

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50277623 (CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of JNK2alpha2 (unknown origin) by by exchange curve binding kinetic analysis | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50218689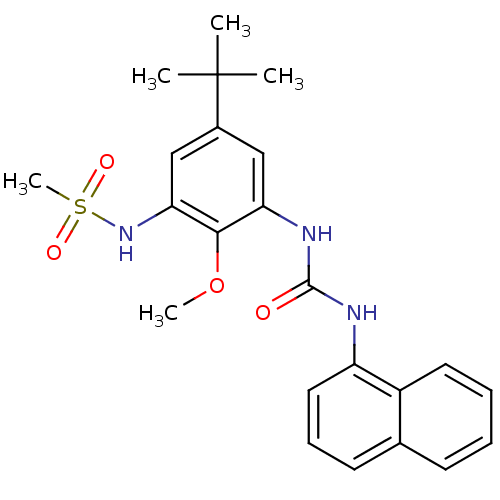 (CHEMBL245230 | N-(5-tert-butyl-2-methoxy-3-(3-naph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Selectivity against c-Jun N-terminal kinase 2-alpha 2 protein kinase | J Med Chem 45: 2994-3008 (2002) BindingDB Entry DOI: 10.7270/Q21G0KMV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50277623 (CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of Abl (unknown origin) | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50277623 (CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of craf (unknown origin) | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Selectivity against RAF proto-oncogene serine/threonine-protein kinase (c-Raf-1) | J Med Chem 45: 2994-3008 (2002) BindingDB Entry DOI: 10.7270/Q21G0KMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM50277623 (CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of Lyn (unknown origin) | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50218677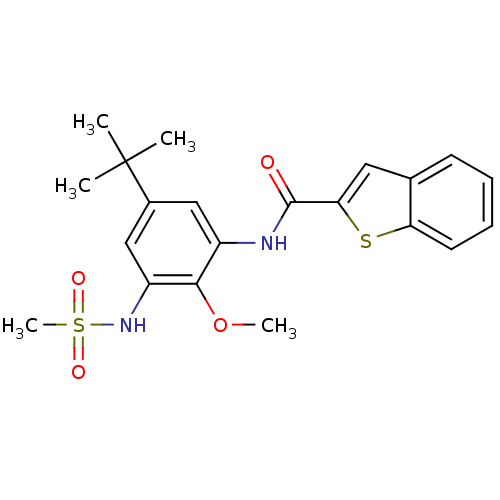 (CHEMBL242005 | N-(5-tert-butyl-2-methoxy-3-(methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 (Homo sapiens (Human)) | BDBM50277623 (CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of HEK (unknown origin) | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50277627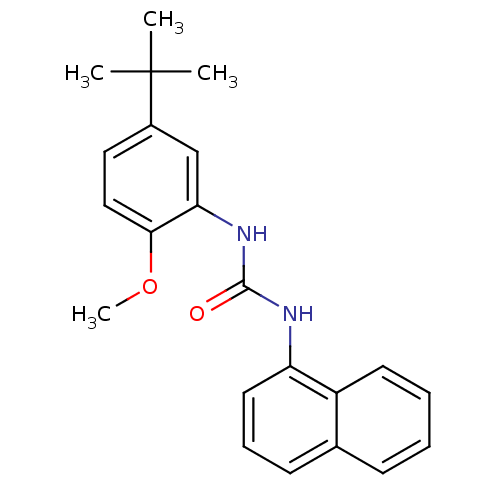 (1-(5-tert-butyl-2-methoxyphenyl)-3-(naphthalen-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50218695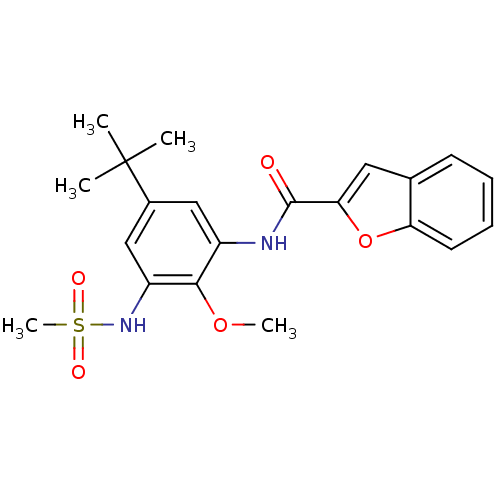 (CHEMBL390254 | N-(5-tert-butyl-2-methoxy-3-(methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50277623 (CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of ECK (unknown origin) | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50277623 (CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50277623 (CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of PKBalpha/Akt1 (unknown origin) | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50277623 (CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of bRaf (unknown origin) | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50277662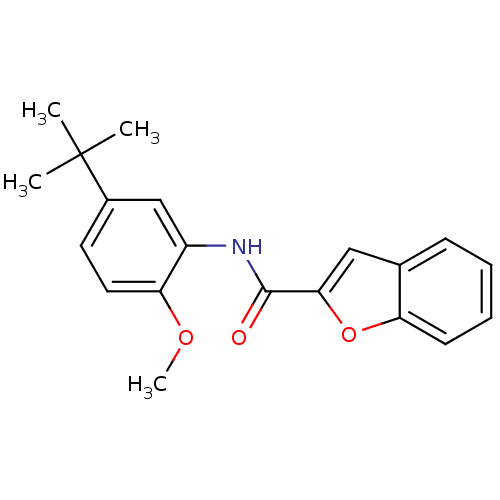 (CHEMBL484405 | N-(5-tert-butyl-2-methoxyphenyl)ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50277663 (CHEMBL519511 | N-(5-tert-butyl-2-methoxyphenyl)ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Selectivity against I-kappa-B-kinase beta | J Med Chem 45: 2994-3008 (2002) BindingDB Entry DOI: 10.7270/Q21G0KMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Selectivity against Zeta-chain (TCR) associated protein kinase 70 kDa (ZAP70) | J Med Chem 45: 2994-3008 (2002) BindingDB Entry DOI: 10.7270/Q21G0KMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Selectivity against p59 Fyn tyrosine kinase | J Med Chem 45: 2994-3008 (2002) BindingDB Entry DOI: 10.7270/Q21G0KMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Selectivity against Syk protein tyrosine kinase | J Med Chem 45: 2994-3008 (2002) BindingDB Entry DOI: 10.7270/Q21G0KMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Selectivity against p56 Lck tyrosine kinase | J Med Chem 45: 2994-3008 (2002) BindingDB Entry DOI: 10.7270/Q21G0KMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Selectivity against Epidermal growth factor receptor | J Med Chem 45: 2994-3008 (2002) BindingDB Entry DOI: 10.7270/Q21G0KMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 3 (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Selectivity against Extracellular signal-regulated kinase 1 (Erk-1) | J Med Chem 45: 2994-3008 (2002) BindingDB Entry DOI: 10.7270/Q21G0KMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Selectivity against HER2 kinase | J Med Chem 45: 2994-3008 (2002) BindingDB Entry DOI: 10.7270/Q21G0KMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14861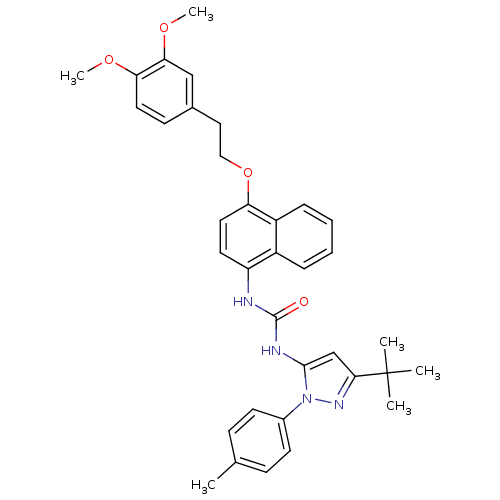 (1-(5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-{4-[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14862 (1-(5-tert-butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50277615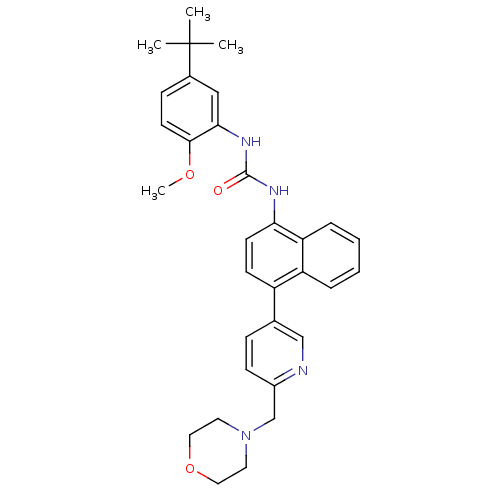 (1-(5-tert-butyl-2-methoxyphenyl)-3-(4-(6-(morpholi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Binding affinity to p38alpha (unknown origin) by exchange curve binding kinetic analysis | Bioorg Med Chem Lett 19: 2386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.03.104 BindingDB Entry DOI: 10.7270/Q22N525R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14860 (1-(5-tert-butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14859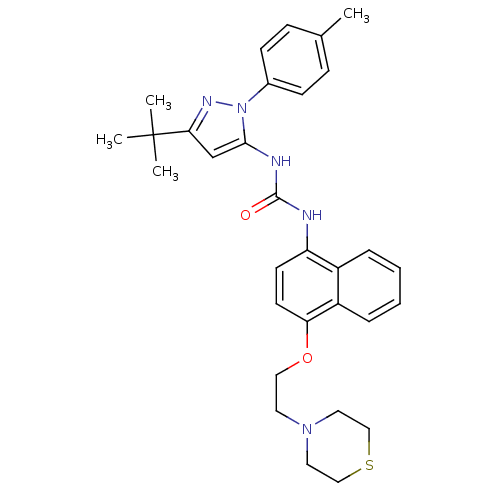 (1-(5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14858 (1-(5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-{4-[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 0.0960 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14857 (1-(5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-{4-[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14856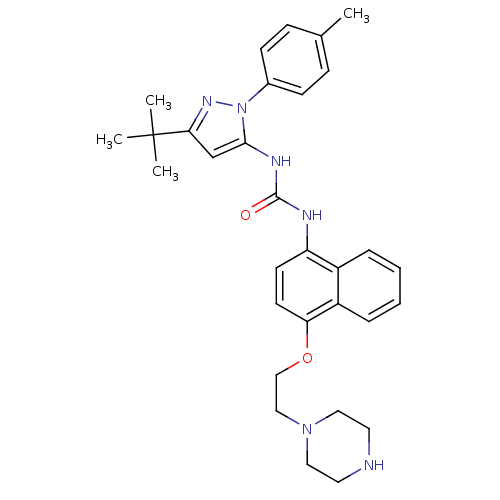 (1-(5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14855 (1-(5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14840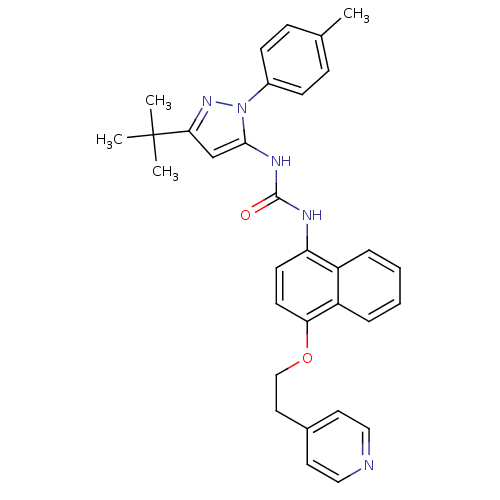 (1-(5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14853 (1-[5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl]-3-[4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14837 (3-[3-tert-butyl-1-(4-methylphenyl)-1H-pyrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14851 (1-[5-tert-Butyl-2-(6-methoxypyridin-3-yl)-2H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14850 (BIRB-796 Analog 41 | N-(4-{3-tert-butyl-5-[({4-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14849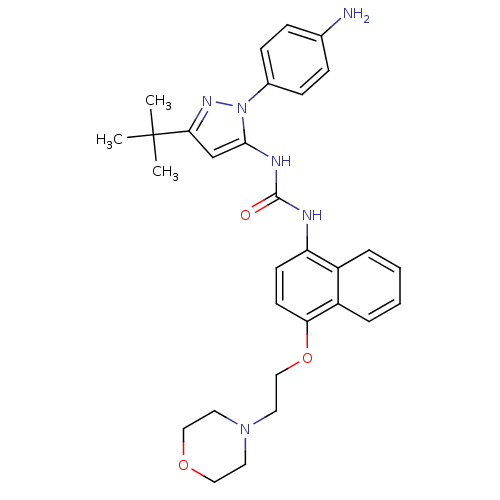 (1-[2-(4-Aminophenyl)-5-tert-butyl-2H-pyrazol-3-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | 7.0 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14848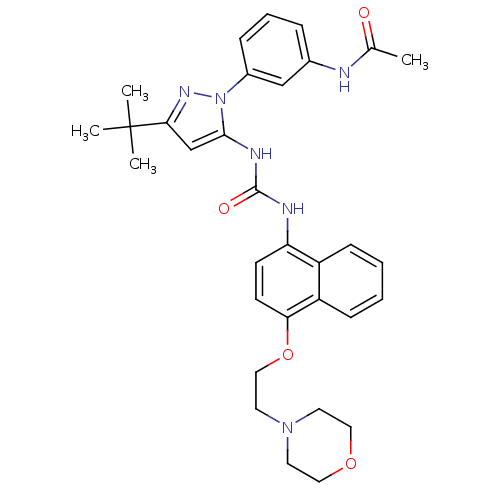 (BIRB-796 Analog 39 | N-(3-{3-tert-butyl-5-[({4-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 9.90 | n/a | n/a | n/a | 7.0 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14847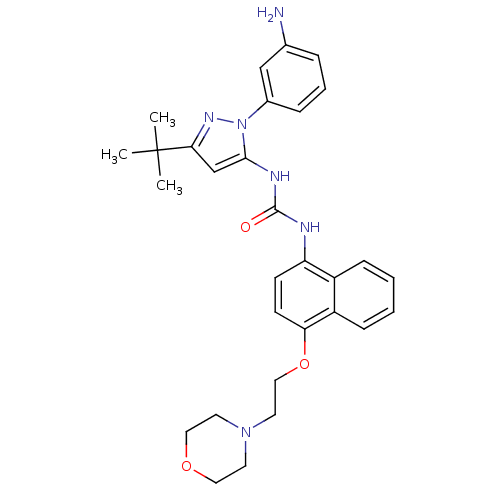 (1-[2-(3-Aminophenyl)-5-tert-butyl-2H-pyrazol-3-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | 7.0 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14846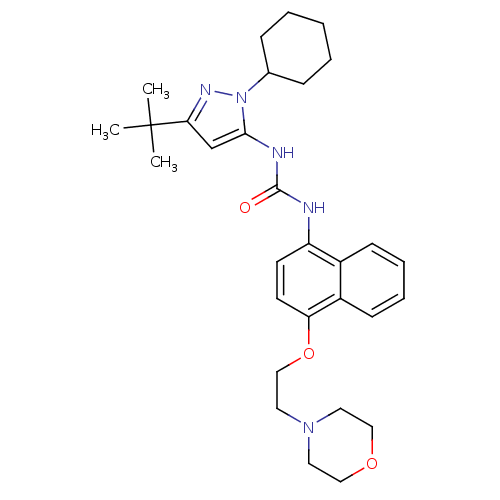 (1-(3-tert-butyl-1-cyclohexyl-1H-pyrazol-5-yl)-3-[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 23 | n/a | n/a | n/a | 7.0 