 Found 266 hits with Last Name = 'nord' and Initial = 'm'
Found 266 hits with Last Name = 'nord' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414549
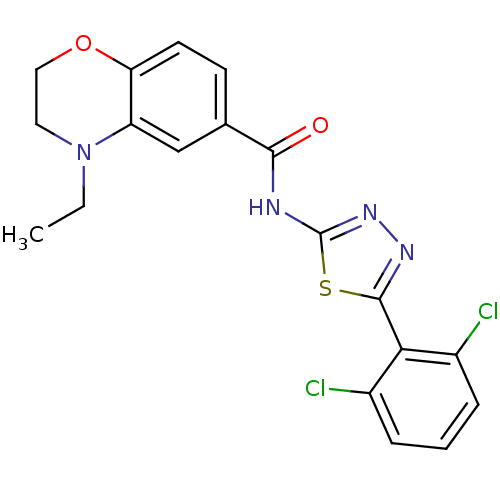
(CHEMBL563480)Show SMILES CCN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C19H16Cl2N4O2S/c1-2-25-8-9-27-15-7-6-11(10-14(15)25)17(26)22-19-24-23-18(28-19)16-12(20)4-3-5-13(16)21/h3-7,10H,2,8-9H2,1H3,(H,22,24,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414547

(CHEMBL558644)Show SMILES CN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C18H14Cl2N4O2S/c1-24-7-8-26-14-6-5-10(9-13(14)24)16(25)21-18-23-22-17(27-18)15-11(19)3-2-4-12(15)20/h2-6,9H,7-8H2,1H3,(H,21,23,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414536
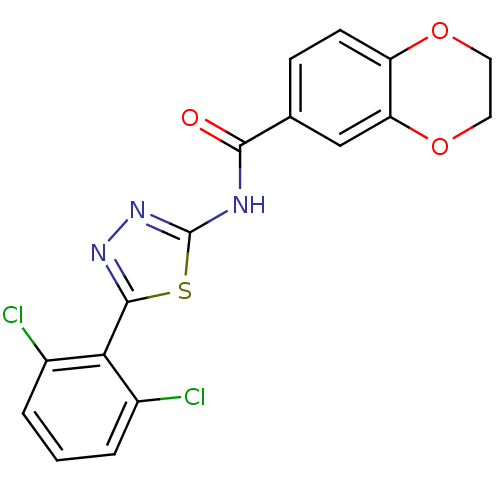
(CHEMBL551202)Show SMILES Clc1cccc(Cl)c1-c1nnc(NC(=O)c2ccc3OCCOc3c2)s1 Show InChI InChI=1S/C17H11Cl2N3O3S/c18-10-2-1-3-11(19)14(10)16-21-22-17(26-16)20-15(23)9-4-5-12-13(8-9)25-7-6-24-12/h1-5,8H,6-7H2,(H,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414543
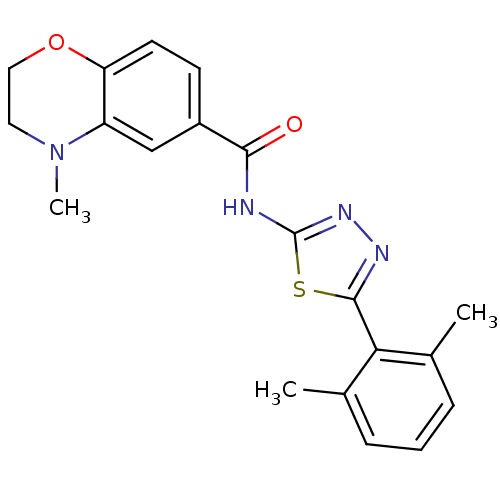
(CHEMBL550316)Show SMILES CN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(C)cccc1C Show InChI InChI=1S/C20H20N4O2S/c1-12-5-4-6-13(2)17(12)19-22-23-20(27-19)21-18(25)14-7-8-16-15(11-14)24(3)9-10-26-16/h4-8,11H,9-10H2,1-3H3,(H,21,23,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50414549

(CHEMBL563480)Show SMILES CCN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C19H16Cl2N4O2S/c1-2-25-8-9-27-15-7-6-11(10-14(15)25)17(26)22-19-24-23-18(28-19)16-12(20)4-3-5-13(16)21/h3-7,10H,2,8-9H2,1H3,(H,22,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat EP3 receptor |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414548

(CHEMBL550055)Show SMILES Clc1cccc(Cl)c1-c1nnc(NC(=O)c2ccc3OCCNc3c2)s1 Show InChI InChI=1S/C17H12Cl2N4O2S/c18-10-2-1-3-11(19)14(10)16-22-23-17(26-16)21-15(24)9-4-5-13-12(8-9)20-6-7-25-13/h1-5,8,20H,6-7H2,(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414537

