

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50041376 ((R)-6-Ethyl-10,13-dimethyl-1,6,7,8,9,10,11,12,13,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9955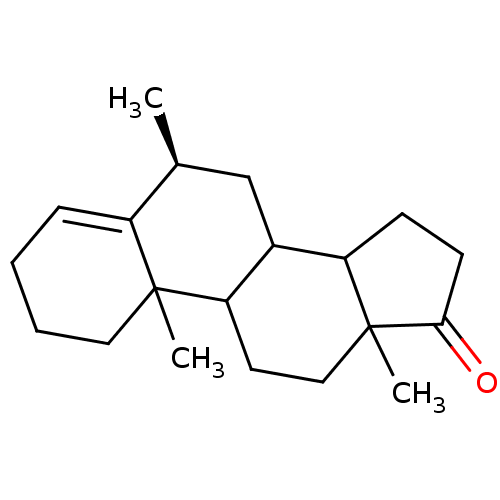 ((8S)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | -50.5 | 37 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9989 ((2S,6S,15S)-6-hydroxy-2-(hydroxymethyl)-15-methylt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40 | -50.3 | 31 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku Pharmaceutical University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 44: 4277-83 (2001) Article DOI: 10.1021/jm010282t BindingDB Entry DOI: 10.7270/Q2SB43ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041369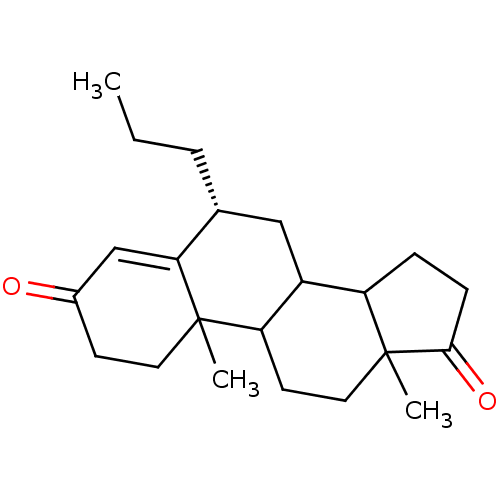 ((R)-10,13-Dimethyl-6-propyl-1,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9948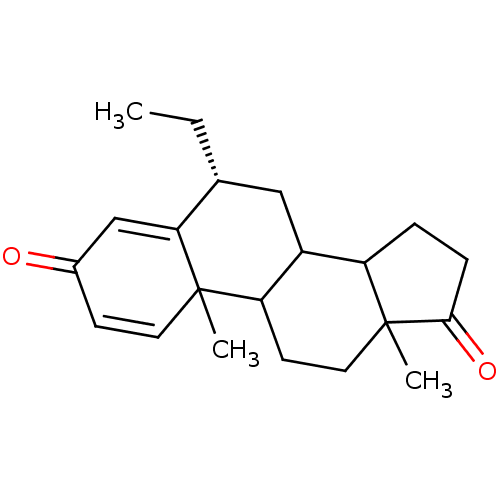 ((8R)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70 | -49.4 | 54 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041361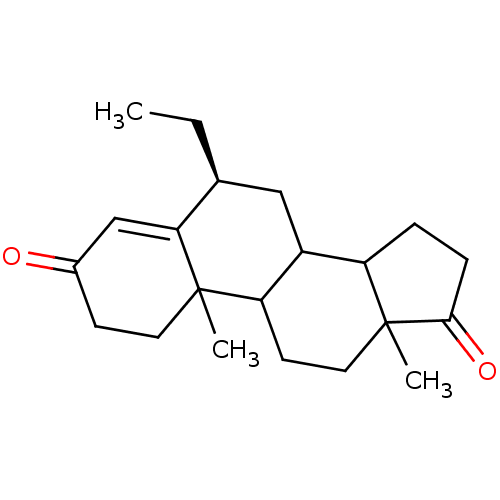 ((S)-6-Ethyl-10,13-dimethyl-1,6,7,8,9,10,11,12,13,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9951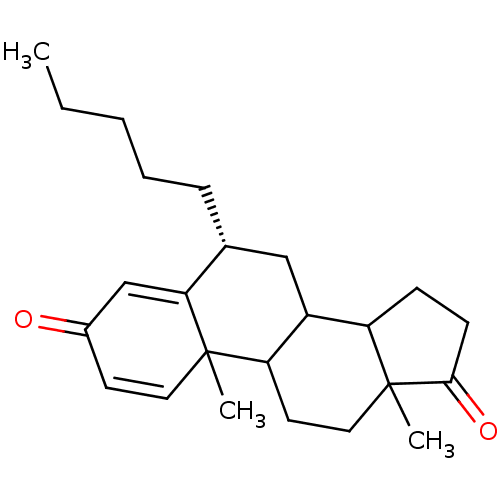 ((8R)-2,15-dimethyl-8-pentyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -49.3 | 54 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041371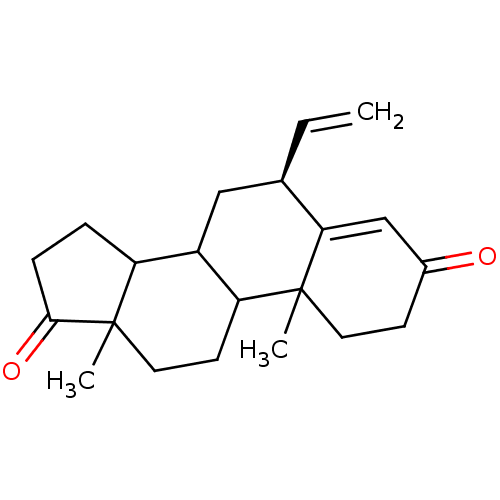 ((S)-10,13-Dimethyl-6-vinyl-1,6,7,8,9,10,11,12,13,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome aromatase Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9960 ((8R)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30 | -49.1 | 49 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041362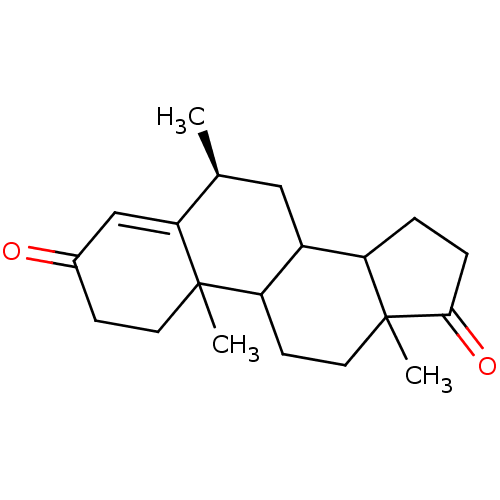 ((S)-6,10,13-Trimethyl-1,6,7,8,9,10,11,12,13,14,15,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9997 ((2S,15S)-2-(hydroxymethyl)-15-methyltetracyclo[8.7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.80 | -48.9 | 49 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku Pharmaceutical University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 44: 4277-83 (2001) Article DOI: 10.1021/jm010282t BindingDB Entry DOI: 10.7270/Q2SB43ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9994 ((2R,8R,15S)-8-hydroxy-2,15-dimethyltetracyclo[8.7....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -48.8 | 50 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku Pharmaceutical University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 44: 4277-83 (2001) Article DOI: 10.1021/jm010282t BindingDB Entry DOI: 