

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50318885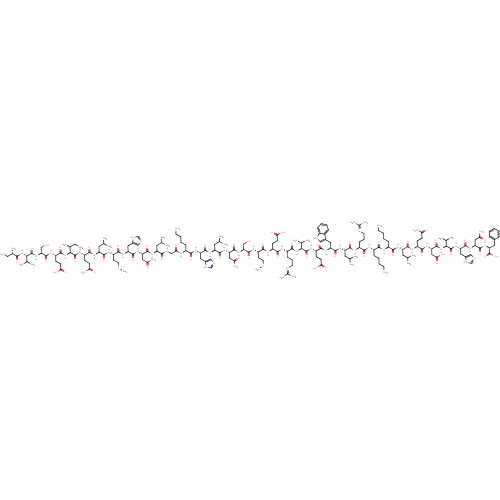 (CHEMBL525610 | teriparatide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of 125I-PTH (1 to 15 residues) from human PTHR1 expressed in African green monkey COS7 cell membranes at 300 uM after 90 mins by gamma c... | J Med Chem 61: 5949-5962 (2018) Article DOI: 10.1021/acs.jmedchem.8b00182 BindingDB Entry DOI: 10.7270/Q2ZC85GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445769 (CHEMBL3104615) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.125 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441476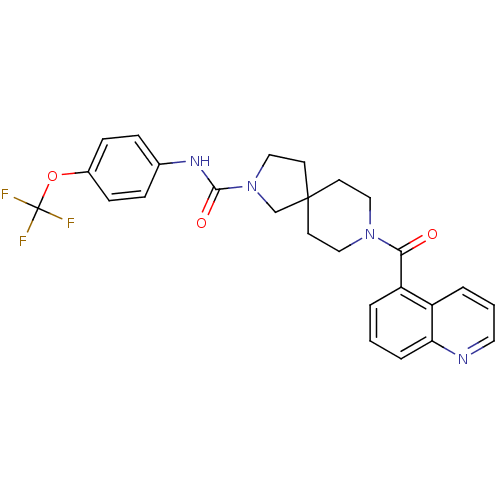 (CHEMBL2436575) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441467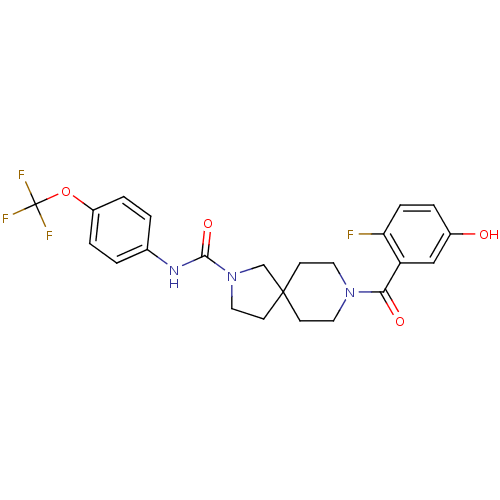 (CHEMBL2436573) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441481 (CHEMBL2436579) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192809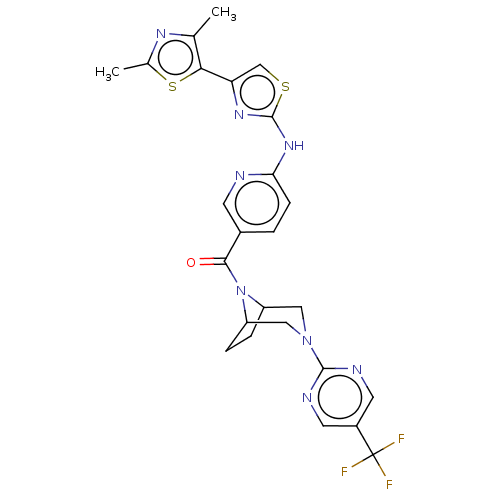 (CHEMBL3941914) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441483 (CHEMBL2436591) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441469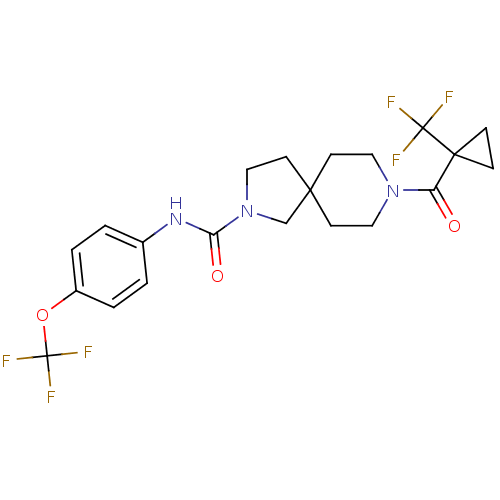 (CHEMBL2436586) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441470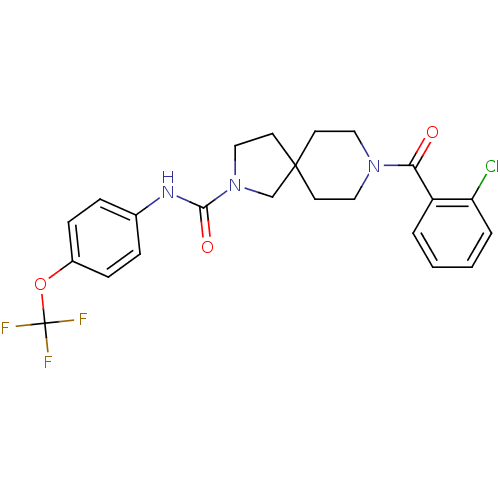 (CHEMBL2436574) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441474 (CHEMBL2436563) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192811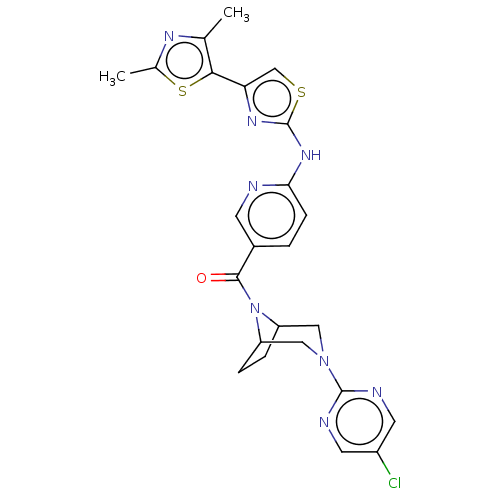 (CHEMBL3971502) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192807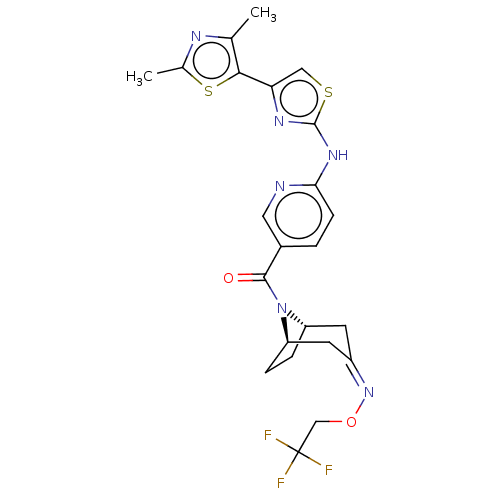 (CHEMBL3984947) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441486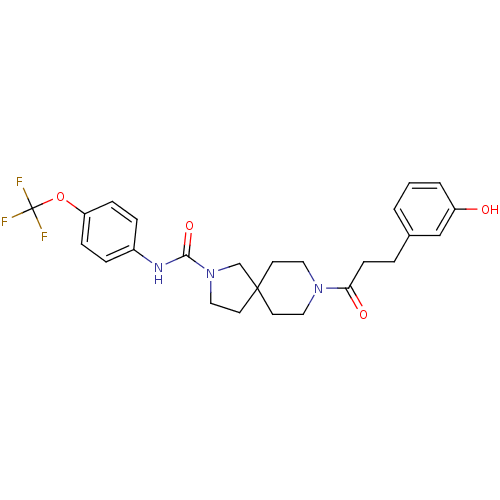 (CHEMBL2436593) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441472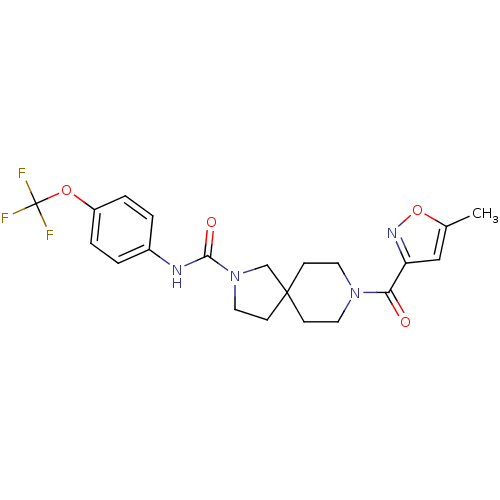 (CHEMBL2436578) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441468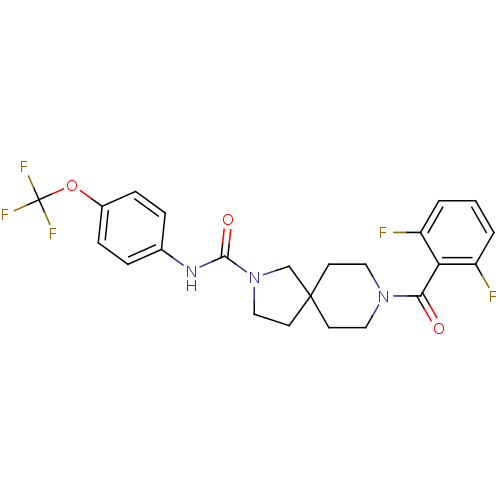 (CHEMBL2436564) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192809 (CHEMBL3941914) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of hypotonicity-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445752 (CHEMBL3104441) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441482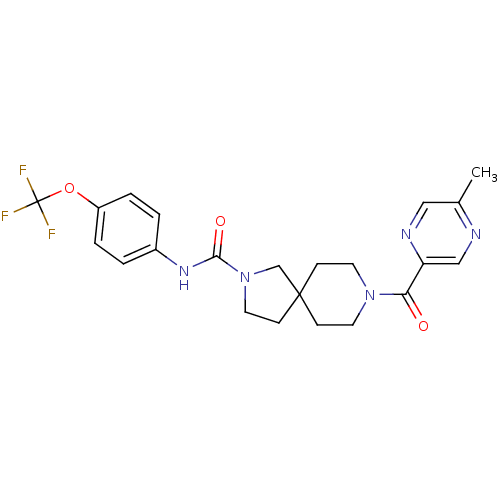 (CHEMBL2436577) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441492 (CHEMBL2436580) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445751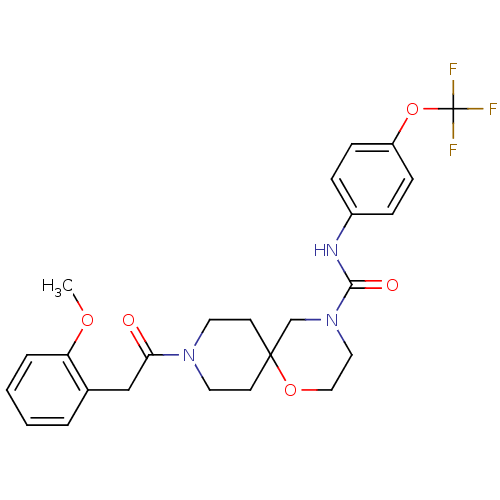 (CHEMBL3104442) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441484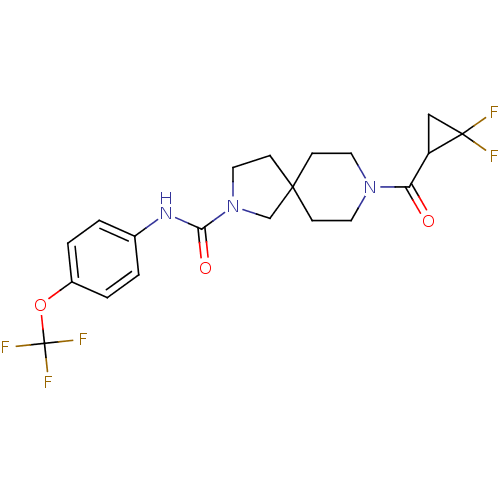 (CHEMBL2436587) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192804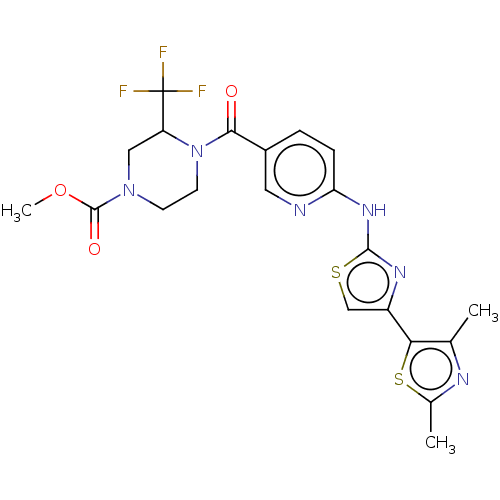 (CHEMBL3933401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441479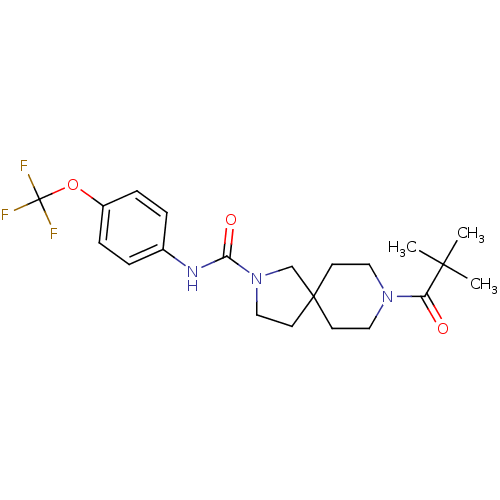 (CHEMBL2436590) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441475 (CHEMBL2436589) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445760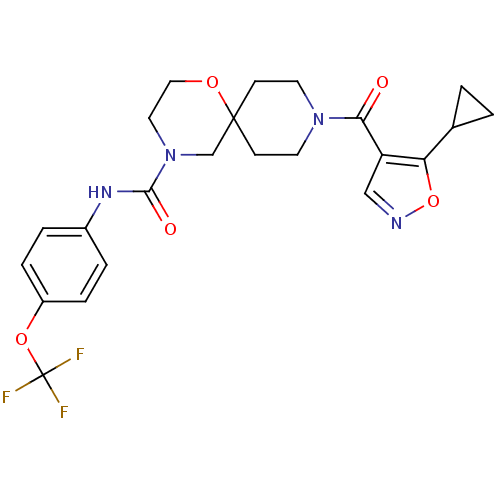 (CHEMBL3104432) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445758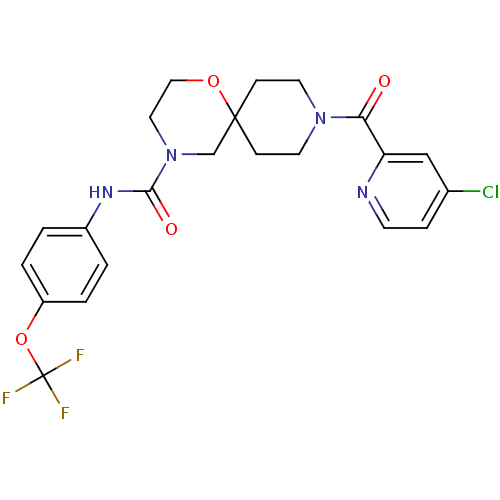 (CHEMBL3104435) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445753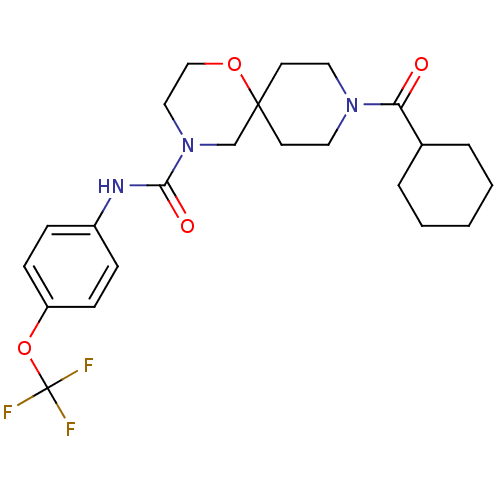 (CHEMBL3104440) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50276045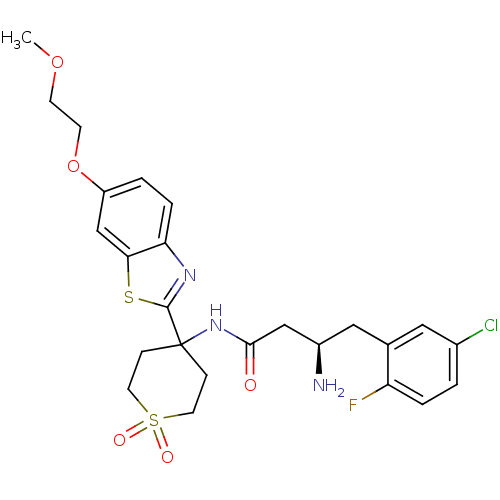 ((R)-3-Amino-4-(5-chloro-2-fluoro-phenyl)-N-{4-[6-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human Caco2 cells-derived DPP4 | Bioorg Med Chem Lett 18: 5435-8 (2008) Article DOI: 10.1016/j.bmcl.2008.09.042 BindingDB Entry DOI: 10.7270/Q2QV3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441471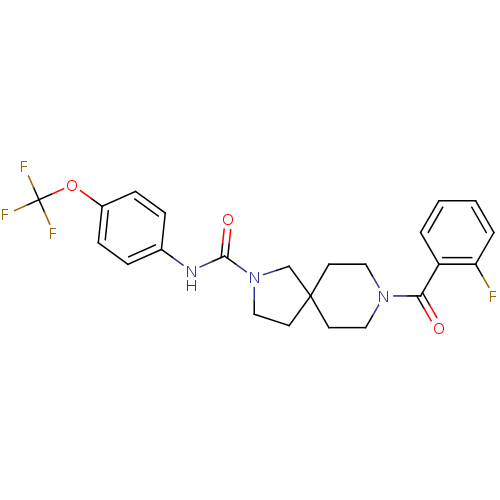 (CHEMBL2436572) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445770 (CHEMBL3104444) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441478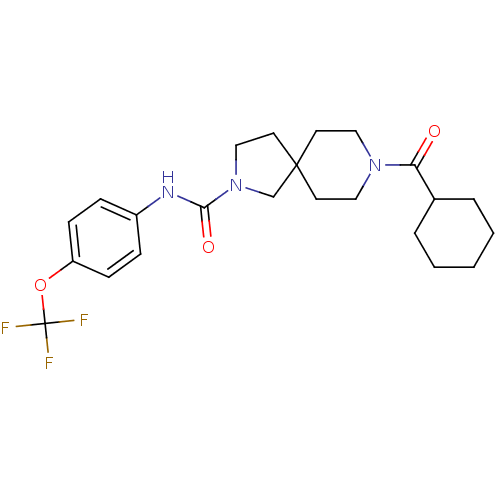 (CHEMBL2436584) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445750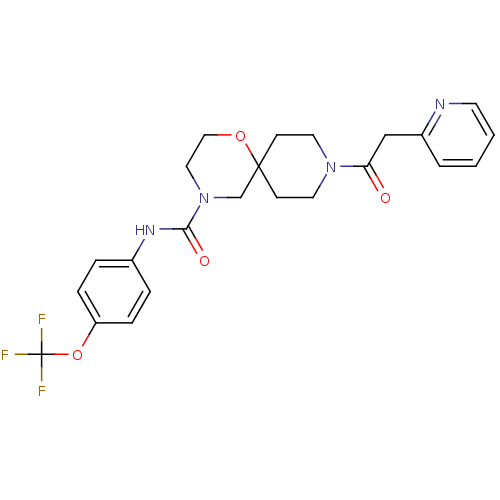 (CHEMBL3104443) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50276047 ((R)-3-Amino-N-{4-[6-(2-morpholin-4-yl-ethoxy)-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human Caco2 cells-derived DPP4 | Bioorg Med Chem Lett 18: 5435-8 (2008) Article DOI: 10.1016/j.bmcl.2008.09.042 BindingDB Entry DOI: 10.7270/Q2QV3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441488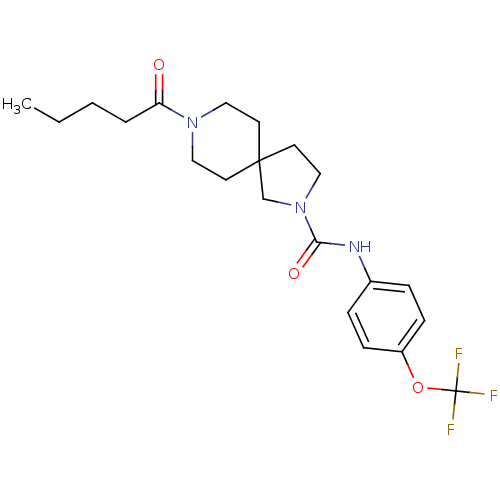 (CHEMBL2436592) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441487 (CHEMBL2436576) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445763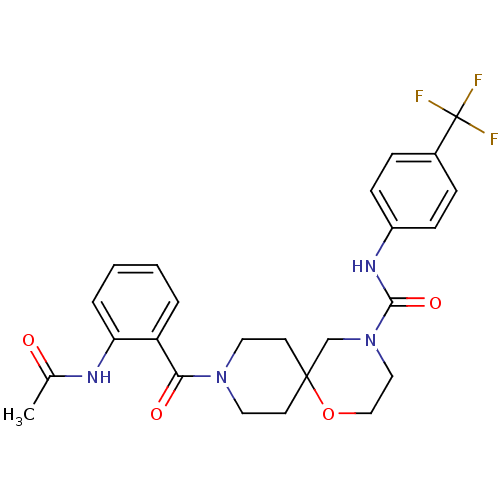 (CHEMBL3104622) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445757 (CHEMBL3104436) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441480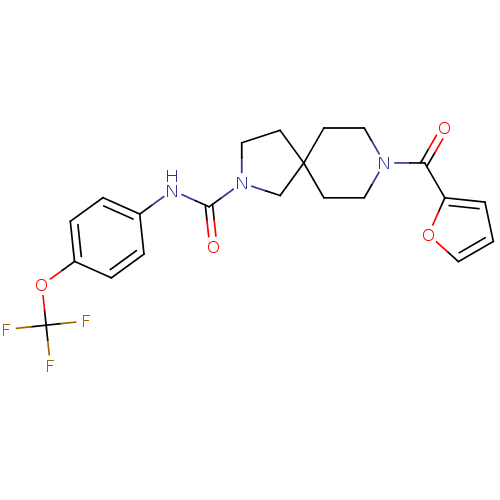 (CHEMBL2436581) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192820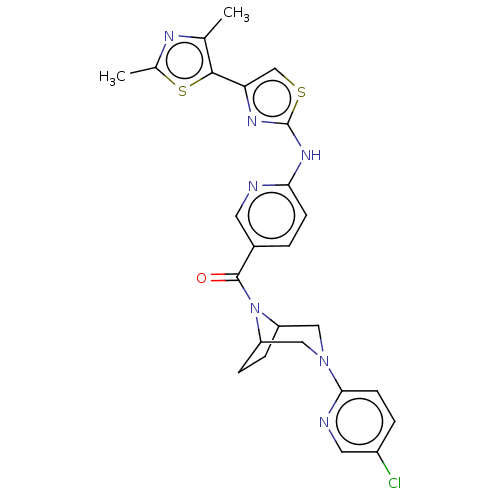 (CHEMBL3950646) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192808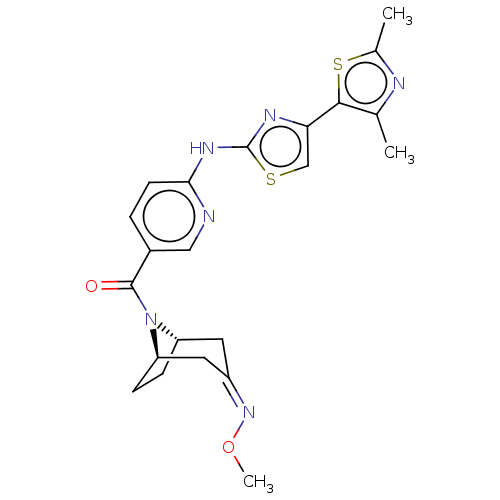 (CHEMBL3915150) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50276001 ((R)-3-Amino-N-{4-[6-(2-methoxy-ethoxy)-benzothiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human Caco2 cells-derived DPP4 | Bioorg Med Chem Lett 18: 5435-8 (2008) Article DOI: 10.1016/j.bmcl.2008.09.042 BindingDB Entry DOI: 10.7270/Q2QV3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445764 (CHEMBL3104621) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441461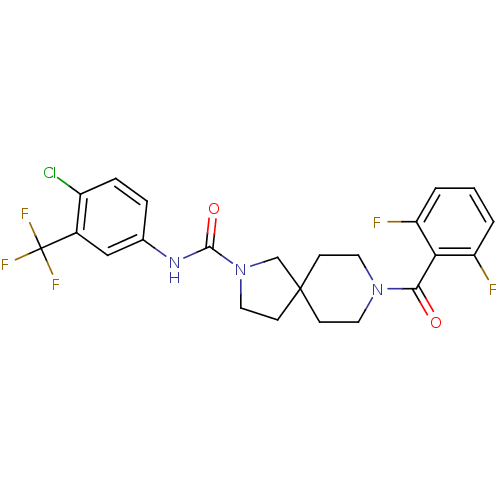 (CHEMBL2436568) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192779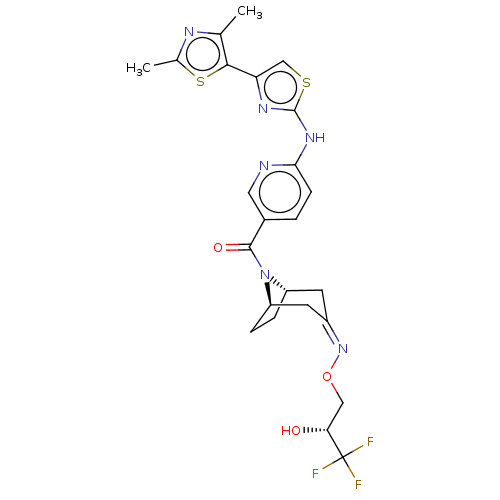 (CHEMBL3957347) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445768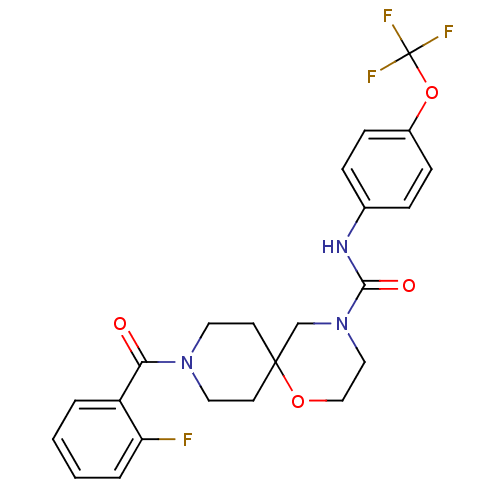 (CHEMBL3102876) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445759 (CHEMBL3104433) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445765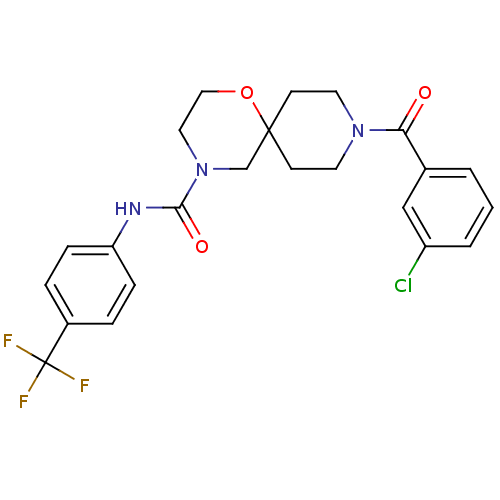 (CHEMBL3104620) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441462 (CHEMBL2436585) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50182869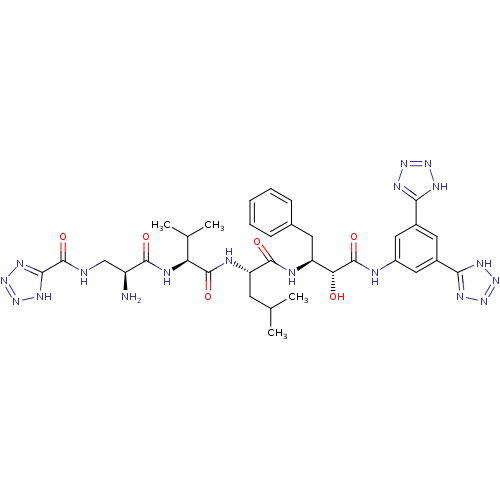 (CHEMBL439521 | N-((S)-2-amino-3-((S)-1-((S)-1-((2S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by FRET assay | Bioorg Med Chem Lett 22: 1130-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.102 BindingDB Entry DOI: 10.7270/Q2BZ66GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445767 (CHEMBL3104617) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 394 total ) | Next | Last >> |