(CHEMBL554297)Show SMILES COc1cc(Cl)c(-c2nnc(NC(=O)c3ccc4OCCOc4c3)s2)c(Cl)c1 Show InChI InChI=1S/C18H13Cl2N3O4S/c1-25-10-7-11(19)15(12(20)8-10)17-22-23-18(28-17)21-16(24)9-2-3-13-14(6-9)27-5-4-26-13/h2-3,6-8H,4-5H2,1H3,(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414533
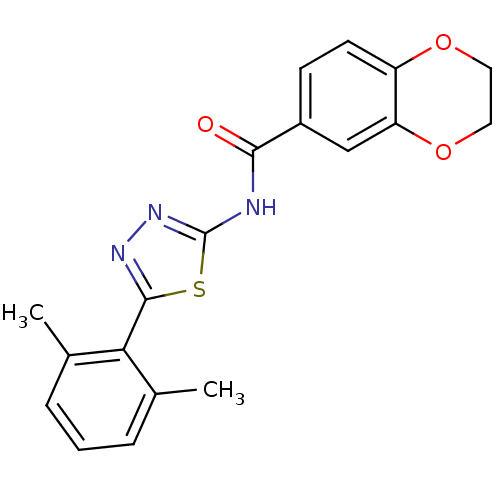
(CHEMBL549437)Show InChI InChI=1S/C19H17N3O3S/c1-11-4-3-5-12(2)16(11)18-21-22-19(26-18)20-17(23)13-6-7-14-15(10-13)25-9-8-24-14/h3-7,10H,8-9H2,1-2H3,(H,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414544
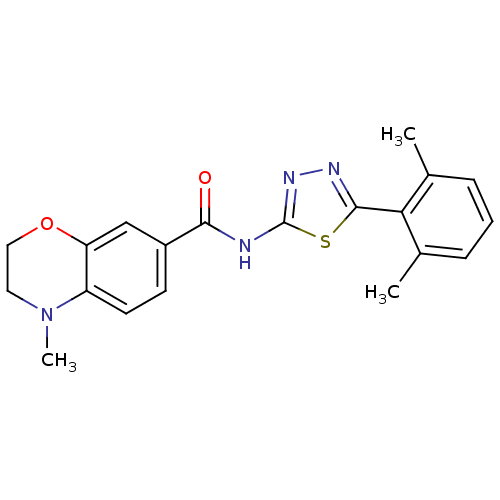
(CHEMBL562329)Show SMILES CN1CCOc2cc(ccc12)C(=O)Nc1nnc(s1)-c1c(C)cccc1C Show InChI InChI=1S/C20H20N4O2S/c1-12-5-4-6-13(2)17(12)19-22-23-20(27-19)21-18(25)14-7-8-15-16(11-14)26-10-9-24(15)3/h4-8,11H,9-10H2,1-3H3,(H,21,23,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414534
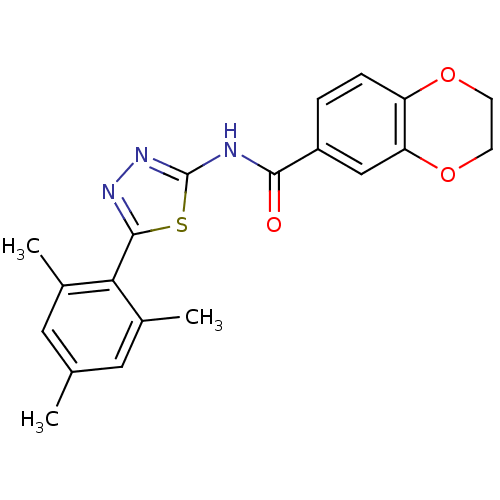
(CHEMBL564190)Show SMILES Cc1cc(C)c(-c2nnc(NC(=O)c3ccc4OCCOc4c3)s2)c(C)c1 Show InChI InChI=1S/C20H19N3O3S/c1-11-8-12(2)17(13(3)9-11)19-22-23-20(27-19)21-18(24)14-4-5-15-16(10-14)26-7-6-25-15/h4-5,8-10H,6-7H2,1-3H3,(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414542

