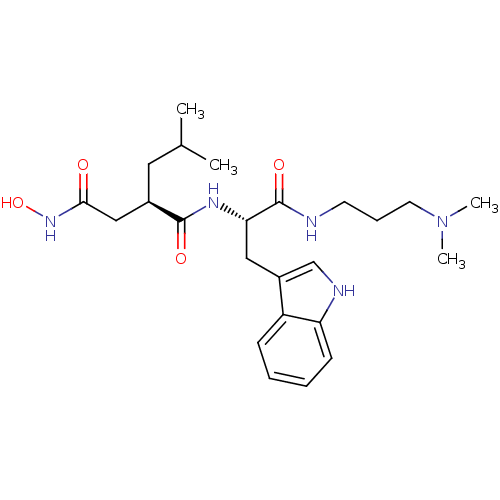 ((R)-N*1*-[(S)-1-(3-Dimethylamino-propylcarbamoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286345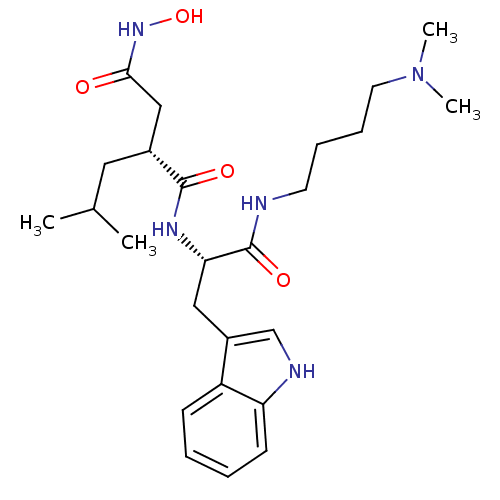 ((R)-N*1*-[(S)-1-(4-Dimethylamino-butylcarbamoyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286350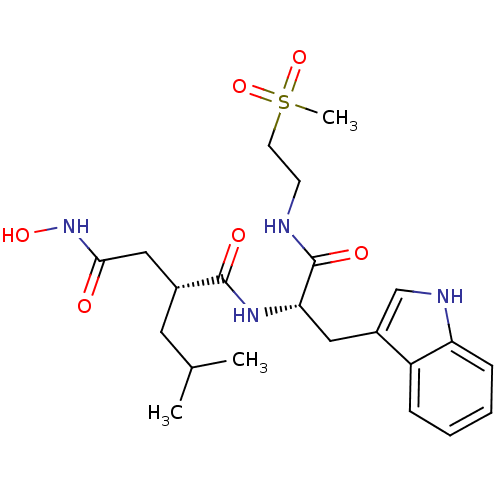 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286347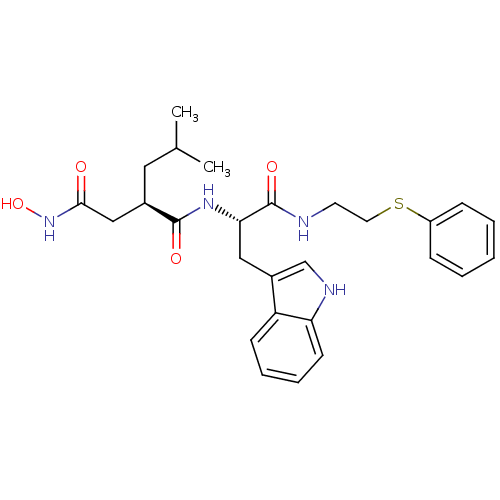 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286339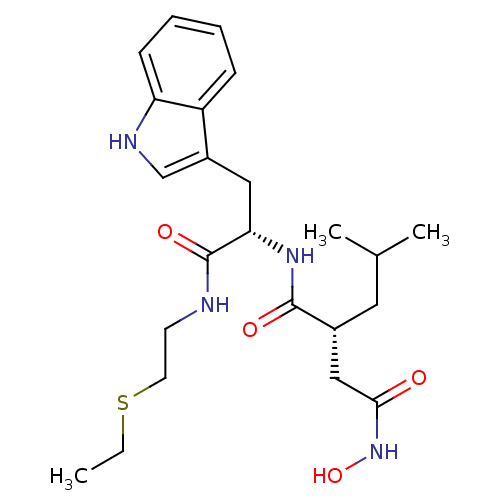 ((R)-N*1*-[(S)-1-(2-Ethylsulfanyl-ethylcarbamoyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286346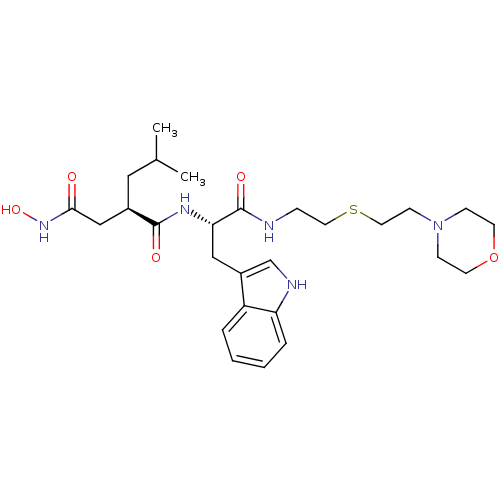 ((R)-N*4*-Hydroxy-N*1*-{(S)-2-(1H-indol-3-yl)-1-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286343 ((R)-N*1*-[(S)-1-(4-Dimethylaminomethyl-benzylcarba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286336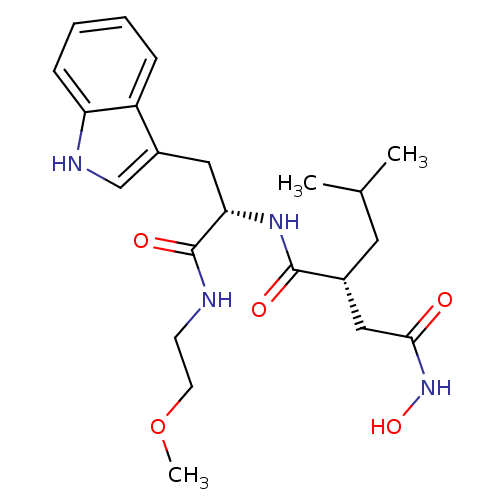 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286342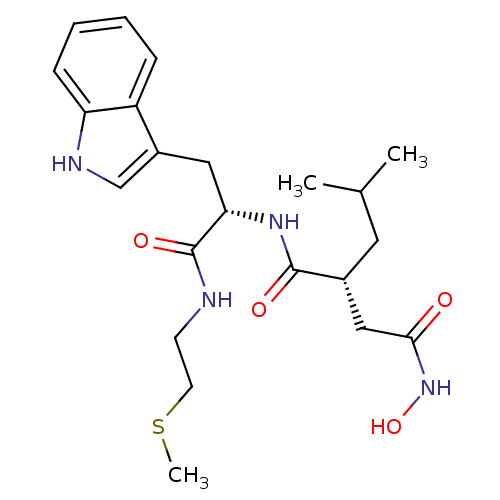 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286348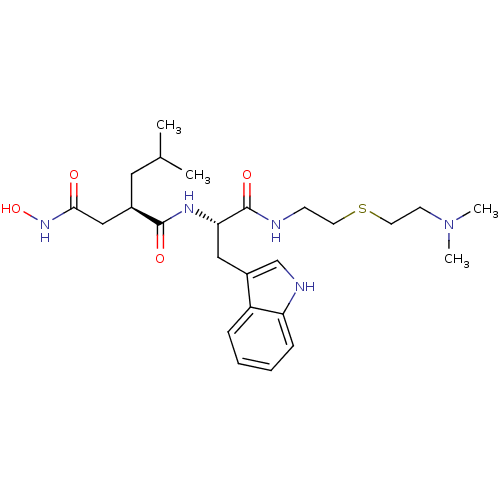 ((R)-N*1*-[(S)-1-[2-(2-Dimethylamino-ethylsulfanyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50065453 ((5S,8S,11S,14R,17S,19aS)-11-(4-Amino-butyl)-5,17-d...