

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM66082 ((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, Universitą di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of DHFR (unknown origin) | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514737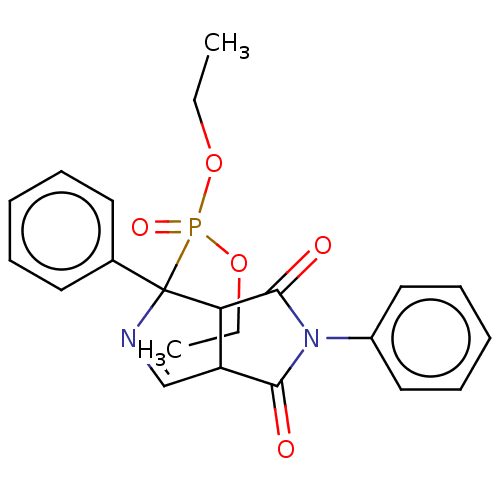 (CHEMBL4482861) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514738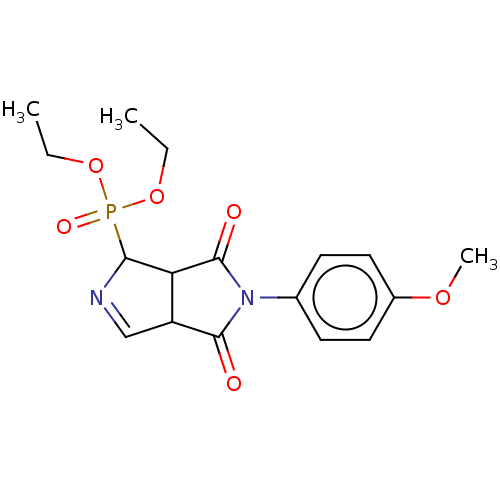 (CHEMBL4536304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514722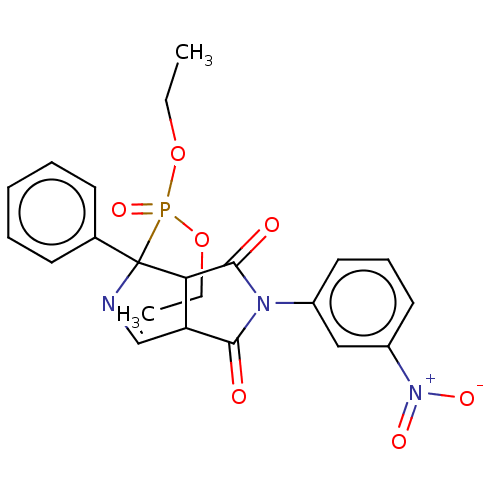 (CHEMBL4438801) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514727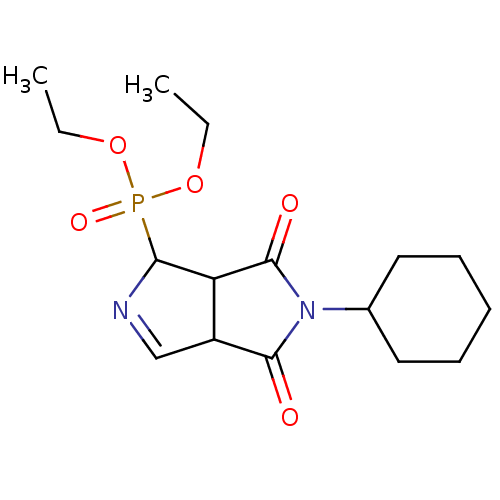 (CHEMBL4483022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514724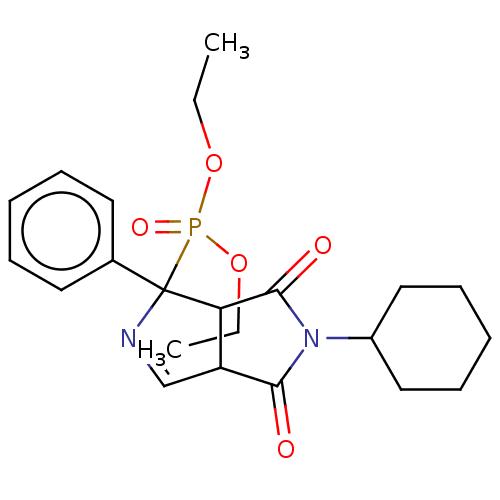 (CHEMBL4535472) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50467705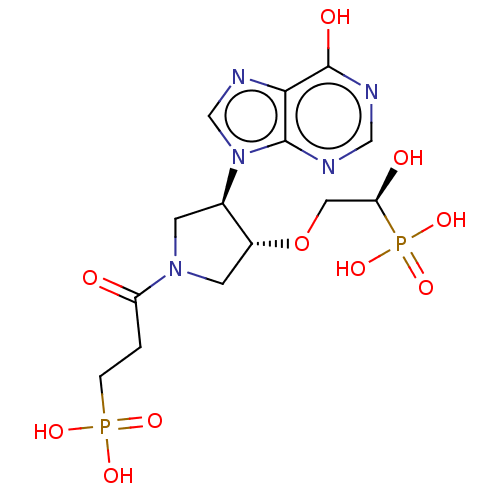 (CHEMBL4282830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by spectrophotometric analysis | Eur J Med Chem 159: 10-22 (2018) Article DOI: 10.1016/j.ejmech.2018.09.039 BindingDB Entry DOI: 10.7270/Q2D22197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50467710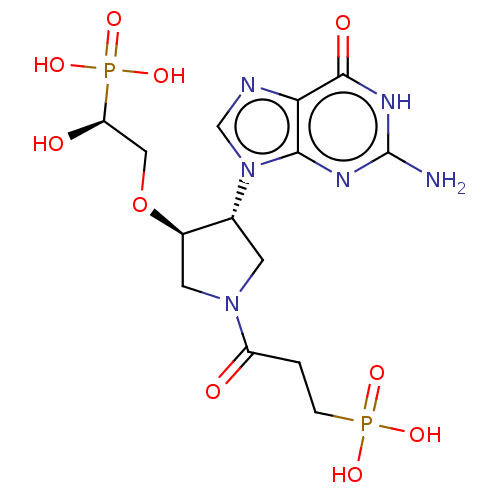 (CHEMBL4290716) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by Hanes plot analysis | Eur J Med Chem 159: 10-22 (2018) Article DOI: 10.1016/j.ejmech.2018.09.039 BindingDB Entry DOI: 10.7270/Q2D22197 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514745 (CHEMBL4439953) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514739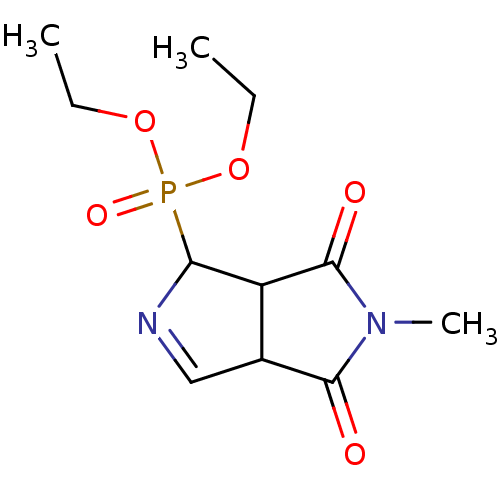 (CHEMBL4467833) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50258940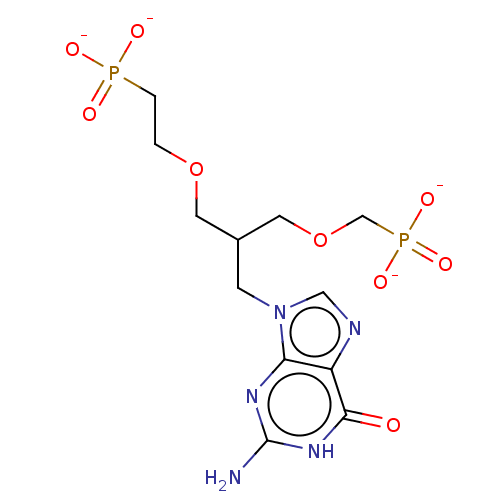 (CHEMBL4086362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human HGPRT using PRib-PP as the substrate by spectrophotometric method | J Med Chem 60: 7539-7554 (2017) Article DOI: 10.1021/acs.jmedchem.7b00926 BindingDB Entry DOI: 10.7270/Q2J968T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514718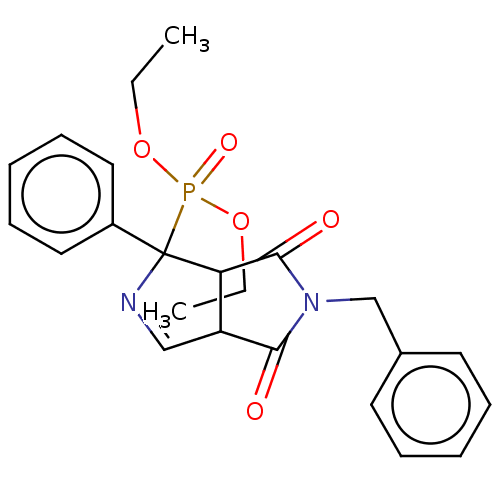 (CHEMBL4438158) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50467712 (CHEMBL4285691) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by spectrophotometric analysis | Eur J Med Chem 159: 10-22 (2018) Article DOI: 10.1016/j.ejmech.2018.09.039 BindingDB Entry DOI: 10.7270/Q2D22197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50467708 (CHEMBL4277753) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by Hanes plot analysis | Eur J Med Chem 159: 10-22 (2018) Article DOI: 10.1016/j.ejmech.2018.09.039 BindingDB Entry DOI: 10.7270/Q2D22197 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50089450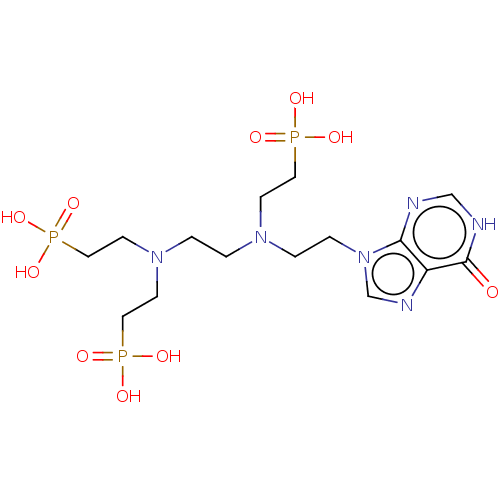 (CHEMBL3578115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT | J Med Chem 58: 4822-38 (2015) Article DOI: 10.1021/acs.jmedchem.5b00611 BindingDB Entry DOI: 10.7270/Q2JH3NXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50433811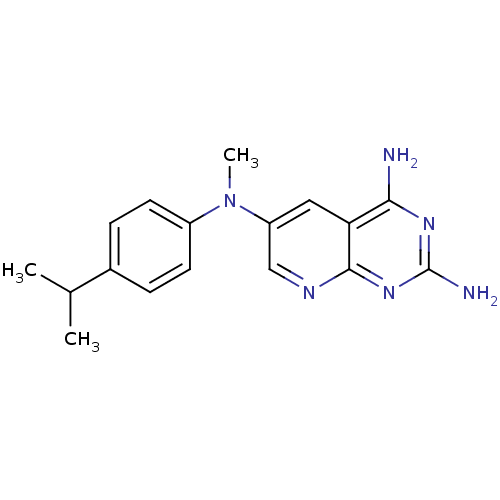 (CHEMBL2382332) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, Universitą di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50467707 (CHEMBL4280490) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by spectrophotometric analysis | Eur J Med Chem 159: 10-22 (2018) Article DOI: 10.1016/j.ejmech.2018.09.039 BindingDB Entry DOI: 10.7270/Q2D22197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50427810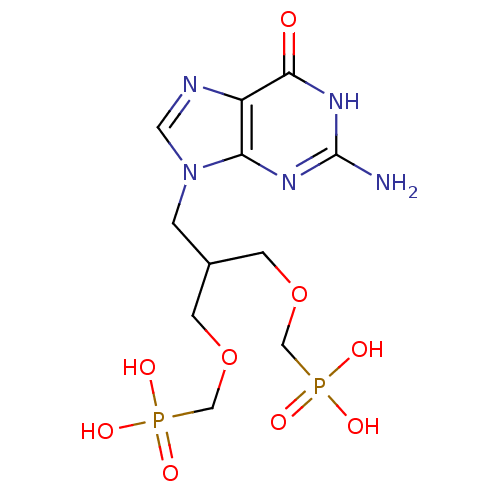 (CHEMBL2325752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human N-terminal hexahistidine-tagged HGPRT | J Med Chem 56: 2513-26 (2013) Article DOI: 10.1021/jm301893b BindingDB Entry DOI: 10.7270/Q2MW2JGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50089449 (CHEMBL3578114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT | J Med Chem 58: 4822-38 (2015) Article DOI: 10.1021/acs.jmedchem.5b00611 BindingDB Entry DOI: 10.7270/Q2JH3NXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50089444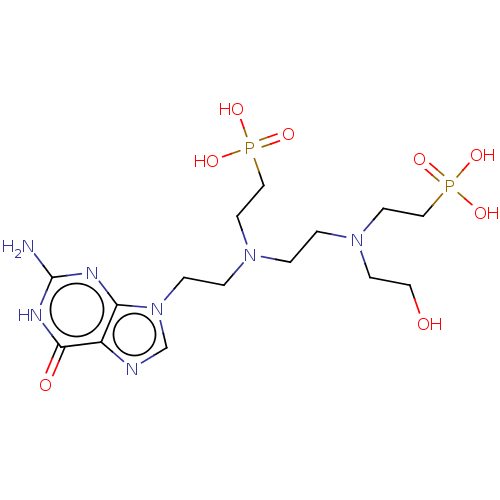 (CHEMBL3578110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT | J Med Chem 58: 4822-38 (2015) Article DOI: 10.1021/acs.jmedchem.5b00611 BindingDB Entry DOI: 10.7270/Q2JH3NXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50059910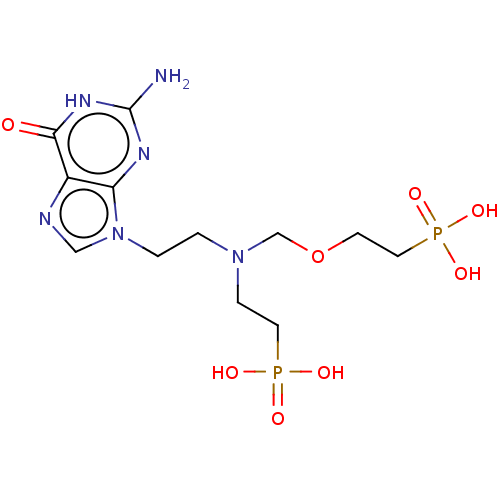 (CHEMBL3394315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT | J Med Chem 58: 827-46 (2015) Article DOI: 10.1021/jm501416t BindingDB Entry DOI: 