(CHEMBL562994)Show InChI InChI=1S/C21H21N3OS/c1-13-6-5-7-14(2)18(13)20-23-24-21(26-20)22-19(25)17-11-10-15-8-3-4-9-16(15)12-17/h5-7,10-12H,3-4,8-9H2,1-2H3,(H,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50296437
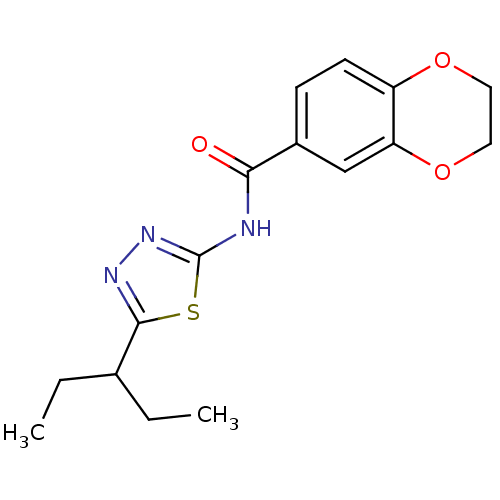
(CHEMBL557109 | N-(5-(pentan-3-yl)-1,3,4-thiadiazol...)Show InChI InChI=1S/C16H19N3O3S/c1-3-10(4-2)15-18-19-16(23-15)17-14(20)11-5-6-12-13(9-11)22-8-7-21-12/h5-6,9-10H,3-4,7-8H2,1-2H3,(H,17,19,20) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414550
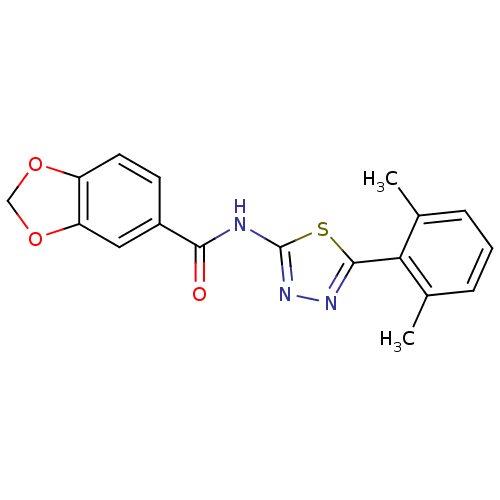
(CHEMBL564317)Show InChI InChI=1S/C18H15N3O3S/c1-10-4-3-5-11(2)15(10)17-20-21-18(25-17)19-16(22)12-6-7-13-14(8-12)24-9-23-13/h3-8H,9H2,1-2H3,(H,19,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50296437

(CHEMBL557109 | N-(5-(pentan-3-yl)-1,3,4-thiadiazol...)Show InChI InChI=1S/C16H19N3O3S/c1-3-10(4-2)15-18-19-16(23-15)17-14(20)11-5-6-12-13(9-11)22-8-7-21-12/h5-6,9-10H,3-4,7-8H2,1-2H3,(H,17,19,20) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human EP3 receptor |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414529
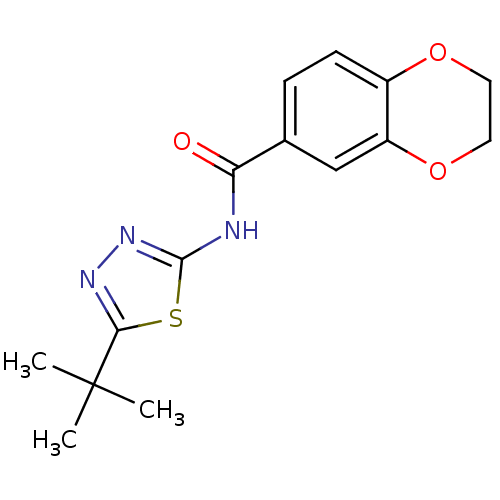
(CHEMBL564305)Show InChI InChI=1S/C15H17N3O3S/c1-15(2,3)13-17-18-14(22-13)16-12(19)9-4-5-10-11(8-9)21-7-6-20-10/h4-5,8H,6-7H2,1-3H3,(H,16,18,19) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414528