10.7270/Q2SB43ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9968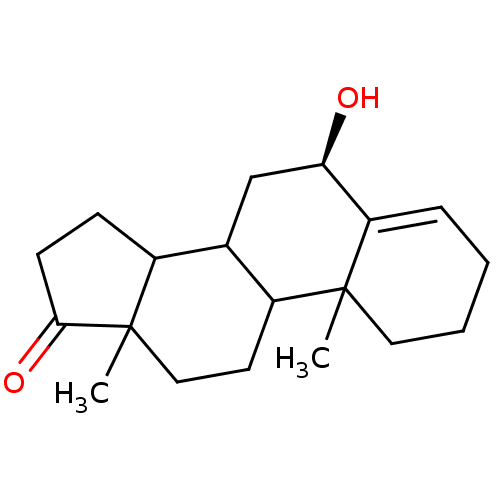 ((8R)-8-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -48.8 | 50 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041366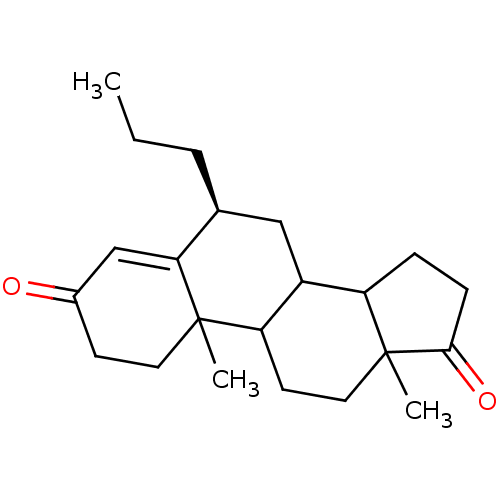 ((S)-10,13-Dimethyl-6-propyl-1,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome aromatase Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9996 ((2R,15S)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | -48.5 | 60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku Pharmaceutical University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 44: 4277-83 (2001) Article DOI: 10.1021/jm010282t BindingDB Entry DOI: 10.7270/Q2SB43ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9981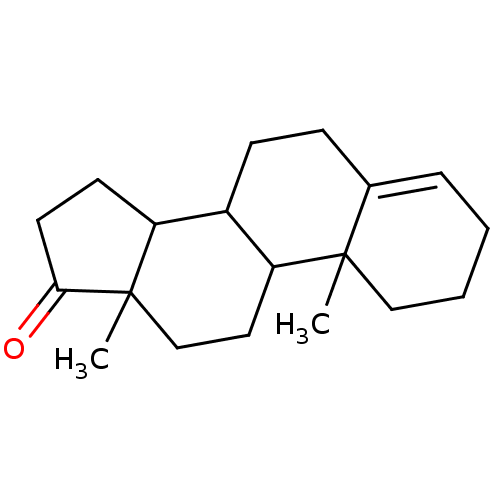 (2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9949 ((8R)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -48.4 | 60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9953 ((8R)-8-heptyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.80 | -48.1 | 60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9952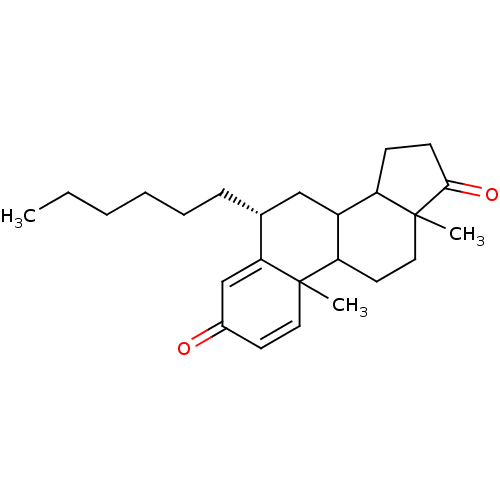 ((8R)-8-hexyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -48.1 | 76 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041365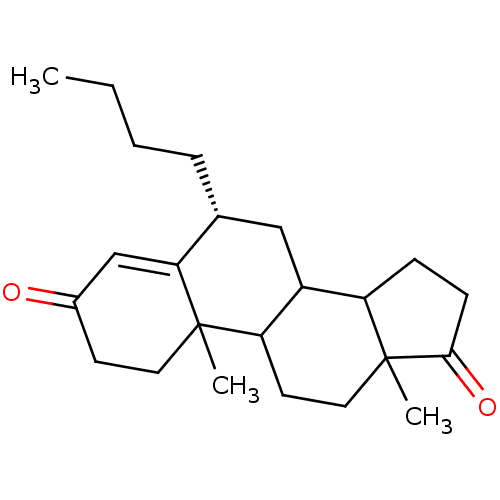 ((R)-6-Butyl-10,13-dimethyl-1,6,7,8,9,10,11,12,13,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041367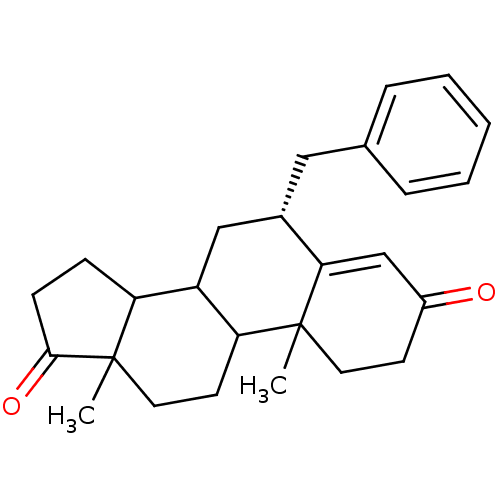 ((R)-6-Benzyl-10,13-dimethyl-1,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome aromatase Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500457 (CHEMBL3746815) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate assessed as free enzyme preincubated for 5 mins followed by su... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9944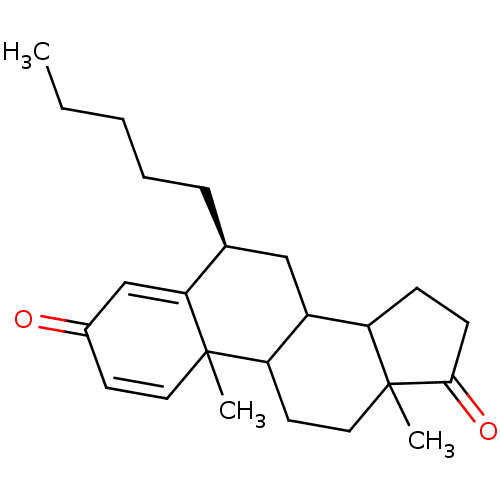 ((8S)-2,15-dimethyl-8-pentyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -47.3 | 140 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041375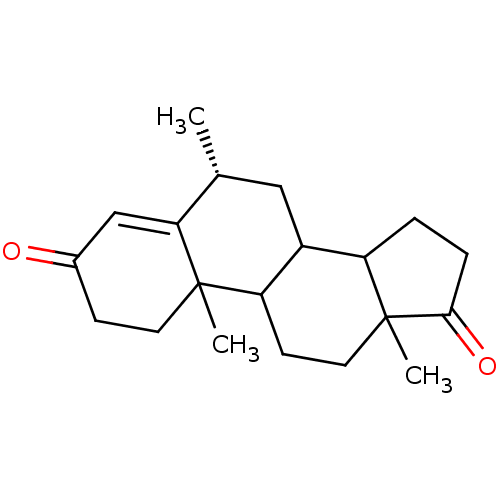 ((R)-6,10,13-Trimethyl-1,6,7,8,9,10,11,12,13,14,15,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9967 ((8S)-8-methoxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -47.0 | 120 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9970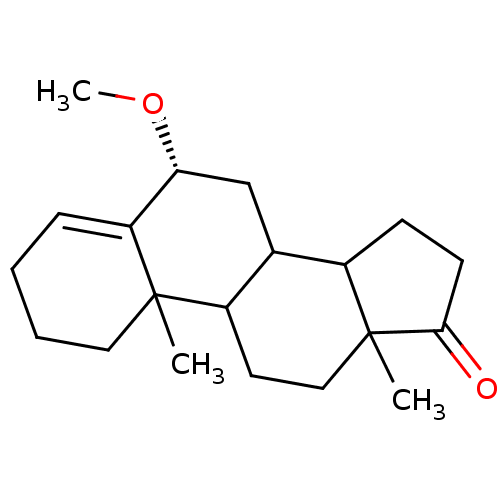 ((8R)-8-methoxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041372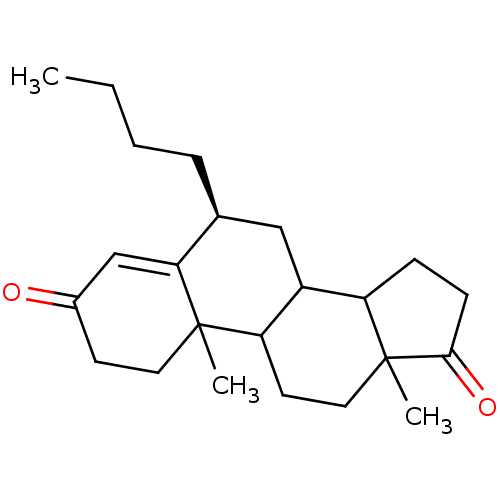 ((S)-6-Butyl-10,13-dimethyl-1,6,7,8,9,10,11,12,13,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome aromatase Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332807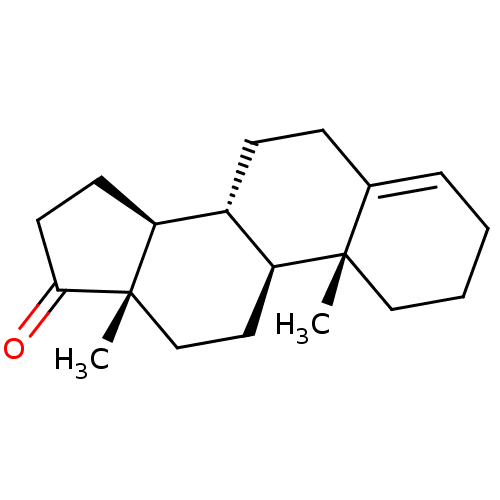 ((8R,9S,10R,13S,14S)-10,13-dimethyl-2,3,7,8,9,10,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332808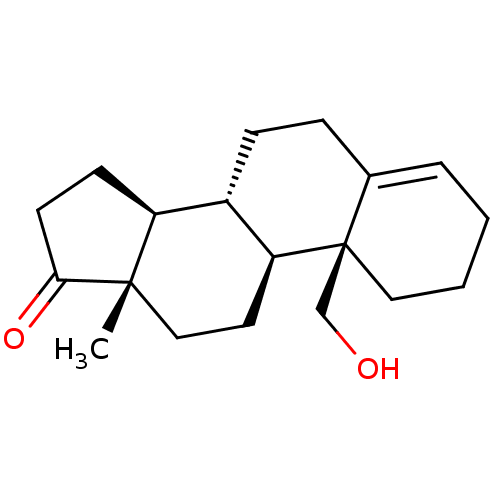 ((8R,9S,10S,13S,14S)-10-(hydroxymethyl)-13-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of 1 uM [1-beta-3H]-androstenedione binding to human placental microsome Cytochrome P450 19A1 | J Med Chem 34: 2496-504 (1991) BindingDB Entry DOI: 10.7270/Q2MG7Q45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9956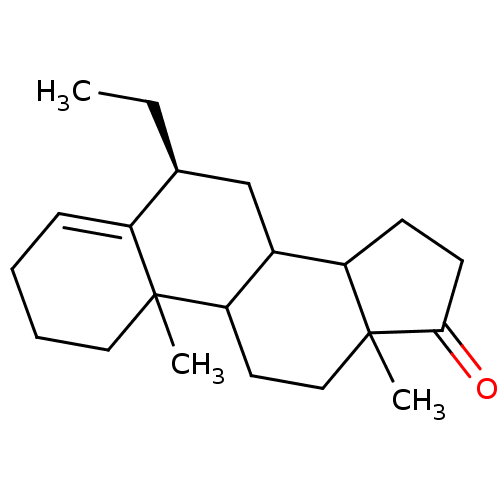 ((8S)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -46.6 | 110 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9950 ((8R)-8-butyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -46.6 | 180 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50394657 (CHEMBL270067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of reductase activity of N-terminal 6His-tagged human AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as pyridine-3-aldehyde redu... | J Nat Prod 74: 1201-6 (2011) Article DOI: 10.1021/np200118q BindingDB Entry DOI: 10.7270/Q25D8SXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9943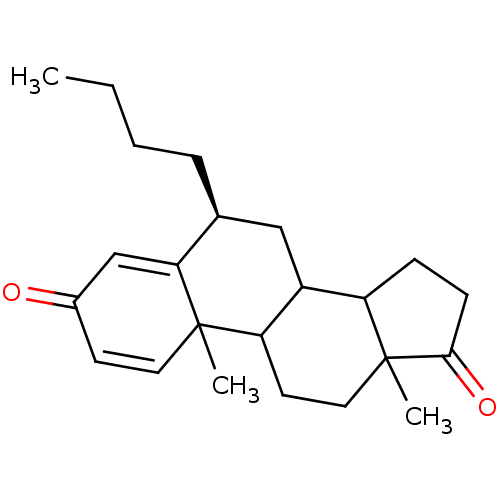 ((8S)-8-butyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | -46.3 | 200 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9966 ((8S)-2,15-dimethyl-14-oxotetracyclo[8.7.0.0^{2,7}....