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14835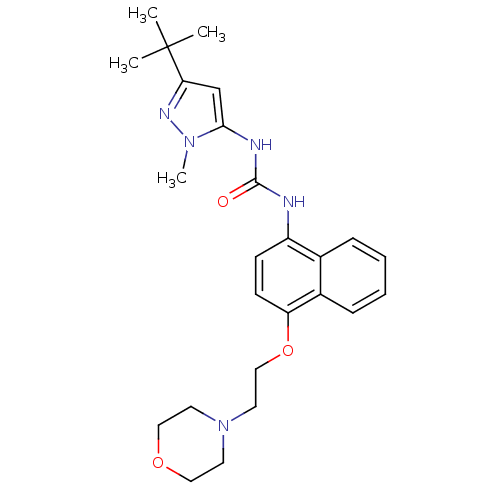 (1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-[4-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 26 | n/a | n/a | n/a | 7.0 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14845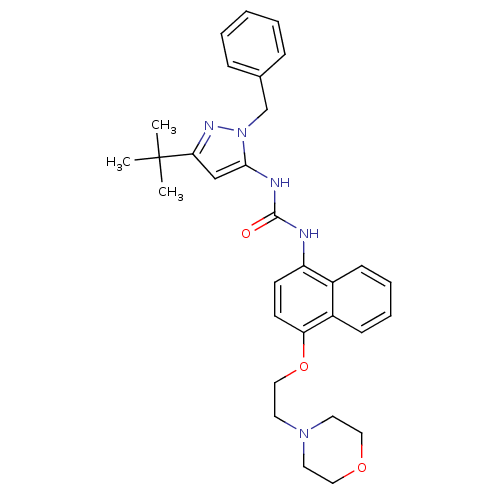 (1-(2-Benzyl-5-tert-butyl-2H-pyrazol-3-yl)-3-[4-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 64 | n/a | n/a | n/a | 7.0 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14844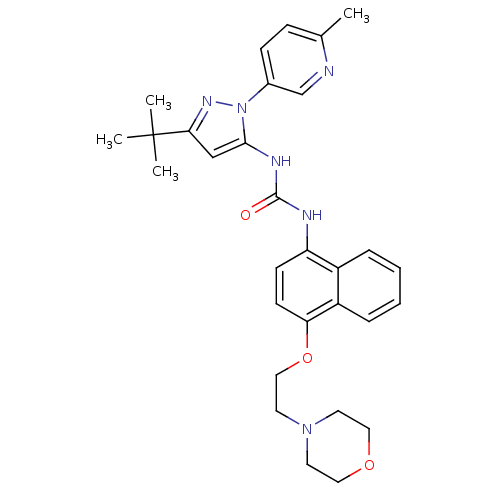 (1-[5-tert-Butyl-2-(6-methyl-pyridin-3-yl)-2H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | 7.0 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | 0.0460 | n/a | n/a | n/a | 7.0 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14843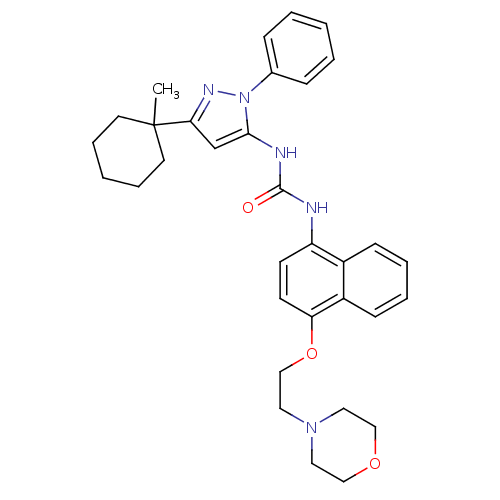 (1-[5-(1-Methylcyclohexyl)-2-phenyl-2H-pyrazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | 7.0 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14839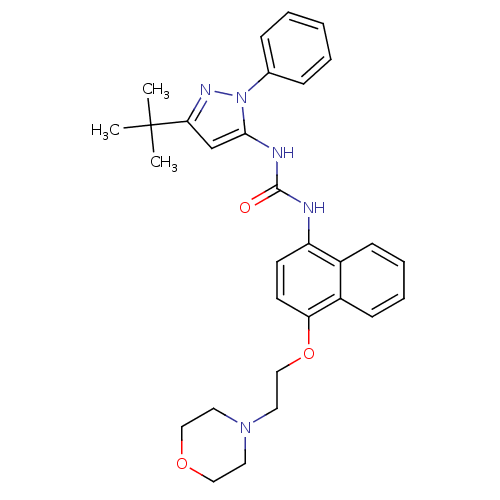 (1-(5-tert-Butyl-2-phenyl-2H-pyrazol-3-yl)-3-[4-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | 7.0 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM14838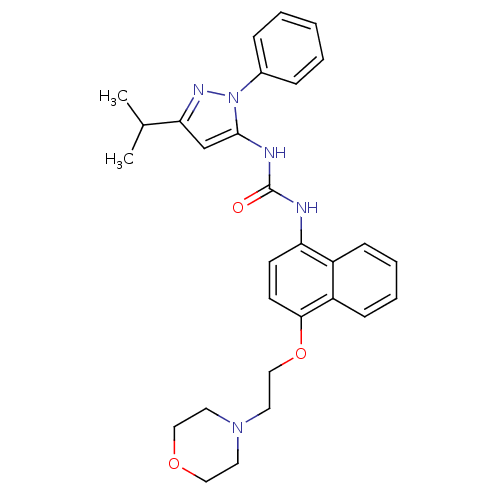 (1-(5-iso-Propyl-2-phenyl-2H-pyrazol-3-yl)-3-[4-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | 7.0 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description UV thermal melt experiments were carried out in a quartz cuvette loaded with sample containing enzyme and each test compound using a spectrophotomete... | J Med Chem 46: 4676-86 (2003) Article DOI: 10.1021/jm030121k BindingDB Entry DOI: 10.7270/Q24F1P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |