 Found 192 hits with Last Name = 'nisbet' and Initial = 'l'
Found 192 hits with Last Name = 'nisbet' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418185
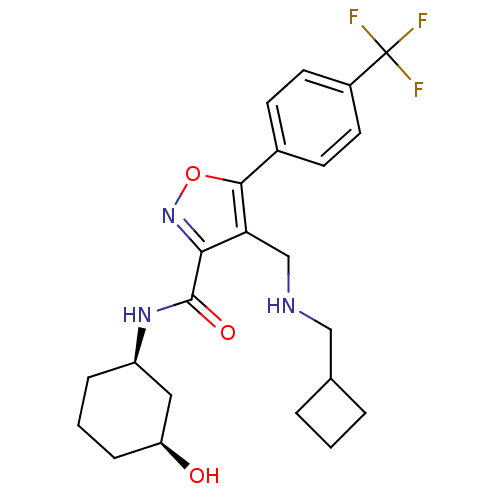
(CHEMBL1761695)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1CNCC1CCC1)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C23H28F3N3O3/c24-23(25,26)16-9-7-15(8-10-16)21-19(13-27-12-14-3-1-4-14)20(29-32-21)22(31)28-17-5-2-6-18(30)11-17/h7-10,14,17-18,27,30H,1-6,11-13H2,(H,28,31)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418184

(CHEMBL1761694)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1CNCC1CC1)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C22H26F3N3O3/c23-22(24,25)15-8-6-14(7-9-15)20-18(12-26-11-13-4-5-13)19(28-31-20)21(30)27-16-2-1-3-17(29)10-16/h6-9,13,16-17,26,29H,1-5,10-12H2,(H,27,30)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418183
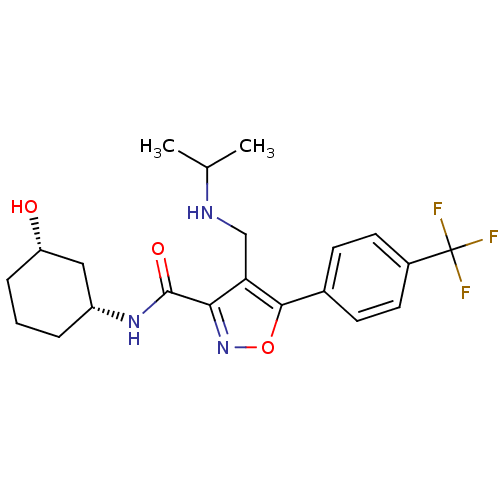
(CHEMBL1761688)Show SMILES CC(C)NCc1c(noc1-c1ccc(cc1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C21H26F3N3O3/c1-12(2)25-11-17-18(20(29)26-15-4-3-5-16(28)10-15)27-30-19(17)13-6-8-14(9-7-13)21(22,23)24/h6-9,12,15-16,25,28H,3-5,10-11H2,1-2H3,(H,26,29)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled-dofetilide from human ERG |
Bioorg Med Chem Lett 21: 4652-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.051
BindingDB Entry DOI: 10.7270/Q2M32X1M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418183

(CHEMBL1761688)Show SMILES CC(C)NCc1c(noc1-c1ccc(cc1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C21H26F3N3O3/c1-12(2)25-11-17-18(20(29)26-15-4-3-5-16(28)10-15)27-30-19(17)13-6-8-14(9-7-13)21(22,23)24/h6-9,12,15-16,25,28H,3-5,10-11H2,1-2H3,(H,26,29)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418189
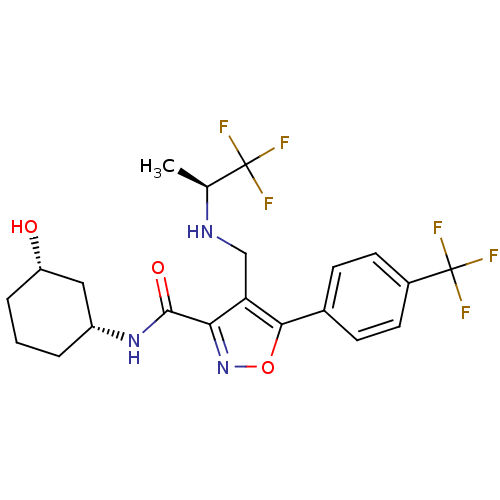
(CHEMBL1761696)Show SMILES C[C@H](NCc1c(noc1-c1ccc(cc1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C21H23F6N3O3/c1-11(20(22,23)24)28-10-16-17(19(32)29-14-3-2-4-15(31)9-14)30-33-18(16)12-5-7-13(8-6-12)21(25,26)27/h5-8,11,14-15,28,31H,2-4,9-10H2,1H3,(H,29,32)/t11-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ influx by FLIPR assay |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50336144