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | -46.0 | 160 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9941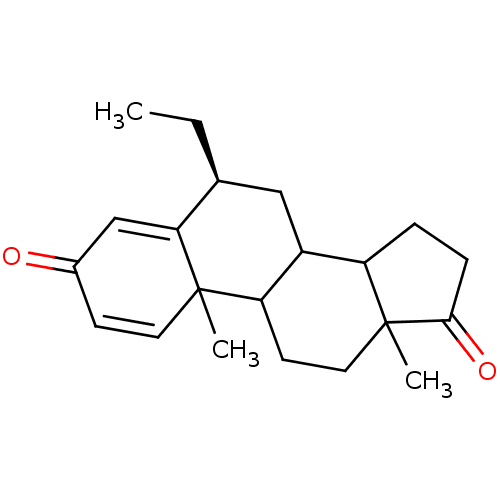 ((8S)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | -45.8 | 250 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500457 (CHEMBL3746815) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate assessed as enzyme-substrate complex preincubated for 5 mins f... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9946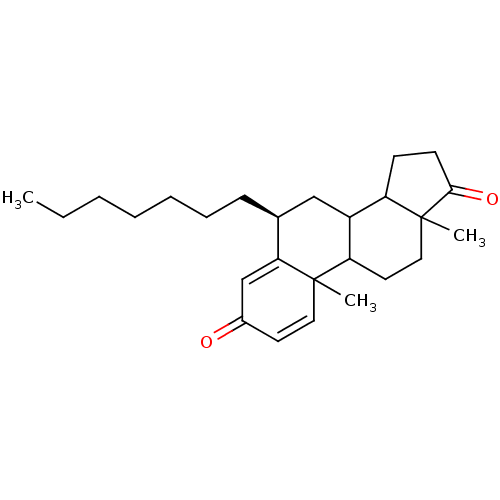 ((8S)-8-heptyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -45.7 | 250 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8592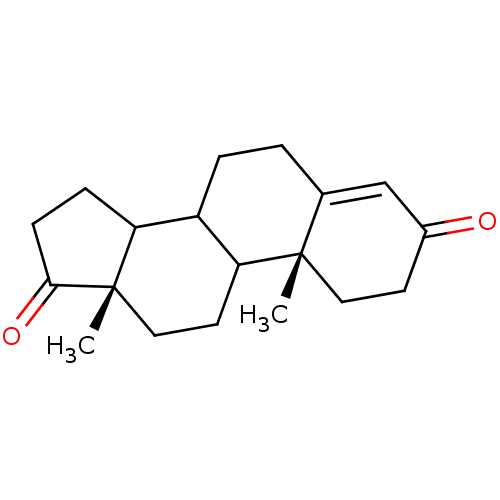 ((2R,15S)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | -45.7 | 300 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025428 (10,13-Dimethyl-1,6,7,8,9,10,11,12,13,14,15,16-dode...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for aromatase cytochrome P45019A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9965 ((8S)-8-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -45.6 | 190 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9992 ((2R,8S,15S)-8-hydroxy-2,15-dimethyltetracyclo[8.7....