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst2) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286338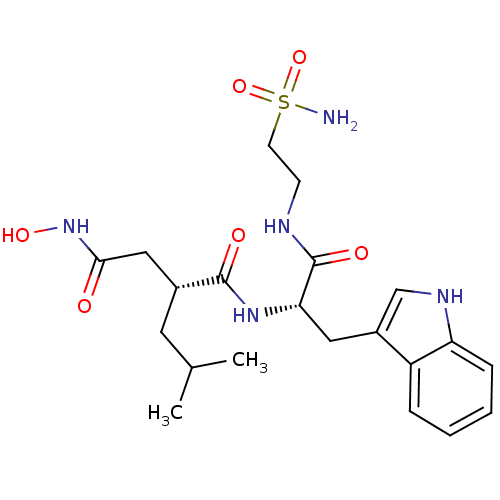 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50104969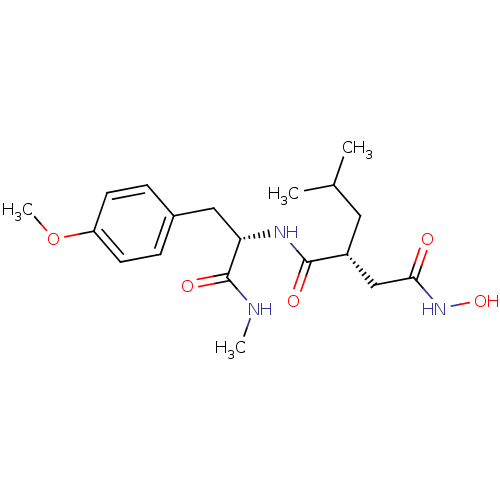 ((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50065450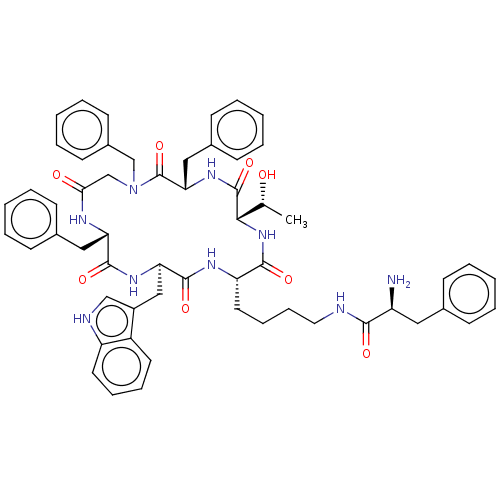 (2-Amino-3-phenyl-N-{4-[(2S,5S,8S,14S,17R)-8,10,14-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst2) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50286341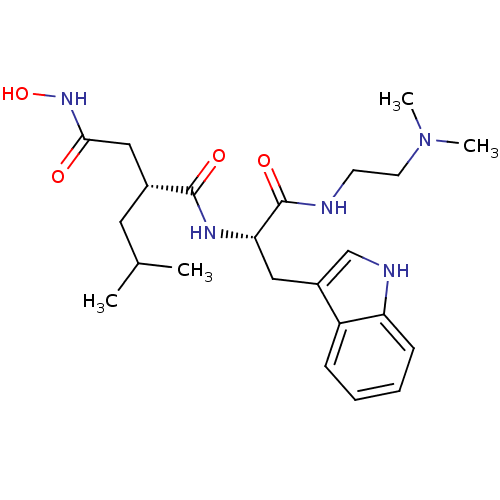 ((R)-N*1*-[(S)-1-(2-Dimethylamino-ethylcarbamoyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) | Bioorg Med Chem Lett 5: 337-342 (1995) Article DOI: 10.1016/0960-894X(95)00031-N BindingDB Entry DOI: 10.7270/Q2P55NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50065453 ((5S,8S,11S,14R,17S,19aS)-11-(4-Amino-butyl)-5,17-d...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human Somatostatin receptor type 5 expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50065452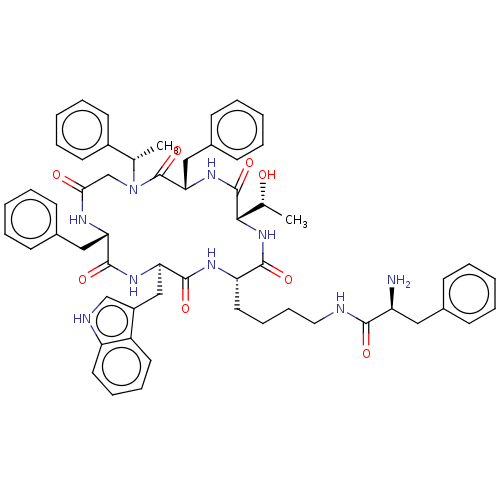 (2-Amino-N-{4-[(2S,5S,8S,14S,17R)-8,14-dibenzyl-5-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst2) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50065451 (2-Amino-N-{4-[(2S,5S,8S,14S,17R)-8,14-dibenzyl-5-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human