(CHEMBL562465)Show InChI InChI=1S/C16H17N3O3S/c20-14(11-5-6-12-13(9-11)22-8-7-21-12)17-16-19-18-15(23-16)10-3-1-2-4-10/h5-6,9-10H,1-4,7-8H2,(H,17,19,20) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414541
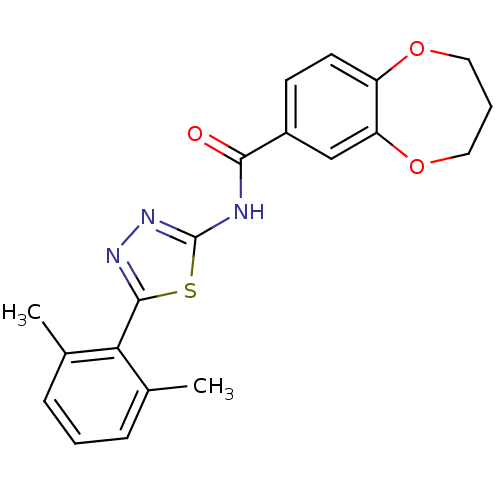
(CHEMBL562132)Show SMILES Cc1cccc(C)c1-c1nnc(NC(=O)c2ccc3OCCCOc3c2)s1 Show InChI InChI=1S/C20H19N3O3S/c1-12-5-3-6-13(2)17(12)19-22-23-20(27-19)21-18(24)14-7-8-15-16(11-14)26-10-4-9-25-15/h3,5-8,11H,4,9-10H2,1-2H3,(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414540
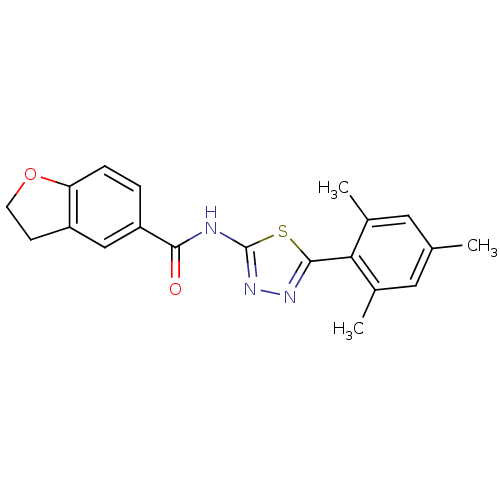
(CHEMBL562692)Show SMILES Cc1cc(C)c(-c2nnc(NC(=O)c3ccc4OCCc4c3)s2)c(C)c1 Show InChI InChI=1S/C20H19N3O2S/c1-11-8-12(2)17(13(3)9-11)19-22-23-20(26-19)21-18(24)15-4-5-16-14(10-15)6-7-25-16/h4-5,8-10H,6-7H2,1-3H3,(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414535
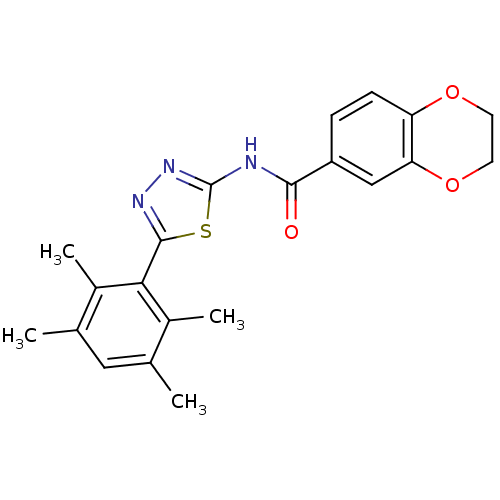
(CHEMBL549909)Show SMILES Cc1cc(C)c(C)c(-c2nnc(NC(=O)c3ccc4OCCOc4c3)s2)c1C Show InChI InChI=1S/C21H21N3O3S/c1-11-9-12(2)14(4)18(13(11)3)20-23-24-21(28-20)22-19(25)15-5-6-16-17(10-15)27-8-7-26-16/h5-6,9-10H,7-8H2,1-4H3,(H,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414532
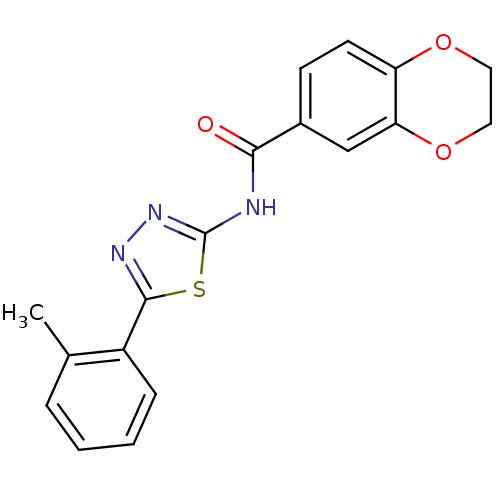
(CHEMBL557312)Show InChI InChI=1S/C18H15N3O3S/c1-11-4-2-3-5-13(11)17-20-21-18(25-17)19-16(22)12-6-7-14-15(10-12)24-9-8-23-14/h2-7,10H,8-9H2,1H3,(H,19,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414538