((1R,3S)-4-chloro-N-(3-hydroxycyclohexyl)-5-(4-(tri...)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1Cl)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C17H16ClF3N2O3/c18-13-14(16(25)22-11-2-1-3-12(24)8-11)23-26-15(13)9-4-6-10(7-5-9)17(19,20)21/h4-7,11-12,24H,1-3,8H2,(H,22,25)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of calcium flux by FLIPR assay |
Bioorg Med Chem Lett 21: 4652-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.051
BindingDB Entry DOI: 10.7270/Q2M32X1M |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418959
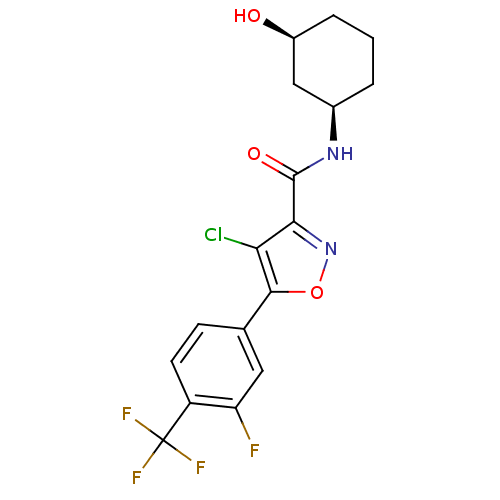
(CHEMBL1807875)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1Cl)-c1ccc(c(F)c1)C(F)(F)F |r| Show InChI InChI=1S/C17H15ClF4N2O3/c18-13-14(16(26)23-9-2-1-3-10(25)7-9)24-27-15(13)8-4-5-11(12(19)6-8)17(20,21)22/h4-6,9-10,25H,1-3,7H2,(H,23,26)/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of calcium flux by FLIPR assay |
Bioorg Med Chem Lett 21: 4652-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.051
BindingDB Entry DOI: 10.7270/Q2M32X1M |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418961
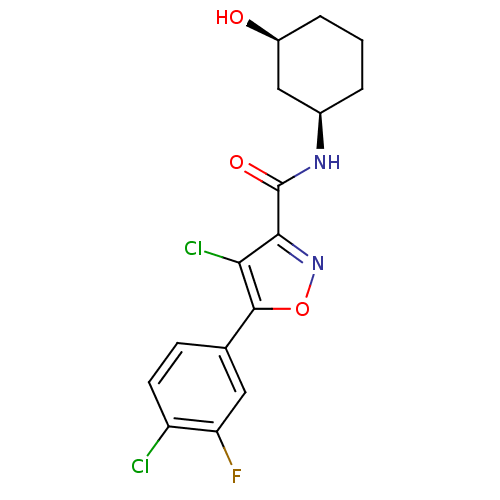
(CHEMBL1807877)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1Cl)-c1ccc(Cl)c(F)c1 |r| Show InChI InChI=1S/C16H15Cl2FN2O3/c17-11-5-4-8(6-12(11)19)15-13(18)14(21-24-15)16(23)20-9-2-1-3-10(22)7-9/h4-6,9-10,22H,1-3,7H2,(H,20,23)/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of calcium flux by FLIPR assay |
Bioorg Med Chem Lett 21: 4652-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.051
BindingDB Entry DOI: 10.7270/Q2M32X1M |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418188
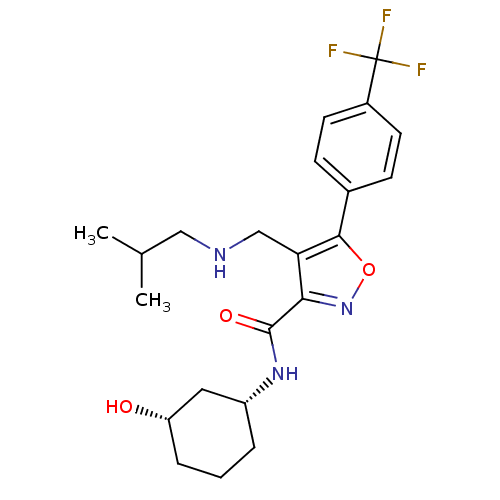
(CHEMBL1761693)Show SMILES CC(C)CNCc1c(noc1-c1ccc(cc1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C22H28F3N3O3/c1-13(2)11-26-12-18-19(21(30)27-16-4-3-5-17(29)10-16)28-31-20(18)14-6-8-15(9-7-14)22(23,24)25/h6-9,13,16-17,26,29H,3-5,10-12H2,1-2H3,(H,27,30)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ influx by FLIPR assay |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411047
(CHEMBL211441)Show SMILES CCC(CC)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C28H31F3N2O4/c1-4-19(5-2)26(14-8-10-18-9-6-7-11-23(18)26)16-27(36,28(29,30)31)25(35)32-20-12-13-21-22(15-20)17(3)33-37-24(21)34/h6-7,9,11-13,15,19,36H,4-5,8,10,14,16H2,1-3H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418185