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -45.6 | 190 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku Pharmaceutical University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 44: 4277-83 (2001) Article DOI: 10.1021/jm010282t BindingDB Entry DOI: 10.7270/Q2SB43ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041370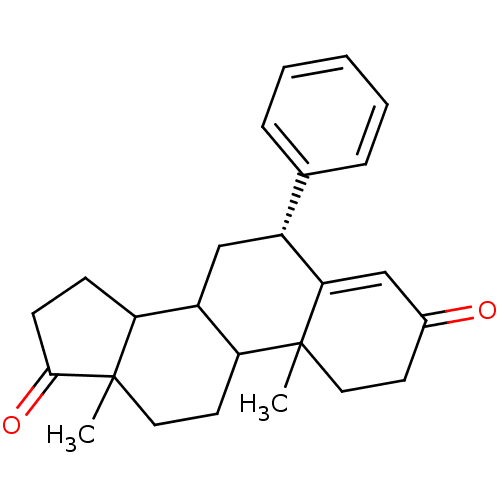 ((R)-10,13-Dimethyl-6-phenyl-1,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome aromatase Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9942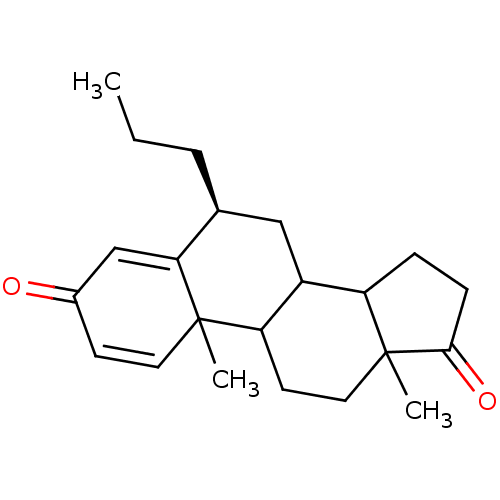 ((8S)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | -45.5 | 270 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041364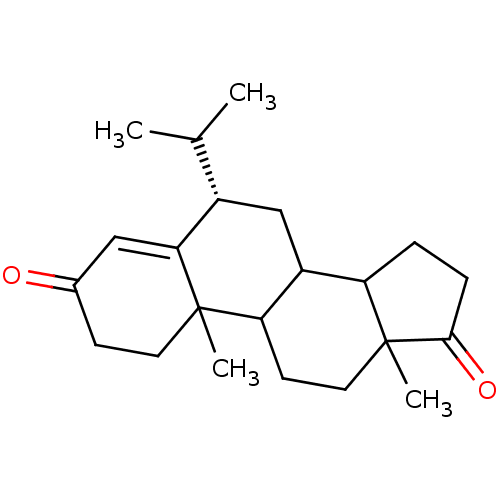 ((S)-6-Isopropyl-10,13-dimethyl-1,6,7,8,9,10,11,12,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome aromatase Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9969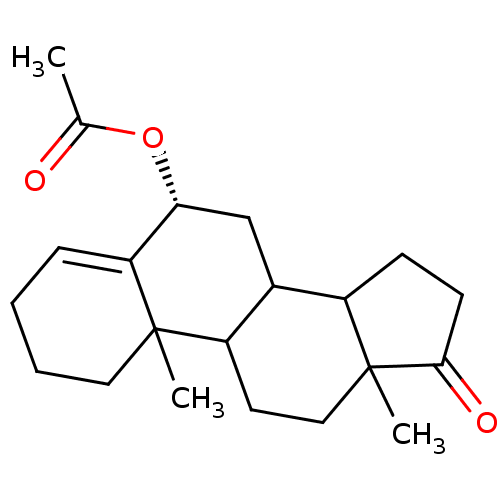 ((8R)-2,15-dimethyl-14-oxotetracyclo[8.7.0.0^{2,7}....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9987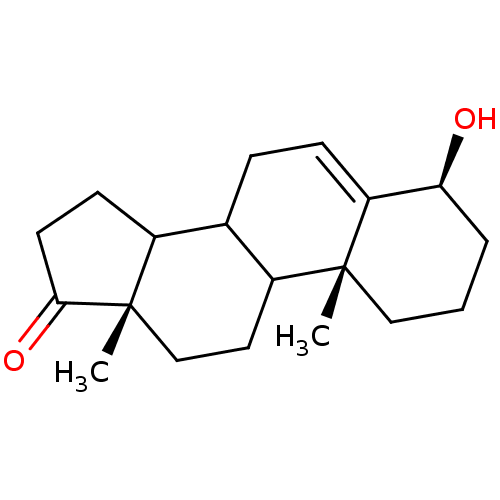 ((2R,6S,15S)-6-hydroxy-2,15-dimethyltetracyclo[8.7....