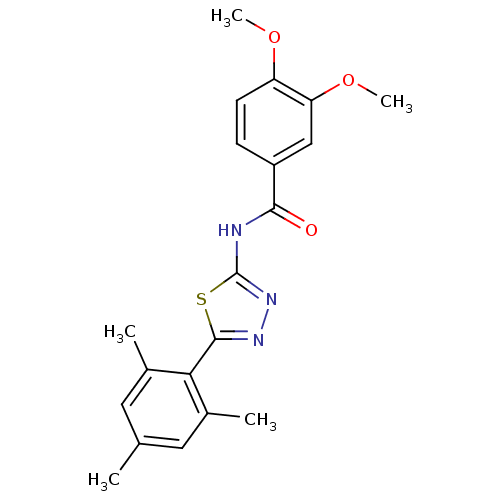
(CHEMBL551203)Show SMILES COc1ccc(cc1OC)C(=O)Nc1nnc(s1)-c1c(C)cc(C)cc1C Show InChI InChI=1S/C20H21N3O3S/c1-11-8-12(2)17(13(3)9-11)19-22-23-20(27-19)21-18(24)14-6-7-15(25-4)16(10-14)26-5/h6-10H,1-5H3,(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414545
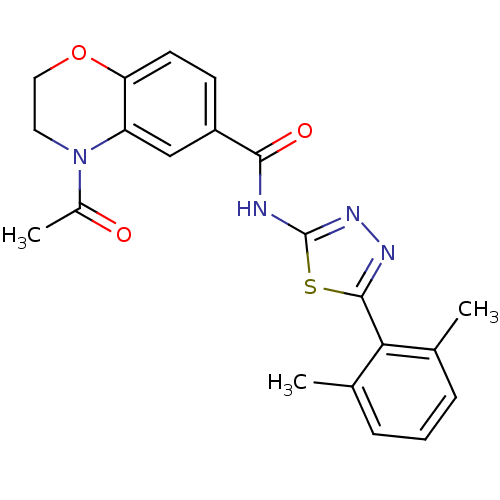
(CHEMBL560868)Show SMILES CC(=O)N1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(C)cccc1C Show InChI InChI=1S/C21H20N4O3S/c1-12-5-4-6-13(2)18(12)20-23-24-21(29-20)22-19(27)15-7-8-17-16(11-15)25(14(3)26)9-10-28-17/h4-8,11H,9-10H2,1-3H3,(H,22,24,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414539
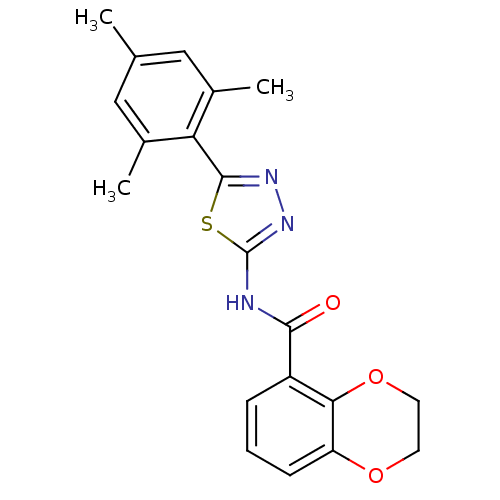
(CHEMBL557857)Show SMILES Cc1cc(C)c(-c2nnc(NC(=O)c3cccc4OCCOc34)s2)c(C)c1 Show InChI InChI=1S/C20H19N3O3S/c1-11-9-12(2)16(13(3)10-11)19-22-23-20(27-19)21-18(24)14-5-4-6-15-17(14)26-8-7-25-15/h4-6,9-10H,7-8H2,1-3H3,(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414546
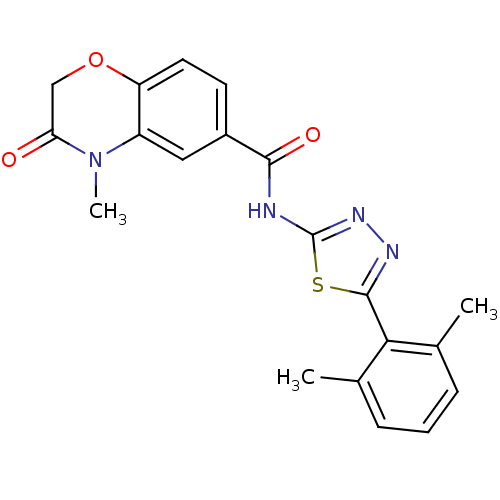
(CHEMBL560388)Show SMILES CN1C(=O)COc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(C)cccc1C Show InChI InChI=1S/C20H18N4O3S/c1-11-5-4-6-12(2)17(11)19-22-23-20(28-19)21-18(26)13-7-8-15-14(9-13)24(3)16(25)10-27-15/h4-9H,10H2,1-3H3,(H,21,23,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50414549