(CHEMBL1761695)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1CNCC1CCC1)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C23H28F3N3O3/c24-23(25,26)16-9-7-15(8-10-16)21-19(13-27-12-14-3-1-4-14)20(29-32-21)22(31)28-17-5-2-6-18(30)11-17/h7-10,14,17-18,27,30H,1-6,11-13H2,(H,28,31)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ influx by FLIPR assay |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418981
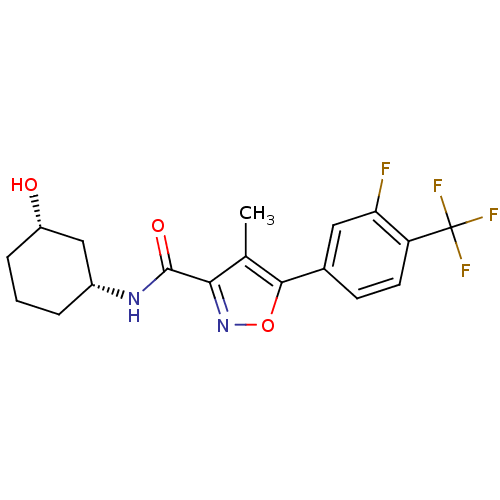
(CHEMBL1807878)Show SMILES Cc1c(noc1-c1ccc(c(F)c1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C18H18F4N2O3/c1-9-15(17(26)23-11-3-2-4-12(25)8-11)24-27-16(9)10-5-6-13(14(19)7-10)18(20,21)22/h5-7,11-12,25H,2-4,8H2,1H3,(H,23,26)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of calcium flux by FLIPR assay |
Bioorg Med Chem Lett 21: 4652-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.051
BindingDB Entry DOI: 10.7270/Q2M32X1M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411024

(CHEMBL208996)Show SMILES Cc1noc(=O)c2ccc(NC(=O)[C@](O)(C[C@]3(CCCc4ccccc34)C3CCCC3)C(F)(F)F)cc12 Show InChI InChI=1S/C28H29F3N2O4/c1-17-22-15-20(12-13-21(22)24(34)37-33-17)32-25(35)27(36,28(29,30)31)16-26(19-9-3-4-10-19)14-6-8-18-7-2-5-11-23(18)26/h2,5,7,11-13,15,19,36H,3-4,6,8-10,14,16H2,1H3,(H,32,35)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418193
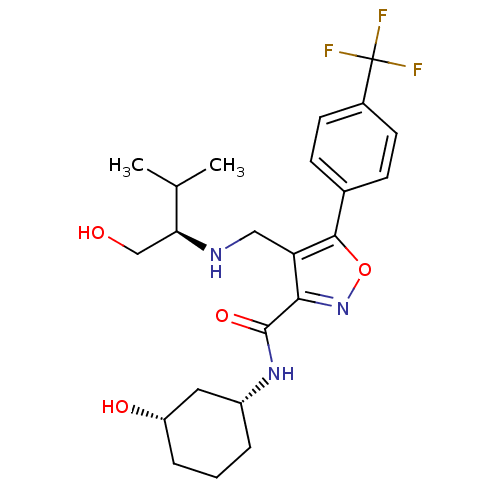
(CHEMBL1761700)Show SMILES CC(C)[C@H](CO)NCc1c(noc1-c1ccc(cc1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C23H30F3N3O4/c1-13(2)19(12-30)27-11-18-20(22(32)28-16-4-3-5-17(31)10-16)29-33-21(18)14-6-8-15(9-7-14)23(24,25)26/h6-9,13,16-17,19,27,30-31H,3-5,10-12H2,1-2H3,(H,28,32)/t16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ influx by FLIPR assay |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411026
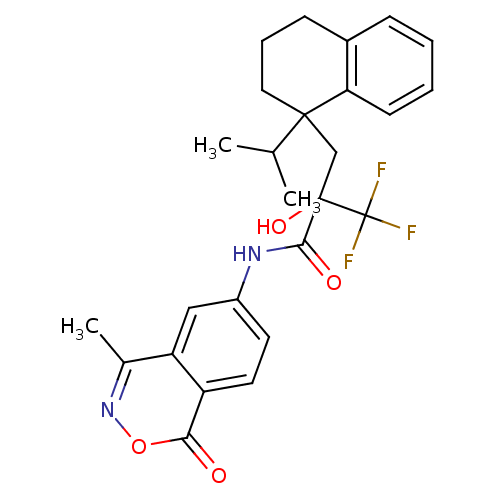
(CHEMBL214336)Show SMILES CC(C)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H27F3N2O4/c1-15(2)24(12-6-8-17-7-4-5-9-21(17)24)14-25(34,26(27,28)29)23(33)30-18-10-11-19-20(13-18)16(3)31-35-22(19)32/h4-5,7,9-11,13,15,34H,6,8,12,14H2,1-3H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50336144