Somatostatin receptor type 5 expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50065452 (2-Amino-N-{4-[(2S,5S,8S,14S,17R)-8,14-dibenzyl-5-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human Somatostatin receptor type 5 expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50065450 (2-Amino-3-phenyl-N-{4-[(2S,5S,8S,14S,17R)-8,10,14-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human Somatostatin receptor type 5 expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50065453 ((5S,8S,11S,14R,17S,19aS)-11-(4-Amino-butyl)-5,17-d...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst3) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50065450 (2-Amino-3-phenyl-N-{4-[(2S,5S,8S,14S,17R)-8,10,14-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 253 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst3) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50065451 (2-Amino-N-{4-[(2S,5S,8S,14S,17R)-8,14-dibenzyl-5-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst3) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50065452 (2-Amino-N-{4-[(2S,5S,8S,14S,17R)-8,14-dibenzyl-5-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 797 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst3) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50065452 (2-Amino-N-{4-[(2S,5S,8S,14S,17R)-8,14-dibenzyl-5-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 987 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst4) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50065452 (2-Amino-N-{4-[(2S,5S,8S,14S,17R)-8,14-dibenzyl-5-(...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst1) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50065451 (2-Amino-N-{4-[(2S,5S,8S,14S,17R)-8,14-dibenzyl-5-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst4) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50065451 (2-Amino-N-{4-[(2S,5S,8S,14S,17R)-8,14-dibenzyl-5-(...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst1) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50065453 ((5S,8S,11S,14R,17S,19aS)-11-(4-Amino-butyl)-5,17-d...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst1) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50065450 (2-Amino-3-phenyl-N-{4-[(2S,5S,8S,14S,17R)-8,10,14-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst4) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50065450 (2-Amino-3-phenyl-N-{4-[(2S,5S,8S,14S,17R)-8,10,14-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst1) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50065453 ((5S,8S,11S,14R,17S,19aS)-11-(4-Amino-butyl)-5,17-d...