(CHEMBL563480)Show SMILES CCN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C19H16Cl2N4O2S/c1-2-25-8-9-27-15-7-6-11(10-14(15)25)17(26)22-19-24-23-18(28-19)16-12(20)4-3-5-13(16)21/h3-7,10H,2,8-9H2,1H3,(H,22,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human TP receptor by radioligand binding assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50414549

(CHEMBL563480)Show SMILES CCN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C19H16Cl2N4O2S/c1-2-25-8-9-27-15-7-6-11(10-14(15)25)17(26)22-19-24-23-18(28-19)16-12(20)4-3-5-13(16)21/h3-7,10H,2,8-9H2,1H3,(H,22,24,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human DP receptor |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50414549

(CHEMBL563480)Show SMILES CCN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C19H16Cl2N4O2S/c1-2-25-8-9-27-15-7-6-11(10-14(15)25)17(26)22-19-24-23-18(28-19)16-12(20)4-3-5-13(16)21/h3-7,10H,2,8-9H2,1H3,(H,22,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human EP4 receptor by radioligand binding assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50414549

(CHEMBL563480)Show SMILES CCN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C19H16Cl2N4O2S/c1-2-25-8-9-27-15-7-6-11(10-14(15)25)17(26)22-19-24-23-18(28-19)16-12(20)4-3-5-13(16)21/h3-7,10H,2,8-9H2,1H3,(H,22,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human EP2 receptor by radioligand binding assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50296437

(CHEMBL557109 | N-(5-(pentan-3-yl)-1,3,4-thiadiazol...)Show InChI InChI=1S/C16H19N3O3S/c1-3-10(4-2)15-18-19-16(23-15)17-14(20)11-5-6-12-13(9-11)22-8-7-21-12/h5-6,9-10H,3-4,7-8H2,1-2H3,(H,17,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human FP receptor |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50296437

(CHEMBL557109 | N-(5-(pentan-3-yl)-1,3,4-thiadiazol...)Show InChI InChI=1S/C16H19N3O3S/c1-3-10(4-2)15-18-19-16(23-15)17-14(20)11-5-6-12-13(9-11)22-8-7-21-12/h5-6,9-10H,3-4,7-8H2,1-2H3,(H,17,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human TP receptor |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50296437

(CHEMBL557109 | N-(5-(pentan-3-yl)-1,3,4-thiadiazol...)Show InChI InChI=1S/C16H19N3O3S/c1-3-10(4-2)15-18-19-16(23-15)17-14(20)11-5-6-12-13(9-11)22-8-7-21-12/h5-6,9-10H,3-4,7-8H2,1-2H3,(H,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human EP4 receptor |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50296437

(CHEMBL557109 | N-(5-(pentan-3-yl)-1,3,4-thiadiazol...)Show InChI InChI=1S/C16H19N3O3S/c1-3-10(4-2)15-18-19-16(23-15)17-14(20)11-5-6-12-13(9-11)22-8-7-21-12/h5-6,9-10H,3-4,7-8H2,1-2H3,(H,17,19,20) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human EP1 receptor |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50296437

(CHEMBL557109 | N-(5-(pentan-3-yl)-1,3,4-thiadiazol...)Show InChI InChI=1S/C16H19N3O3S/c1-3-10(4-2)15-18-19-16(23-15)17-14(20)11-5-6-12-13(9-11)22-8-7-21-12/h5-6,9-10H,3-4,7-8H2,1-2H3,(H,17,19,20) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human DP receptor |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50296437

(CHEMBL557109 | N-(5-(pentan-3-yl)-1,3,4-thiadiazol...)Show InChI InChI=1S/C16H19N3O3S/c1-3-10(4-2)15-18-19-16(23-15)17-14(20)11-5-6-12-13(9-11)22-8-7-21-12/h5-6,9-10H,3-4,7-8H2,1-2H3,(H,17,19,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human IP receptor |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414530
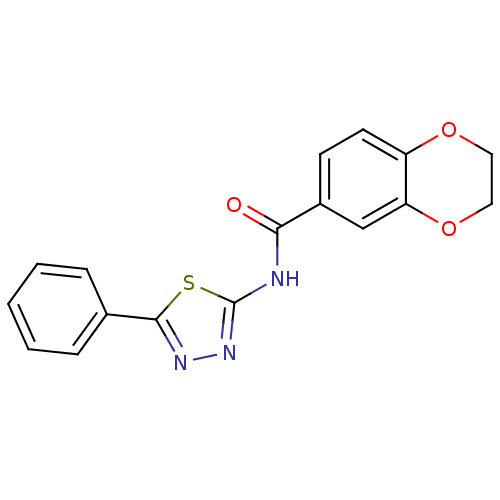
(CHEMBL538370)Show InChI InChI=1S/C17H13N3O3S/c21-15(12-6-7-13-14(10-12)23-9-8-22-13)18-17-20-19-16(24-17)11-4-2-1-3-5-11/h1-7,10H,8-9H2,(H,18,20,21) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50414549