10.7270/Q2HM5B3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50059908 (CHEMBL3394327) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT | J Med Chem 58: 827-46 (2015) Article DOI: 10.1021/jm501416t BindingDB Entry DOI: 10.7270/Q2HM5B3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50089446 (CHEMBL3578112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT | J Med Chem 58: 4822-38 (2015) Article DOI: 10.1021/acs.jmedchem.5b00611 BindingDB Entry DOI: 10.7270/Q2JH3NXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467707 (CHEMBL4280490) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... | Eur J Med Chem 159: 10-22 (2018) Article DOI: 10.1016/j.ejmech.2018.09.039 BindingDB Entry DOI: 10.7270/Q2D22197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467705 (CHEMBL4282830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... | Eur J Med Chem 159: 10-22 (2018) Article DOI: 10.1016/j.ejmech.2018.09.039 BindingDB Entry DOI: 10.7270/Q2D22197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467710 (CHEMBL4290716) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... | Eur J Med Chem 159: 10-22 (2018) Article DOI: 10.1016/j.ejmech.2018.09.039 BindingDB Entry DOI: 10.7270/Q2D22197 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514734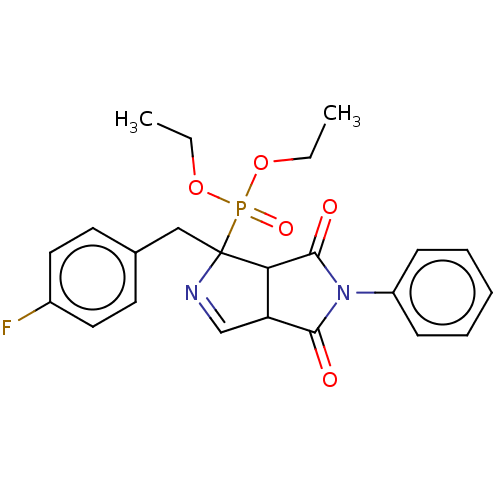 (CHEMBL4568994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50392261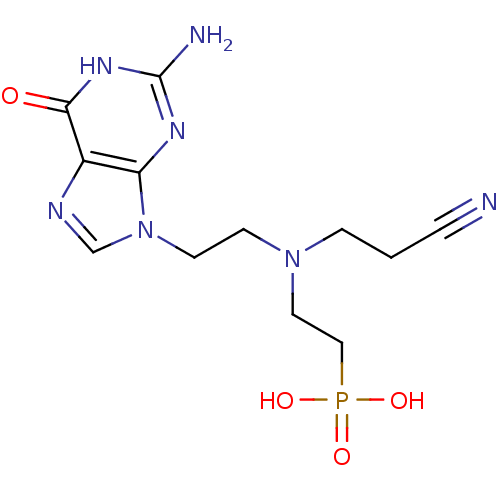 (CHEMBL2153480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT | J Med Chem 58: 827-46 (2015) Article DOI: 10.1021/jm501416t BindingDB Entry DOI: 10.7270/Q2HM5B3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405066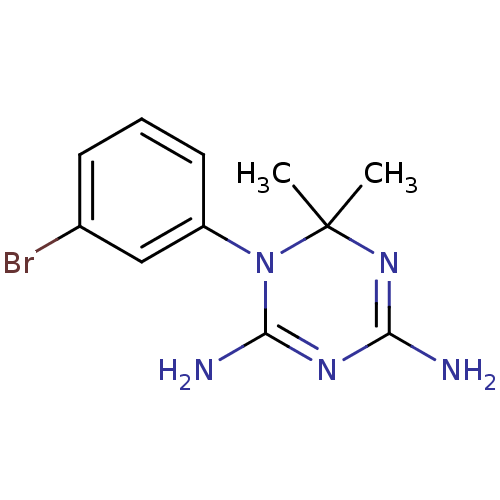 (CHEMBL268088) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, Universitą di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50059909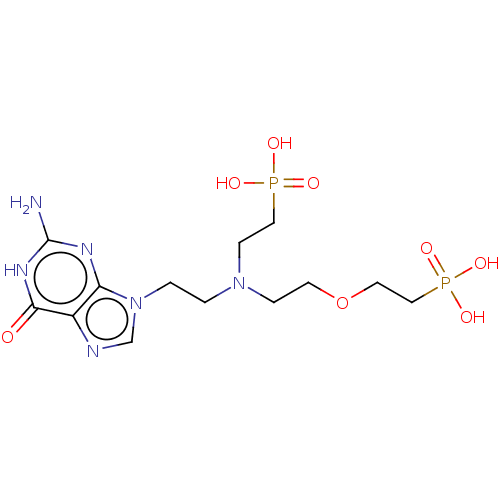 (CHEMBL3394316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT | J Med Chem 58: 827-46 (2015) Article DOI: 10.1021/jm501416t BindingDB Entry DOI: 10.7270/Q2HM5B3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50268142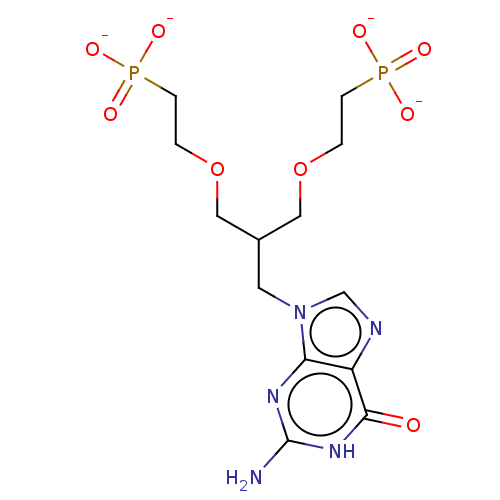 (CHEMBL4098313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Flemingovo nįm. 2, CZ-16610 Prague 6, Czech Republic. Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-histidine-tagged human HGPRT using PRib-PP as substrate in presence of guanine by Hanes plot analysis | Bioorg Med Chem 25: 4008-4030 (2017) Article DOI: 10.1016/j.bmc.2017.05.048 BindingDB Entry DOI: 10.7270/Q2NS0XCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50258937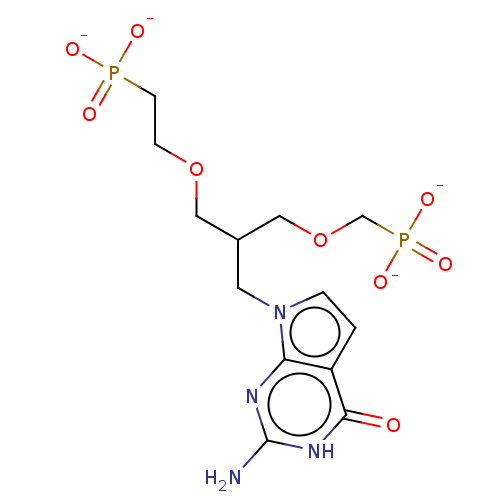 (CHEMBL4067618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human HGPRT using PRib-PP as the substrate by spectrophotometric method | J Med Chem 60: 7539-7554 (2017) Article DOI: 10.1021/acs.jmedchem.7b00926 BindingDB Entry DOI: 10.7270/Q2J968T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50090054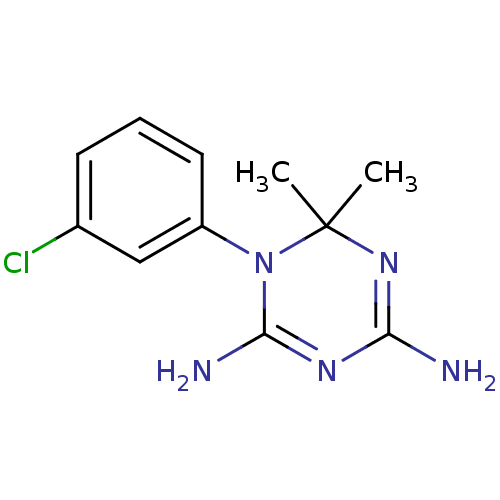 (1-(3-Chloro-phenyl)-6,6-dimethyl-1,6-dihydro-[1,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, Universitą di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50258941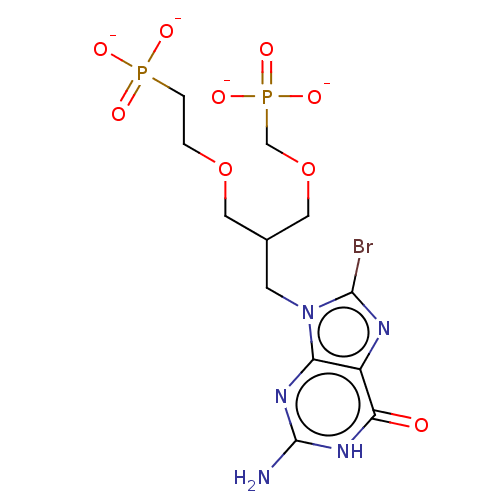 (CHEMBL4078513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human HGPRT using PRib-PP as the substrate by spectrophotometric method | J Med Chem 60: 7539-7554 (2017) Article DOI: 10.1021/acs.jmedchem.7b00926 BindingDB Entry DOI: 10.7270/Q2J968T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50090069 (1-(3,4-Dichloro-phenyl)-6,6-dimethyl-1,6-dihydro-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, Universitą di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467708 (CHEMBL4277753) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... | Eur J Med Chem 159: 10-22 (2018) Article DOI: 10.1016/j.ejmech.2018.09.039 BindingDB Entry DOI: 10.7270/Q2D22197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50291793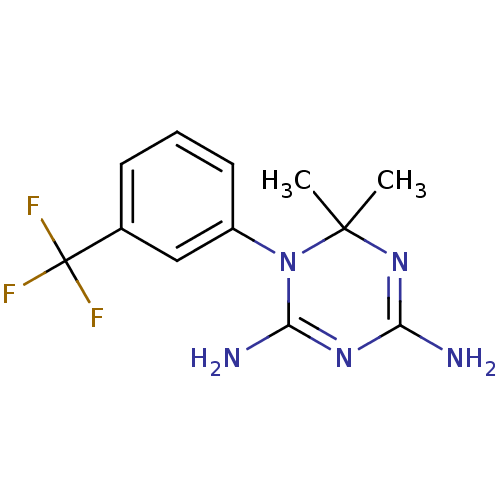 (6,6-Dimethyl-1-(3-trifluoromethyl-phenyl)-1,6-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, Universitą di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514743 (CHEMBL4454147) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514751 (CHEMBL4554450) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50059913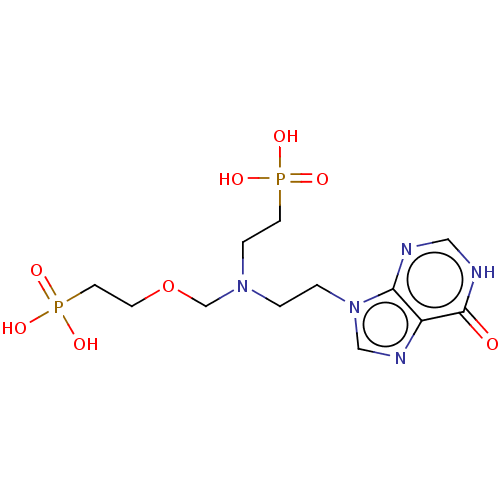 (CHEMBL3394312) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT | J Med Chem 58: 827-46 (2015) Article DOI: 10.1021/jm501416t BindingDB