((1R,3S)-4-chloro-N-(3-hydroxycyclohexyl)-5-(4-(tri...)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1Cl)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C17H16ClF3N2O3/c18-13-14(16(25)22-11-2-1-3-12(24)8-11)23-26-15(13)9-4-6-10(7-5-9)17(19,20)21/h4-7,11-12,24H,1-3,8H2,(H,22,25)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, MSD, Newhouse, Lanarkshire, UK. ronnie.palin@gmail.com
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced calcium influx by fluorimetric assay |
Bioorg Med Chem Lett 21: 892-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.092
BindingDB Entry DOI: 10.7270/Q2K074JC |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411039

(CHEMBL385450)Show SMILES CCCCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C27H29F3N2O4/c1-3-4-13-25(14-7-9-18-8-5-6-10-22(18)25)16-26(35,27(28,29)30)24(34)31-19-11-12-20-21(15-19)17(2)32-36-23(20)33/h5-6,8,10-12,15,35H,3-4,7,9,13-14,16H2,1-2H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411034
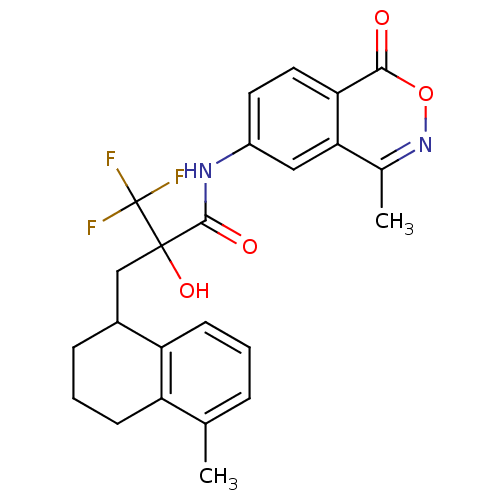
(CHEMBL378785)Show SMILES Cc1cccc2C(CC(O)(C(=O)Nc3ccc4c(c3)c(C)noc4=O)C(F)(F)F)CCCc12 Show InChI InChI=1S/C24H23F3N2O4/c1-13-5-3-8-18-15(6-4-7-17(13)18)12-23(32,24(25,26)27)22(31)28-16-9-10-19-20(11-16)14(2)29-33-21(19)30/h3,5,8-11,15,32H,4,6-7,12H2,1-2H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411034

(CHEMBL378785)Show SMILES Cc1cccc2C(CC(O)(C(=O)Nc3ccc4c(c3)c(C)noc4=O)C(F)(F)F)CCCc12 Show InChI InChI=1S/C24H23F3N2O4/c1-13-5-3-8-18-15(6-4-7-17(13)18)12-23(32,24(25,26)27)22(31)28-16-9-10-19-20(11-16)14(2)29-33-21(19)30/h3,5,8-11,15,32H,4,6-7,12H2,1-2H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418194
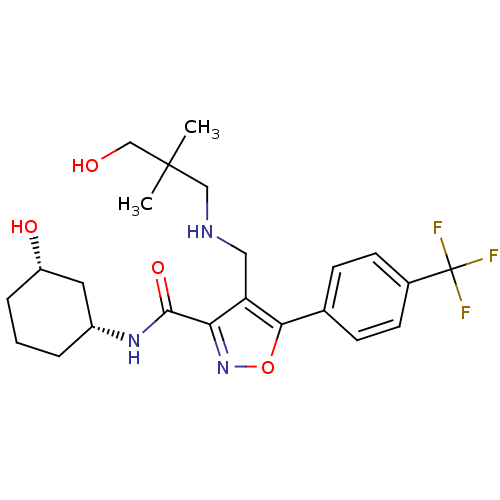
(CHEMBL1761702)Show SMILES CC(C)(CO)CNCc1c(noc1-c1ccc(cc1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C23H30F3N3O4/c1-22(2,13-30)12-27-11-18-19(21(32)28-16-4-3-5-17(31)10-16)29-33-20(18)14-6-8-15(9-7-14)23(24,25)26/h6-9,16-17,27,30-31H,3-5,10-13H2,1-2H3,(H,28,32)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ influx by FLIPR assay |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418187
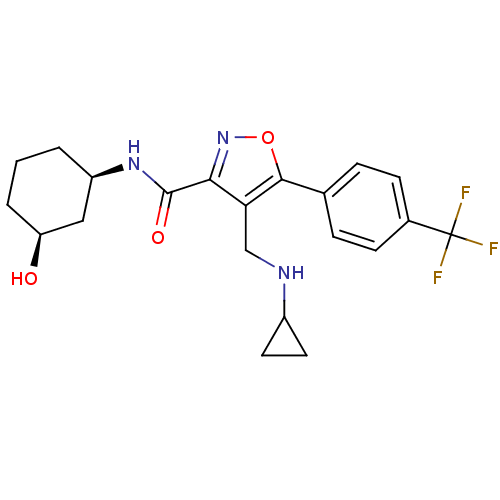
(CHEMBL1761691)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1CNC1CC1)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C21H24F3N3O3/c22-21(23,24)13-6-4-12(5-7-13)19-17(11-25-14-8-9-14)18(27-30-19)20(29)26-15-2-1-3-16(28)10-15/h4-7,14-16,25,28H,1-3,8-11H2,(H,26,29)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ influx by FLIPR assay |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418965