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | -45.1 | 280 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku Pharmaceutical University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 44: 4277-83 (2001) Article DOI: 10.1021/jm010282t BindingDB Entry DOI: 10.7270/Q2SB43ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9945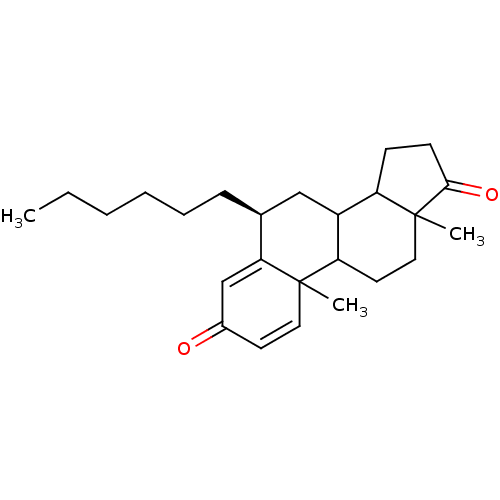 ((8S)-8-hexyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | -45.1 | 280 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50008057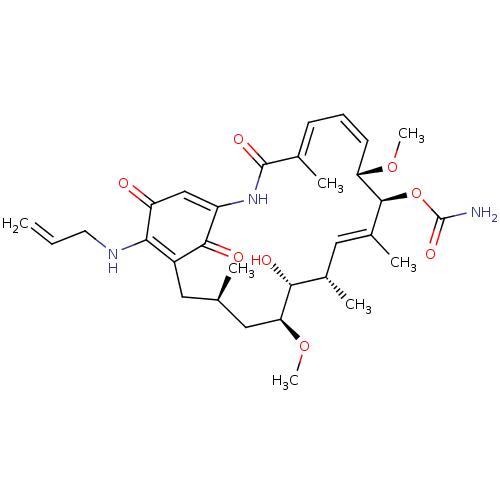 (BMS-722782 | CHEBI:64153 | TANESPIMYCIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of FITC-GA binding to Hsp90alpha (unknown origin) ATPase site after 2 hrs by fluorescence polarization assay | Bioorg Med Chem Lett 25: 1338-42 (2015) Article DOI: 10.1016/j.bmcl.2015.01.023 BindingDB Entry DOI: 10.7270/Q27M09MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9957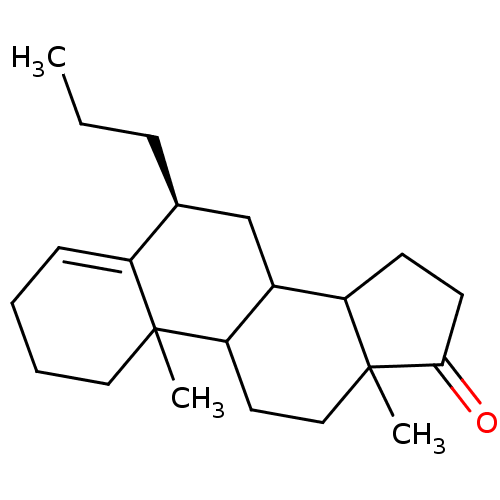 ((8S)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -44.7 | 230 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50041363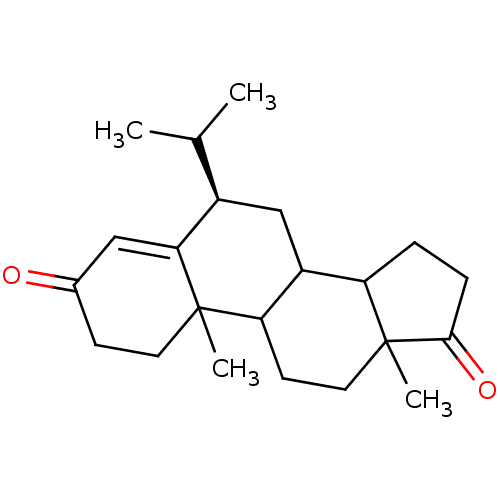 ((R)-6-Isopropyl-10,13-dimethyl-1,6,7,8,9,10,11,12,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome aromatase Cytochrome P450 19A1 | J Med Chem 37: 1312-9 (1994) BindingDB Entry DOI: 10.7270/Q2NZ86PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 454 total ) | Next | Last >> |