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description In vitro inhibition of radioligand binding to human somatostatin receptor (hsst4) expressed in CHO-K1 cells. | J Med Chem 41: 2679-85 (1998) Article DOI: 10.1021/jm970393l BindingDB Entry DOI: 10.7270/Q2Z60N6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527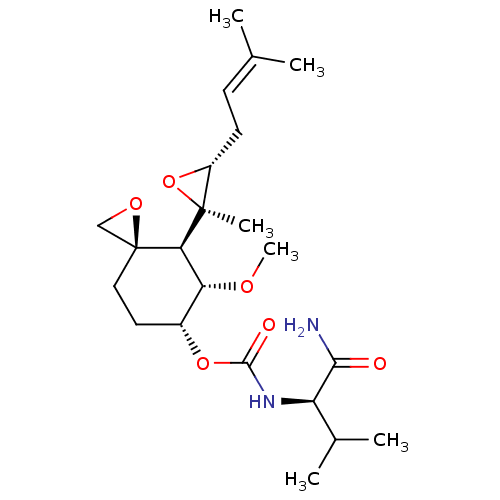 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 2.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 4.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50277149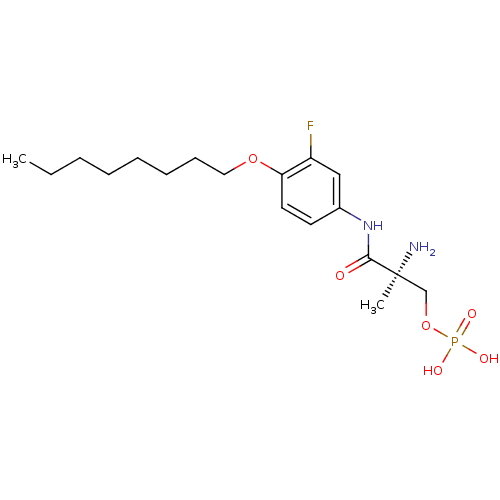 ((S)-2-amino-3-(3-fluoro-4-(octyloxy)phenylamino)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 369-72 (2008) Article DOI: 10.1016/j.bmcl.2008.11.072 BindingDB Entry DOI: 10.7270/Q2H70FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249294 ((R)-2-amino-2-(4-(4-(5-phenylpentyloxy)phenyl)-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249114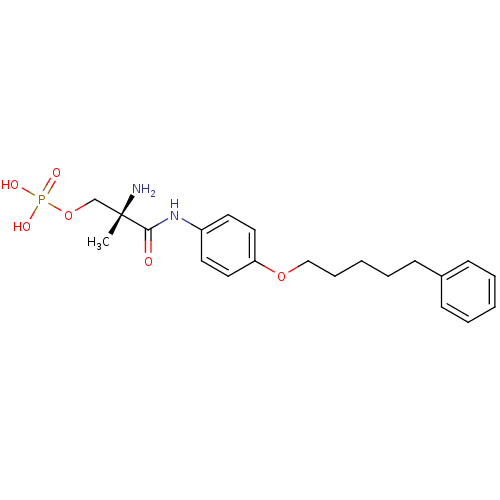 ((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315562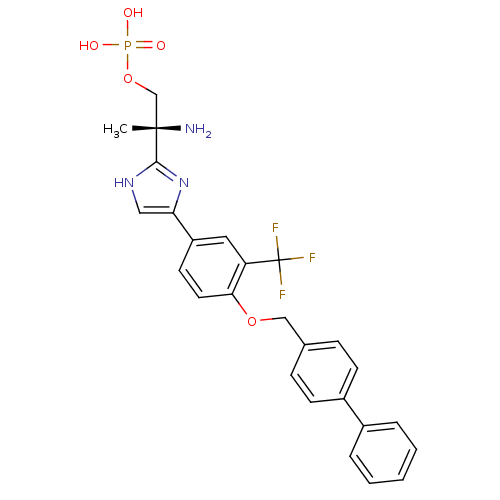 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)-3-(trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315559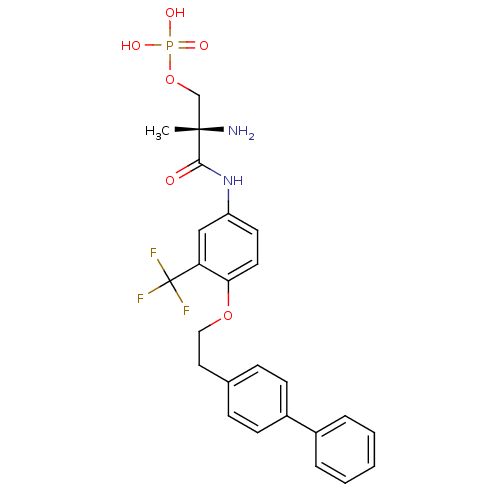 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 535 total ) | Next | Last >> |