(CHEMBL1807883)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1onc(c1Cl)-c1ccc(Cl)c(F)c1 |r| Show InChI InChI=1S/C16H15Cl2FN2O3/c17-11-5-4-8(6-12(11)19)14-13(18)15(24-21-14)16(23)20-9-2-1-3-10(22)7-9/h4-6,9-10,22H,1-3,7H2,(H,20,23)/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of calcium flux by FLIPR assay |
Bioorg Med Chem Lett 21: 4652-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.051
BindingDB Entry DOI: 10.7270/Q2M32X1M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411036
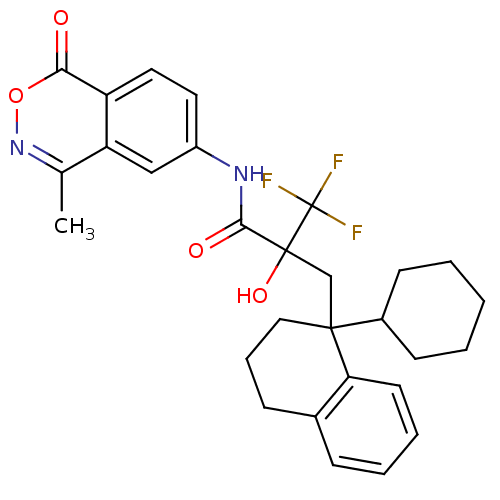
(CHEMBL383890)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3(CCCc4ccccc34)C3CCCCC3)C(F)(F)F)cc12 Show InChI InChI=1S/C29H31F3N2O4/c1-18-23-16-21(13-14-22(23)25(35)38-34-18)33-26(36)28(37,29(30,31)32)17-27(20-10-3-2-4-11-20)15-7-9-19-8-5-6-12-24(19)27/h5-6,8,12-14,16,20,37H,2-4,7,9-11,15,17H2,1H3,(H,33,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411020

(CHEMBL211266)Show SMILES CC(C)CC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C27H29F3N2O4/c1-16(2)14-25(12-6-8-18-7-4-5-9-22(18)25)15-26(35,27(28,29)30)24(34)31-19-10-11-20-21(13-19)17(3)32-36-23(20)33/h4-5,7,9-11,13,16,35H,6,8,12,14-15H2,1-3H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418964

(CHEMBL1807882)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1onc(c1Cl)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C17H16ClF3N2O3/c18-13-14(9-4-6-10(7-5-9)17(19,20)21)23-26-15(13)16(25)22-11-2-1-3-12(24)8-11/h4-7,11-12,24H,1-3,8H2,(H,22,25)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of calcium flux by FLIPR assay |
Bioorg Med Chem Lett 21: 4652-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.051
BindingDB Entry DOI: 10.7270/Q2M32X1M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Activity at GR assessed as ability to antagonize dexamethasone-induced MMTV luciferase reporter gene transactivation in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411048
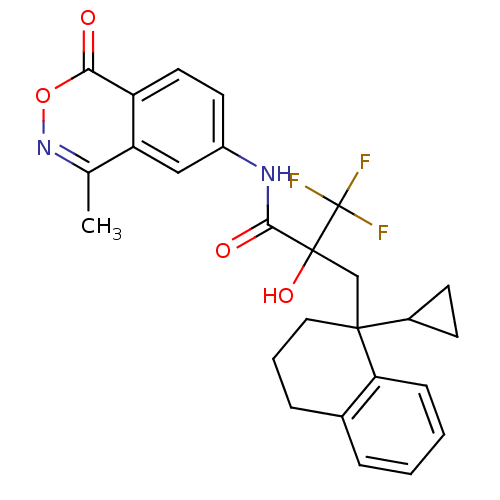
(CHEMBL386448)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3(CCCc4ccccc34)C3CC3)C(F)(F)F)cc12 Show InChI InChI=1S/C26H25F3N2O4/c1-15-20-13-18(10-11-19(20)22(32)35-31-15)30-23(33)25(34,26(27,28)29)14-24(17-8-9-17)12-4-6-16-5-2-3-7-21(16)24/h2-3,5,7,10-11,13,17,34H,4,6,8-9,12,14H2,1H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411037

