

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM27083 (1-(2,6-difluorophenyl)-3-{3-[5-(morpholin-4-ylmeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26503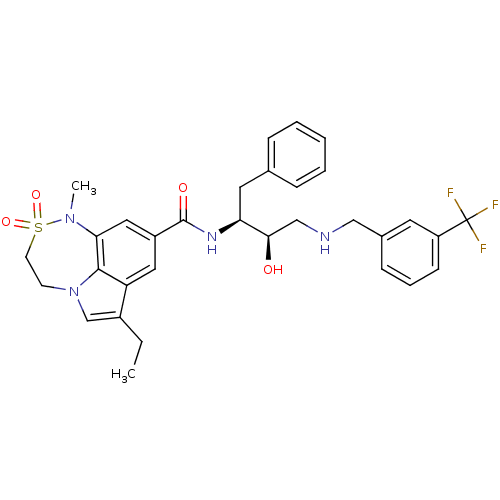 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26788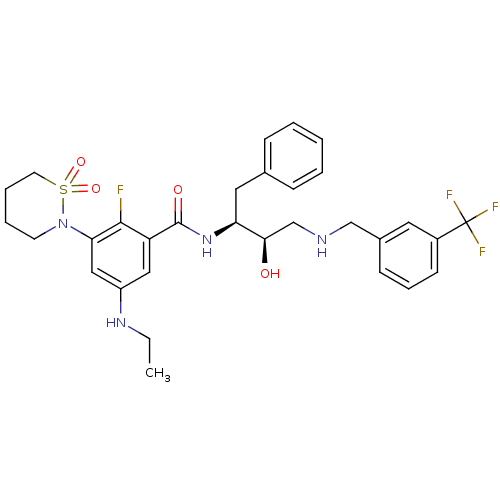 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26503 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26503 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29782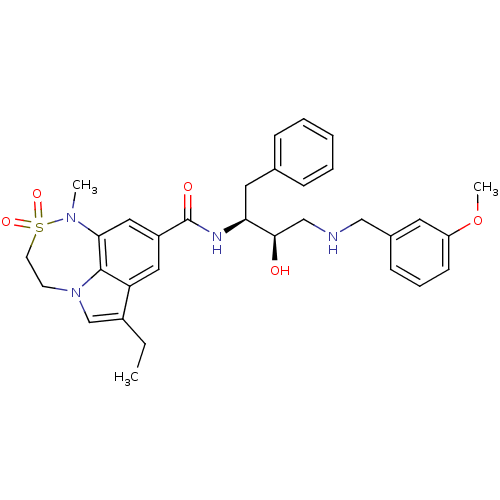 (7,6,5 tricyclic sulfonamide, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM27083 (1-(2,6-difluorophenyl)-3-{3-[5-(morpholin-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM27082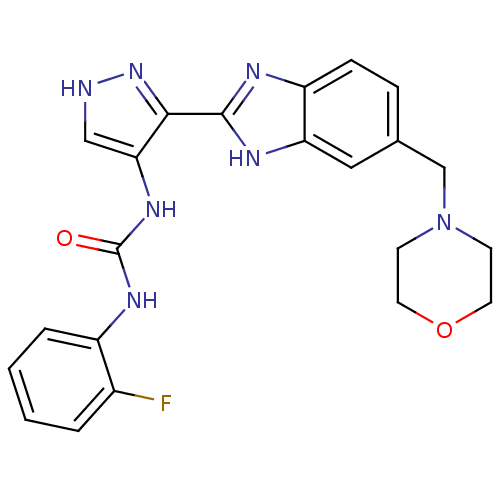 (1-(2-fluorophenyl)-3-{3-[5-(morpholin-4-ylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM24644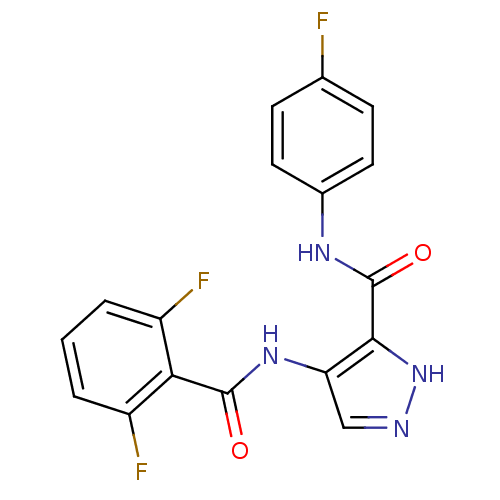 (4-N-(2,6-difluorobenzene)-3-N-(4-fluorophenyl)-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Astex | Assay Description CDK2/cyclin A activity was determined using a radiometric assay to measure the incorporation of gamma-phosphate from [gamma-33P]-ATP