(CHEMBL563480)Show SMILES CCN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C19H16Cl2N4O2S/c1-2-25-8-9-27-15-7-6-11(10-14(15)25)17(26)22-19-24-23-18(28-19)16-12(20)4-3-5-13(16)21/h3-7,10H,2,8-9H2,1H3,(H,22,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF2a from human recombinant FP receptor expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414531
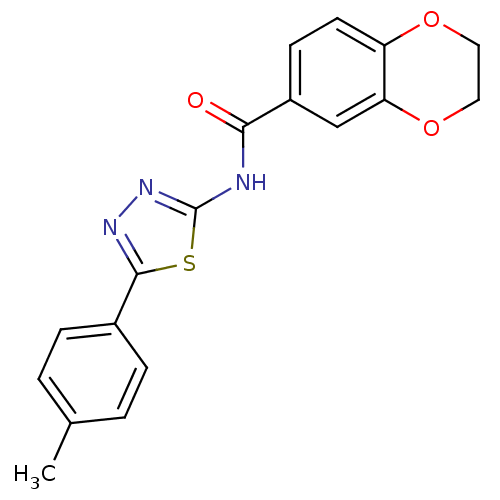
(CHEMBL549708)Show InChI InChI=1S/C18H15N3O3S/c1-11-2-4-12(5-3-11)17-20-21-18(25-17)19-16(22)13-6-7-14-15(10-13)24-9-8-23-14/h2-7,10H,8-9H2,1H3,(H,19,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414549

(CHEMBL563480)Show SMILES CCN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C19H16Cl2N4O2S/c1-2-25-8-9-27-15-7-6-11(10-14(15)25)17(26)22-19-24-23-18(28-19)16-12(20)4-3-5-13(16)21/h3-7,10H,2,8-9H2,1H3,(H,22,24,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human EP1 receptor by radioligand binding assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability to inhibit recombinant human dihydrofolate reductase. |
J Med Chem 43: 3852-61 (2000)
BindingDB Entry DOI: 10.7270/Q2N87BGB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MMP12 catalytic domain (Gly106 to Gly263 residues) expressed in Escherichia coli BL21 Codon Plus cells using 5-FAM/QX... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01285
BindingDB Entry DOI: 10.7270/Q2PK0KTG |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50554436
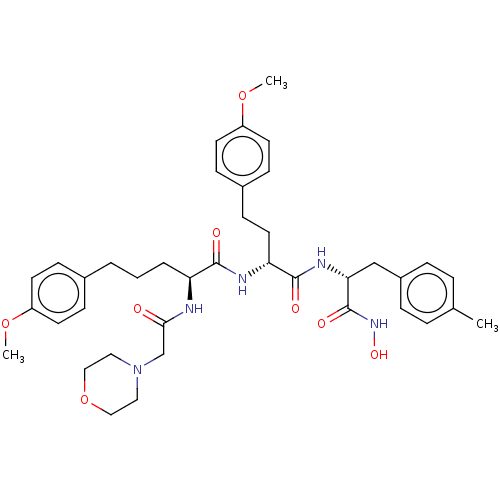
(CHEMBL4787709)Show SMILES COc1ccc(CCC[C@H](NC(=O)CN2CCOCC2)C(=O)N[C@H](CCc2ccc(OC)cc2)C(=O)N[C@H](Cc2ccc(C)cc2)C(=O)NO)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MMP12 catalytic domain (Gly106 to Gly263 residues) expressed in Escherichia coli BL21 Codon Plus cells using 5-FAM/QX... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01285
BindingDB Entry DOI: 10.7270/Q2PK0KTG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against dihydrofolate reductase DHFR from rat liver. |
J Med Chem 44: 2391-402 (2001)
BindingDB Entry DOI: 10.7270/Q21J9911 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50554435
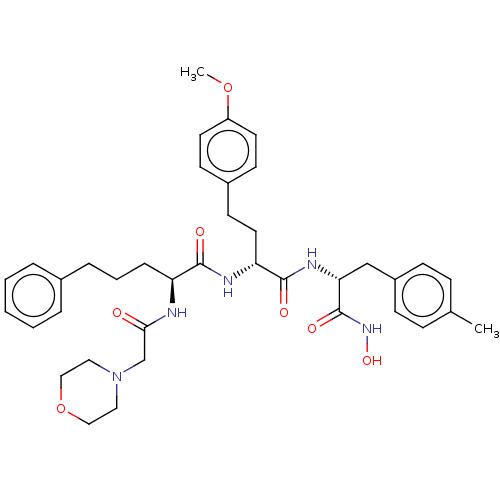
(CHEMBL4755394)Show SMILES COc1ccc(CC[C@@H](NC(=O)[C@H](CCCc2ccccc2)NC(=O)CN2CCOCC2)C(=O)N[C@H](Cc2ccc(C)cc2)C(=O)NO)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MMP12 catalytic domain (Gly106 to Gly263 residues) expressed in Escherichia coli BL21 Codon Plus cells using 5-FAM/QX... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01285
BindingDB Entry DOI: 10.7270/Q2PK0KTG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50296010