(CHEMBL213453)Show SMILES CCCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H27F3N2O4/c1-3-12-24(13-6-8-17-7-4-5-9-21(17)24)15-25(34,26(27,28)29)23(33)30-18-10-11-19-20(14-18)16(2)31-35-22(19)32/h4-5,7,9-11,14,34H,3,6,8,12-13,15H2,1-2H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411026

(CHEMBL214336)Show SMILES CC(C)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H27F3N2O4/c1-15(2)24(12-6-8-17-7-4-5-9-21(17)24)14-25(34,26(27,28)29)23(33)30-18-10-11-19-20(13-18)16(3)31-35-22(19)32/h4-5,7,9-11,13,15,34H,6,8,12,14H2,1-3H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411035
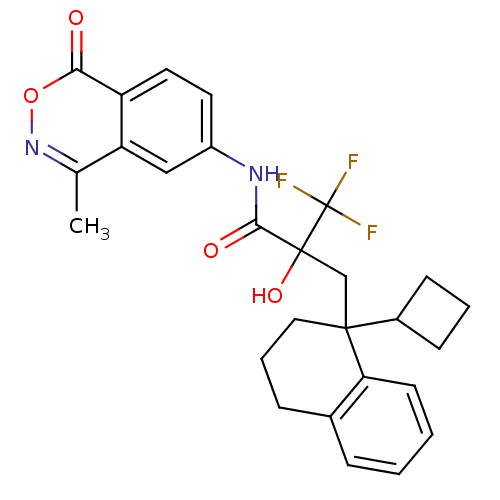
(CHEMBL210185)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3(CCCc4ccccc34)C3CCC3)C(F)(F)F)cc12 Show InChI InChI=1S/C27H27F3N2O4/c1-16-21-14-19(11-12-20(21)23(33)36-32-16)31-24(34)26(35,27(28,29)30)15-25(18-8-4-9-18)13-5-7-17-6-2-3-10-22(17)25/h2-3,6,10-12,14,18,35H,4-5,7-9,13,15H2,1H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411023
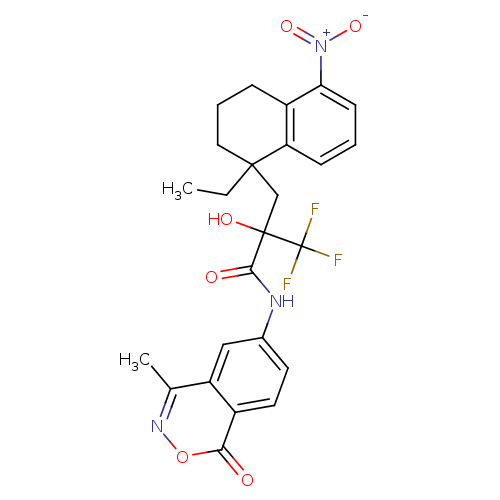
(CHEMBL208779)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2c(cccc12)[N+]([O-])=O Show InChI InChI=1S/C25H24F3N3O6/c1-3-23(11-5-6-17-19(23)7-4-8-20(17)31(35)36)13-24(34,25(26,27)28)22(33)29-15-9-10-16-18(12-15)14(2)30-37-21(16)32/h4,7-10,12,34H,3,5-6,11,13H2,1-2H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411030