into histone H1.... | J Med Chem 51: 4986-99 (2008) Article DOI: 10.1021/jm800382h BindingDB Entry DOI: 10.7270/Q24X563W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM27079 (N-{3-[5-(morpholin-4-ylmethyl)-1H-1,3-benzodiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29796 (sulfonamide tricyclic analogue, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26786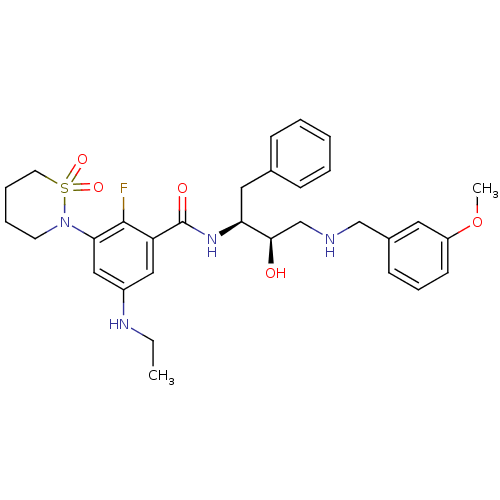 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26788 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29815 (sulfonamide tricyclic analogue, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26774 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(2S,3R)-3-hydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26776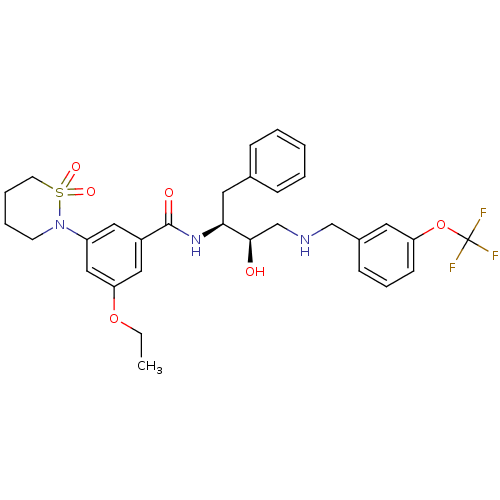 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-ethoxy-N-[(2S,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26777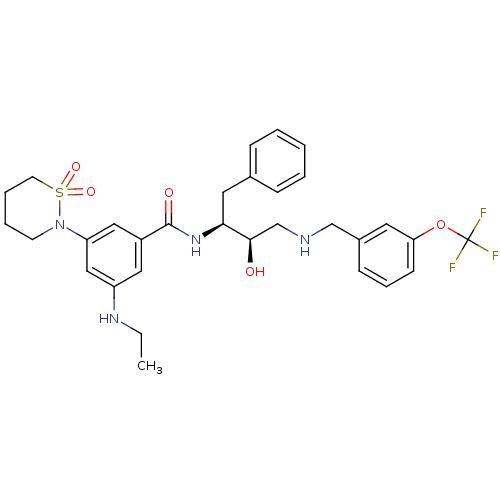 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26782 (N-[(2S,3R)-1-(3,5-difluorophenyl)-3-hydroxy-4-{[(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26506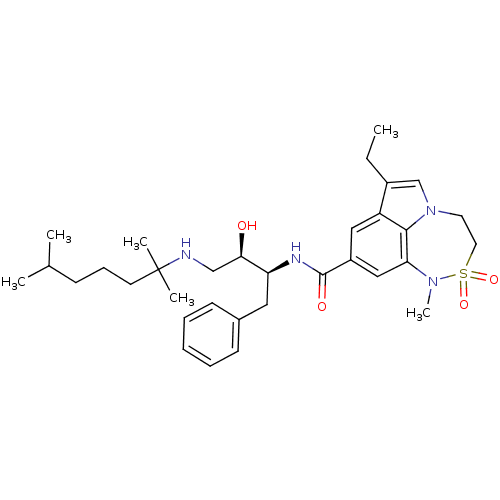 (BMCL193669 Compound 24 | N-[(2S,3R)-4-[(2,6-dimeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29789 (7,6,5 tricyclic sulfonamide, 35) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26506 (BMCL193669 Compound 24 | N-[(2S,3R)-4-[(2,6-dimeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29794 (sulfonamide tricyclic analogue, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM27084 (1-cyclohexyl-3-{3-[5-(morpholin-4-ylmethyl)-1H-1,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM27084 (1-cyclohexyl-3-{3-[5-(morpholin-4-ylmethyl)-1H-1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM27078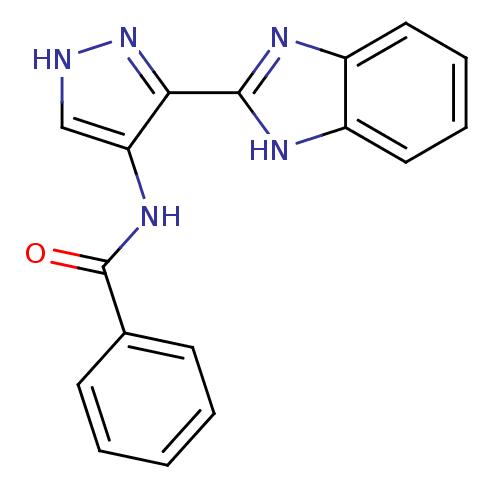 (N-[3-(1H-1,3-benzodiazol-2-yl)-1H-pyrazol-4-yl]ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26773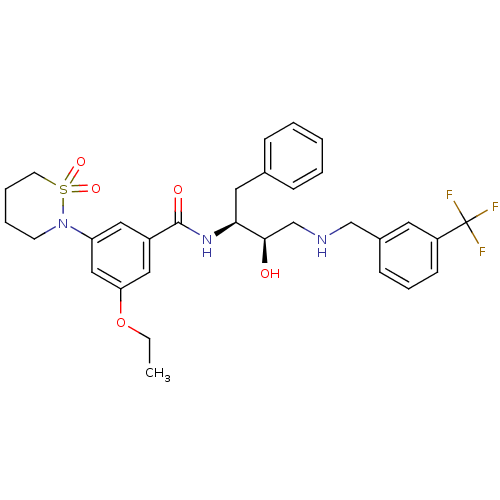 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-ethoxy-N-[(2S,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29770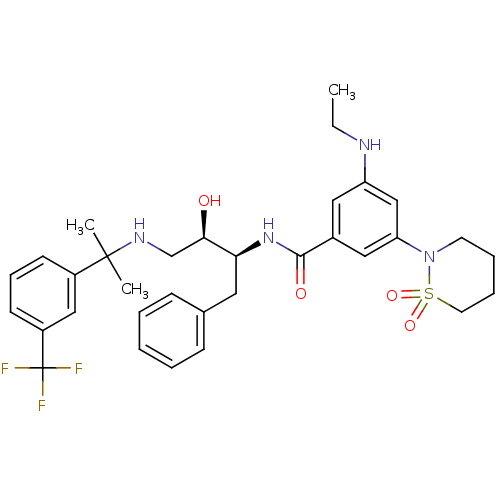 (hydroxyethylamine derivative, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26787 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(2S,3R)-4-{[(1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29777 (7,6,5 tricyclic sulfonamide, 15 | BMCL193674 Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29777 (7,6,5 tricyclic sulfonamide, 15 | BMCL193674 Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29792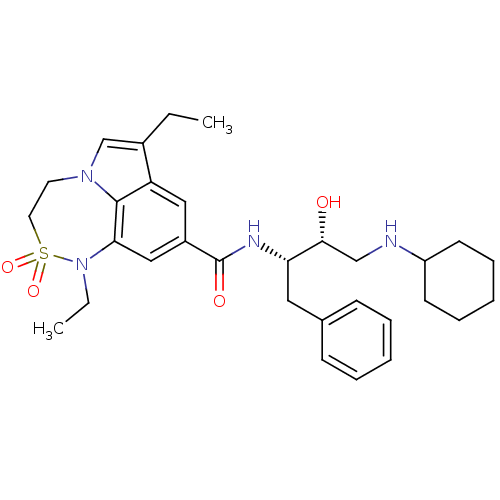 (7,6,5 tricyclic sulfonamide, 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29805 (sulfone tricyclic analogue, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29781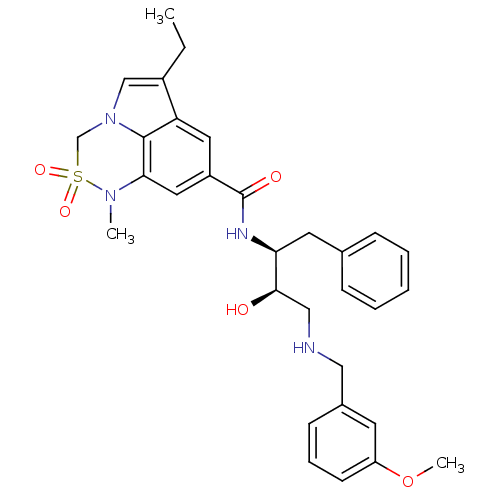 (6,6,5 tricyclic sulfonamide, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26781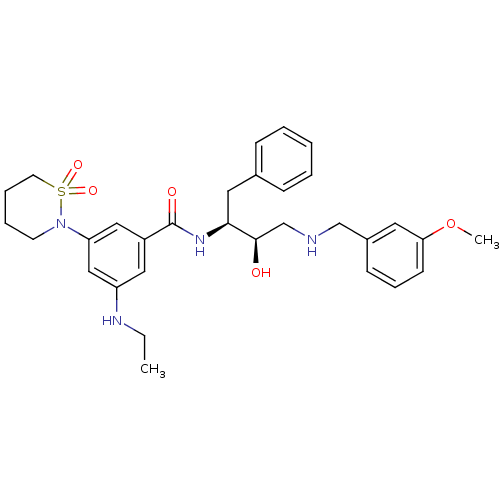 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29809 (sulfonamide tricyclic analogue, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29804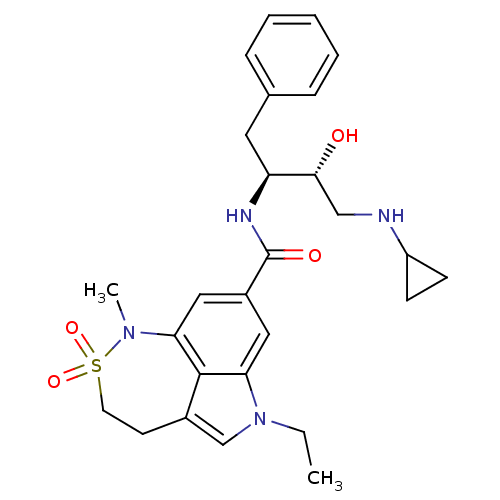 (sulfonamide tricyclic analogue, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26508 (BMCL193669 Compound 26 | N-[(2S,3R)-4-(cyclohexyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26508 (BMCL193669 Compound 26 | N-[(2S,3R)-4-(cyclohexyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29755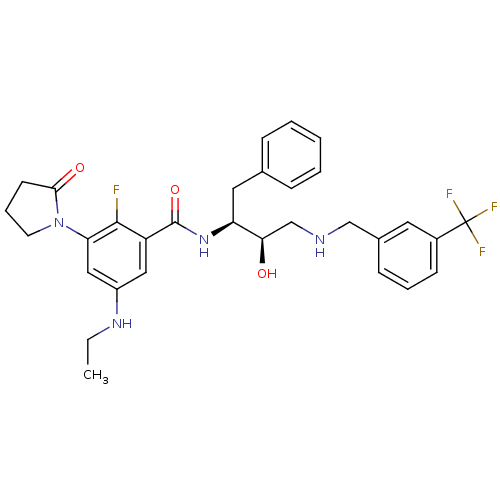 (hydroxyethylamine derivative, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3118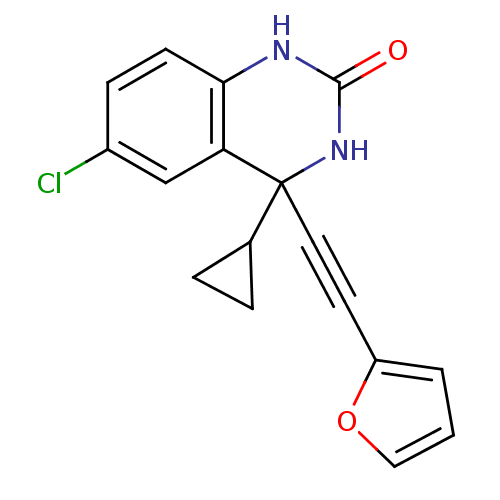 (3,4-Dihydroquinazolinon 4l | 6-chloro-4-cyclopropy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 37: 2437-44 (1994) Article DOI: 10.1021/jm00041a023 BindingDB Entry DOI: 10.7270/Q2416V7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2223 (2-Pyridinone derivative 21 | 3-[2-(4,7-dichloro-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Sharp and Dohme Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2922-5 (1991) Article DOI: 10.1021/jm00113a036 BindingDB Entry DOI: 10.7270/Q2WM1BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM10145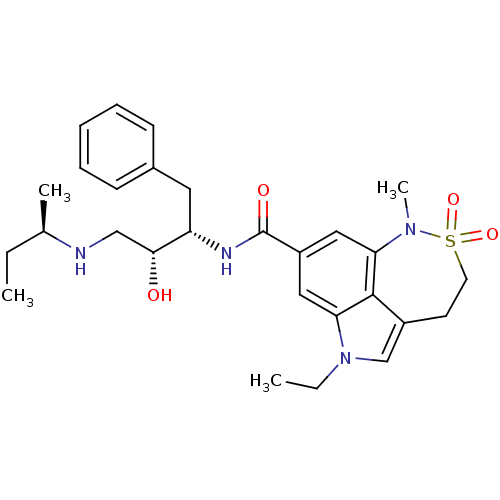 (lysine sulfonamide analogue 9 | methyl N-[(1S)-1-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26780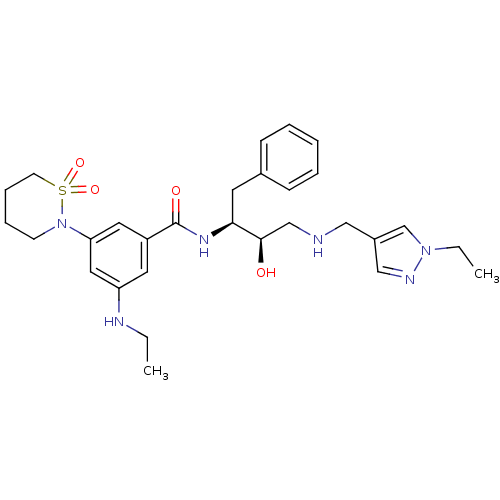 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(2S,3R)-4-{[(1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM27086 (3-{3-[5-(morpholin-4-ylmethyl)-1H-1,3-benzodiazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 449 total ) | Next | Last >> |