Entry DOI: 10.7270/Q2HM5B3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467712 (CHEMBL4285691) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... | Eur J Med Chem 159: 10-22 (2018) Article DOI: 10.1016/j.ejmech.2018.09.039 BindingDB Entry DOI: 10.7270/Q2D22197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50086502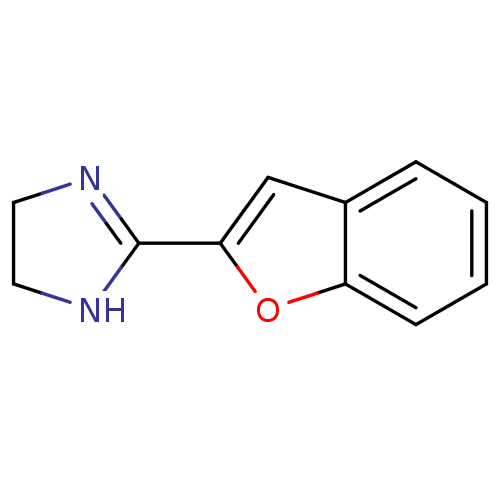 (2-BFi | 2-Benzofuran-2-yl-4,5-dihydro-1H-imidazole...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50268140 (CHEMBL4061954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Flemingovo nįm. 2, CZ-16610 Prague 6, Czech Republic. Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-histidine-tagged human HGPRT using PRib-PP as substrate in presence of guanine by Hanes plot analysis | Bioorg Med Chem 25: 4008-4030 (2017) Article DOI: 10.1016/j.bmc.2017.05.048 BindingDB Entry DOI: 10.7270/Q2NS0XCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50059911 (CHEMBL3394314) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT | J Med Chem 58: 827-46 (2015) Article DOI: 10.1021/jm501416t BindingDB Entry DOI: 10.7270/Q2HM5B3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50268154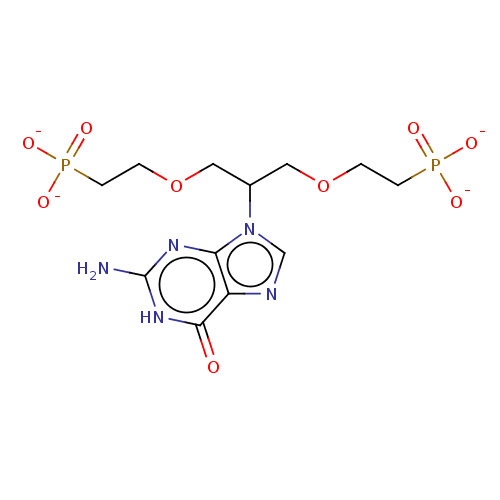 (CHEMBL4090466) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Flemingovo nįm. 2, CZ-16610 Prague 6, Czech Republic. Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-histidine-tagged human HGPRT using PRib-PP as substrate in presence of guanine by Hanes plot analysis | Bioorg Med Chem 25: 4008-4030 (2017) Article DOI: 10.1016/j.bmc.2017.05.048 BindingDB Entry DOI: 10.7270/Q2NS0XCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50258942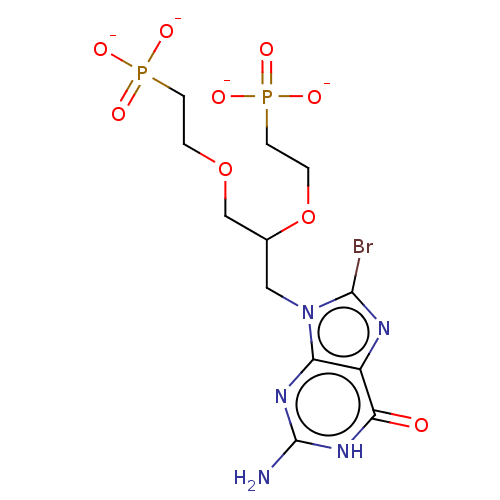 (CHEMBL4064903) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human HGPRT using PRib-PP as the substrate by spectrophotometric method | J Med Chem 60: 7539-7554 (2017) Article DOI: 10.1021/acs.jmedchem.7b00926 BindingDB Entry DOI: 10.7270/Q2J968T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18792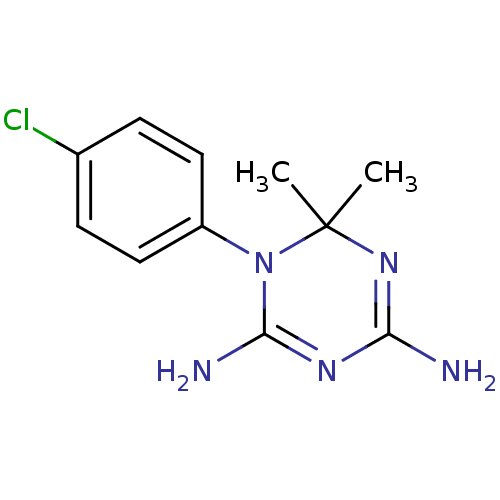 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, Universitą di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467709 (CHEMBL4291117) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... | Eur J Med Chem 159: 10-22 (2018) Article DOI: 10.1016/j.ejmech.2018.09.039 BindingDB Entry DOI: 10.7270/Q2D22197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, Universitą di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis | Eur J Med Chem 135: 467-478 (2017) Article DOI: 10.1016/j.ejmech.2017.04.070 BindingDB Entry DOI: 10.7270/Q2057JD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50089445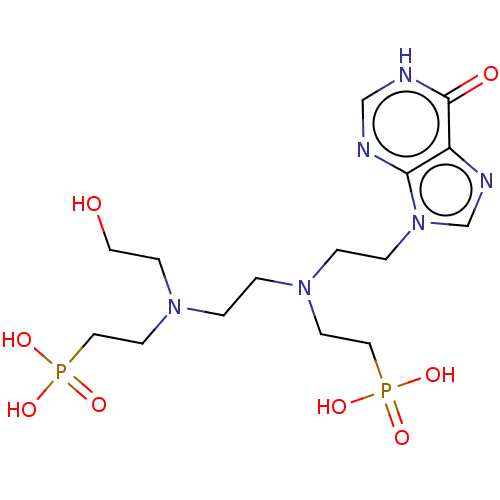 (CHEMBL3578111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human HGPRT | J Med Chem 58: 4822-38 (2015) Article DOI: 10.1021/acs.jmedchem.5b00611 BindingDB Entry DOI: 10.7270/Q2JH3NXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 252 total ) | Next | Last >> |