(CHEMBL210077)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C25H25F3N2O4/c1-3-23(12-6-8-16-7-4-5-9-20(16)23)14-24(33,25(26,27)28)22(32)29-17-10-11-18-19(13-17)15(2)30-34-21(18)31/h4-5,7,9-11,13,33H,3,6,8,12,14H2,1-2H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411033
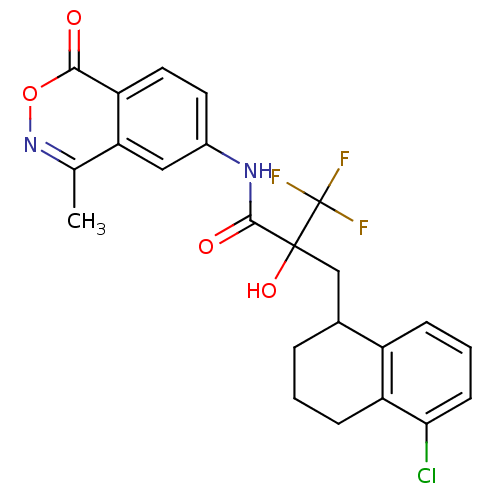
(CHEMBL211387)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3CCCc4c(Cl)cccc34)C(F)(F)F)cc12 Show InChI InChI=1S/C23H20ClF3N2O4/c1-12-18-10-14(8-9-17(18)20(30)33-29-12)28-21(31)22(32,23(25,26)27)11-13-4-2-6-16-15(13)5-3-7-19(16)24/h3,5,7-10,13,32H,2,4,6,11H2,1H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418960
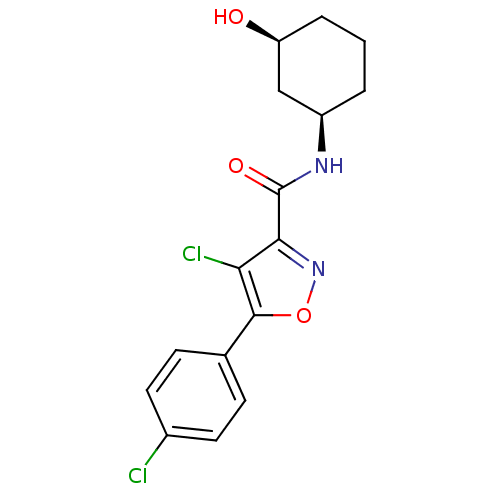
(CHEMBL1807876)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1Cl)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C16H16Cl2N2O3/c17-10-6-4-9(5-7-10)15-13(18)14(20-23-15)16(22)19-11-2-1-3-12(21)8-11/h4-7,11-12,21H,1-3,8H2,(H,19,22)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of calcium flux by FLIPR assay |
Bioorg Med Chem Lett 21: 4652-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.051
BindingDB Entry DOI: 10.7270/Q2M32X1M |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418202
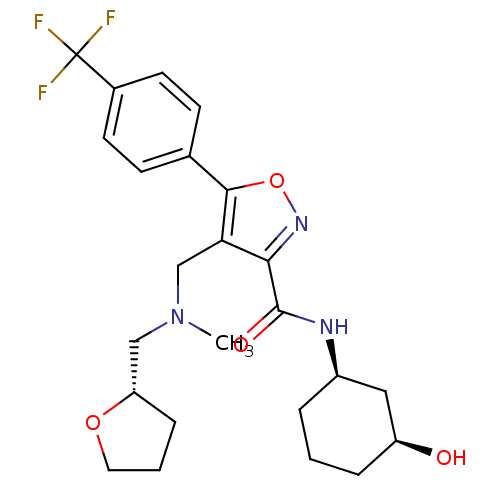
(CHEMBL1761706)Show SMILES CN(C[C@@H]1CCCO1)Cc1c(noc1-c1ccc(cc1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C24H30F3N3O4/c1-30(13-19-6-3-11-33-19)14-20-21(23(32)28-17-4-2-5-18(31)12-17)29-34-22(20)15-7-9-16(10-8-15)24(25,26)27/h7-10,17-19,31H,2-6,11-14H2,1H3,(H,28,32)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ influx by FLIPR assay |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411047

(CHEMBL211441)Show SMILES CCC(CC)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C28H31F3N2O4/c1-4-19(5-2)26(14-8-10-18-9-6-7-11-23(18)26)16-27(36,28(29,30)31)25(35)32-20-12-13-21-22(15-20)17(3)33-37-24(21)34/h6-7,9,11-13,15,19,36H,4-5,8,10,14,16H2,1-3H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411037

(CHEMBL213453)Show SMILES CCCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H27F3N2O4/c1-3-12-24(13-6-8-17-7-4-5-9-21(17)24)15-25(34,26(27,28)29)23(33)30-18-10-11-19-20(14-18)16(2)31-35-22(19)32/h4-5,7,9-11,14,34H,3,6,8,12-13,15H2,1-2H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411030

(CHEMBL210077)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C25H25F3N2O4/c1-3-23(12-6-8-16-7-4-5-9-20(16)23)14-24(33,25(26,27)28)22(32)29-17-10-11-18-19(13-17)15(2)30-34-21(18)31/h4-5,7,9-11,13,33H,3,6,8,12,14H2,1-2H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411041
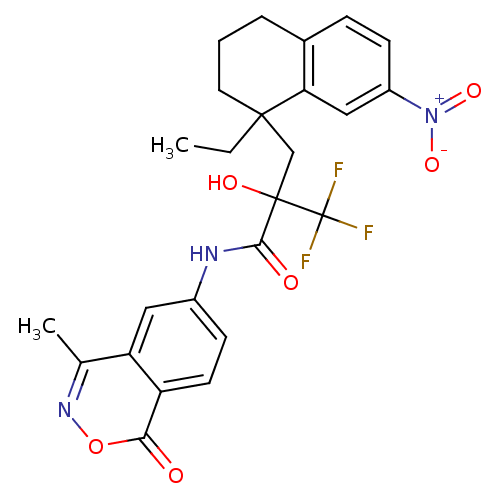
(CHEMBL213621)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccc(cc12)[N+]([O-])=O Show InChI InChI=1S/C25H24F3N3O6/c1-3-23(10-4-5-15-6-8-17(31(35)36)12-20(15)23)13-24(34,25(26,27)28)22(33)29-16-7-9-18-19(11-16)14(2)30-37-21(18)32/h6-9,11-12,34H,3-5,10,13H2,1-2H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411039

(CHEMBL385450)Show SMILES CCCCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C27H29F3N2O4/c1-3-4-13-25(14-7-9-18-8-5-6-10-22(18)25)16-26(35,27(28,29)30)24(34)31-19-11-12-20-21(15-19)17(2)32-36-23(20)33/h5-6,8,10-12,15,35H,3-4,7,9,13-14,16H2,1-2H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50336143
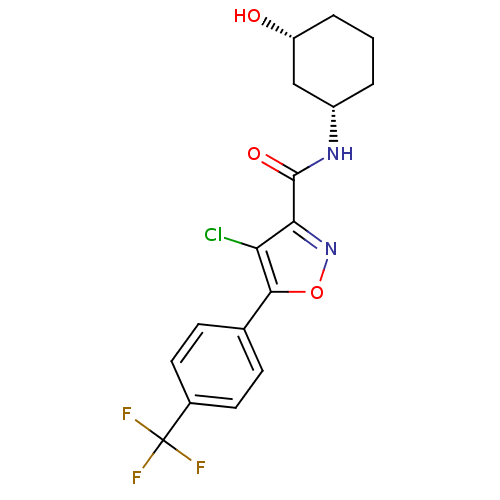
((1S,3R)-4-chloro-N-(3-hydroxycyclohexyl)-5-(4-(tri...)Show SMILES O[C@@H]1CCC[C@@H](C1)NC(=O)c1noc(c1Cl)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C17H16ClF3N2O3/c18-13-14(16(25)22-11-2-1-3-12(24)8-11)23-26-15(13)9-4-6-10(7-5-9)17(19,20)21/h4-7,11-12,24H,1-3,8H2,(H,22,25)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, MSD, Newhouse, Lanarkshire, UK. ronnie.palin@gmail.com
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced calcium influx by fluorimetric assay |
Bioorg Med Chem Lett 21: 892-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.092
BindingDB Entry DOI: 10.7270/Q2K074JC |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411045

(CHEMBL208840)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCCc2ccccc12 Show InChI InChI=1S/C26H27F3N2O4/c1-3-24(13-7-6-9-17-8-4-5-10-21(17)24)15-25(34,26(27,28)29)23(33)30-18-11-12-19-20(14-18)16(2)31-35-22(19)32/h4-5,8,10-12,14,34H,3,6-7,9,13,15H2,1-2H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411049

(CHEMBL190838)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3CCCc4ccccc34)C(F)(F)F)cc12 Show InChI InChI=1S/C23H21F3N2O4/c1-13-19-11-16(9-10-18(19)20(29)32-28-13)27-21(30)22(31,23(24,25)26)12-15-7-4-6-14-5-2-3-8-17(14)15/h2-3,5,8-11,15,31H,4,6-7,12H2,1H3,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411032

(CHEMBL209259)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2cc(ccc12)[N+]([O-])=O Show InChI InChI=1S/C25H24F3N3O6/c1-3-23(10-4-5-15-11-17(31(35)36)7-9-20(15)23)13-24(34,25(26,27)28)22(33)29-16-6-8-18-19(12-16)14(2)30-37-21(18)32/h6-9,11-12,34H,3-5,10,13H2,1-2H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418184

(CHEMBL1761694)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1CNCC1CC1)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C22H26F3N3O3/c23-22(24,25)15-8-6-14(7-9-15)20-18(12-26-11-13-4-5-13)19(28-31-20)21(30)27-16-2-1-3-17(29)10-16/h6-9,13,16-17,26,29H,1-5,10-12H2,(H,27,30)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ influx by FLIPR assay |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418196

(CHEMBL1761704)Show SMILES COCCN(C)Cc1c(noc1-c1ccc(cc1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C22H28F3N3O4/c1-28(10-11-31-2)13-18-19(21(30)26-16-4-3-5-17(29)12-16)27-32-20(18)14-6-8-15(9-7-14)22(23,24)25/h6-9,16-17,29H,3-5,10-13H2,1-2H3,(H,26,30)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ influx by FLIPR assay |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411024

(CHEMBL208996)Show SMILES Cc1noc(=O)c2ccc(NC(=O)[C@](O)(C[C@]3(CCCc4ccccc34)C3CCCC3)C(F)(F)F)cc12 Show InChI InChI=1S/C28H29F3N2O4/c1-17-22-15-20(12-13-21(22)24(34)37-33-17)32-25(35)27(36,28(29,30)31)16-26(19-9-3-4-10-19)14-6-8-18-7-2-5-11-23(18)26/h2,5,7,11-13,15,19,36H,3-4,6,8-10,14,16H2,1H3,(H,32,35)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data