(2-isobutyl-4-(5-phenyl-1H-pyrrolo[2,3-b]pyridin-3-...)Show SMILES CC(C)Cc1cc(ccc1C(O)=O)-c1c[nH]c2ncc(cc12)-c1ccccc1 Show InChI InChI=1S/C24H22N2O2/c1-15(2)10-18-11-17(8-9-20(18)24(27)28)22-14-26-23-21(22)12-19(13-25-23)16-6-4-3-5-7-16/h3-9,11-15H,10H2,1-2H3,(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGK1 by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 4441-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.051
BindingDB Entry DOI: 10.7270/Q2WQ03VC |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50294706

(4-((2-chlorobenzyl)(3-methyl-1-(1-methyl-1H-tetraz...)Show SMILES CC(C)CC(N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1)c1nnnn1C Show InChI InChI=1S/C21H22Cl2N6/c1-14(2)10-20(21-25-26-27-28(21)3)29(13-16-6-4-5-7-18(16)22)17-9-8-15(12-24)19(23)11-17/h4-9,11,14,20H,10,13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay |
Bioorg Med Chem Lett 19: 2637-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.146
BindingDB Entry DOI: 10.7270/Q2FX79GM |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50152761

(3-[3-(2-Bromo-phenyl)-ureido]-6-chloro-2-hydroxy-b...)Show InChI InChI=1S/C13H11BrClN3O4S/c14-7-3-1-2-4-9(7)17-13(20)18-10-6-5-8(15)12(11(10)19)23(16,21)22/h1-6,19H,(H2,16,21,22)(H2,17,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [125I]IL-8 binding towards C-X-C chemokine receptor type 2 of human expressed in CHO cells |
Bioorg Med Chem Lett 14: 4375-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.097
BindingDB Entry DOI: 10.7270/Q2SQ914M |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against dihydrofolate reductase DHFR from rat liver. |
J Med Chem 44: 2391-402 (2001)
BindingDB Entry DOI: 10.7270/Q21J9911 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against dihydrofolate reductase DHFR from T. gondii |
J Med Chem 44: 2391-402 (2001)
BindingDB Entry DOI: 10.7270/Q21J9911 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50152759

(3-[3-(2-Bromo-phenyl)-ureido]-6-chloro-2-hydroxy-b...)Show InChI InChI=1S/C14H11BrClN3O3/c15-7-3-1-2-4-9(7)18-14(22)19-10-6-5-8(16)11(12(10)20)13(17)21/h1-6,20H,(H2,17,21)(H2,18,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [125I]IL-8 binding towards C-X-C chemokine receptor type 2 of human expressed in CHO cells |
Bioorg Med Chem Lett 14: 4375-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.097
BindingDB Entry DOI: 10.7270/Q2SQ914M |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50101643
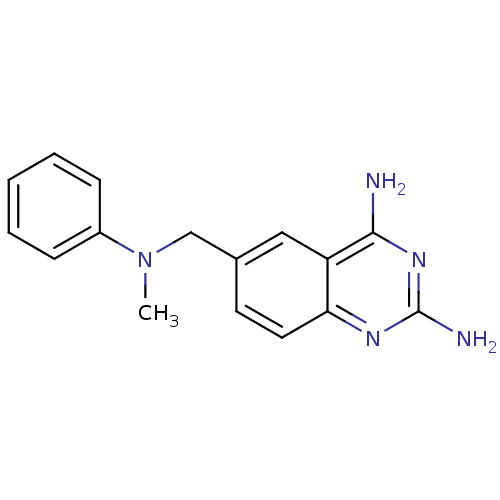
(6-[(Methyl-phenyl-amino)-methyl]-quinazoline-2,4-d...)Show InChI InChI=1S/C16H17N5/c1-21(12-5-3-2-4-6-12)10-11-7-8-14-13(9-11)15(17)20-16(18)19-14/h2-9H,10H2,1H3,(H4,17,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against dihydrofolate reductase DHFR from T. gondii |
J Med Chem 44: 2391-402 (2001)
BindingDB Entry DOI: 10.7270/Q21J9911 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data