

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acyl-CoA (8-3)-desaturase (Homo sapiens (Human)) | BDBM50245999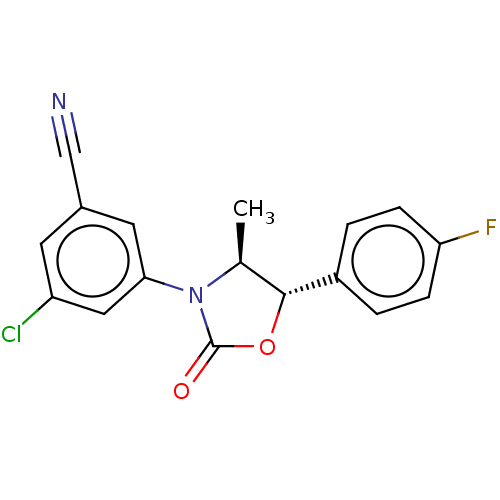 (CHEMBL4084502) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of D5D in human HepG2 cells assessed as [14C]AA formation from [14C]DGLA preincubated for 30 mins followed by [14C]eicosatrienoic acid add... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50245999 (CHEMBL4084502) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Homo sapiens (Human)) | BDBM50245998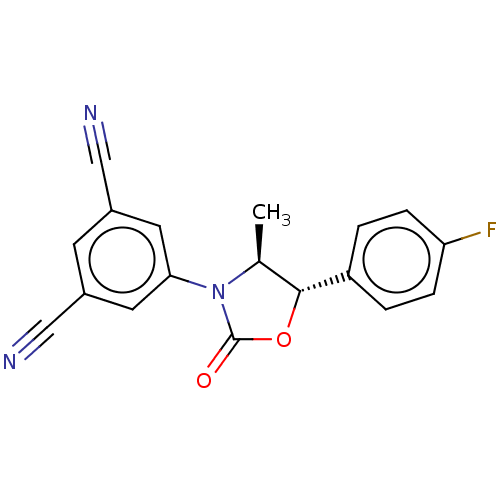 (CHEMBL4064555) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of D5D in human HepG2 cells assessed as [14C]AA formation from [14C]DGLA preincubated for 30 mins followed by [14C]eicosatrienoic acid add... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50245998 (CHEMBL4064555) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Mus musculus) | BDBM50545129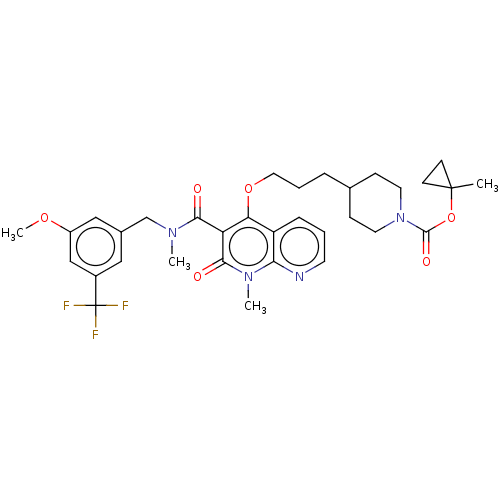 (CHEMBL4645607) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant C-terminal FLAG-tagged SMS2 expressed in mammalian expression system using C14-phosphatidylcholineD72 and C17-ceramid... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Mus musculus) | BDBM50545128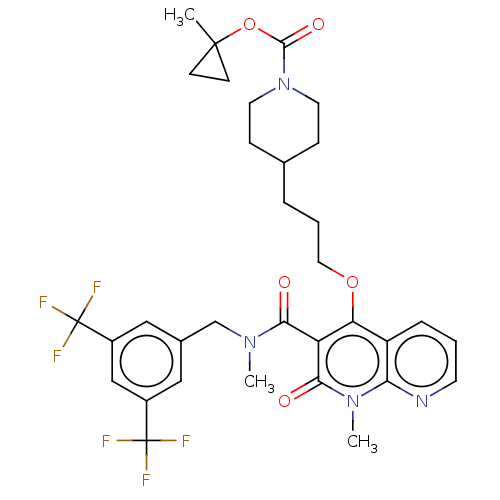 (CHEMBL4641888) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant C-terminal FLAG-tagged SMS2 expressed in mammalian expression system using C14-phosphatidylcholineD72 and C17-ceramid... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Homo sapiens (Human)) | BDBM50246049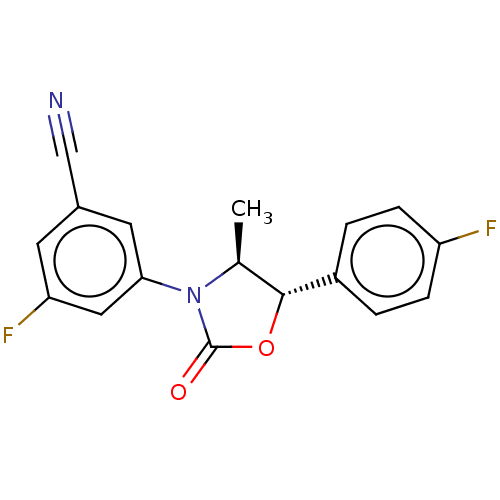 (CHEMBL4102458) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of D5D in human HepG2 cells assessed as [14C]AA formation from [14C]DGLA preincubated for 30 mins followed by [14C]eicosatrienoic acid add... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246049 (CHEMBL4102458) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Homo sapiens (Human)) | BDBM50246023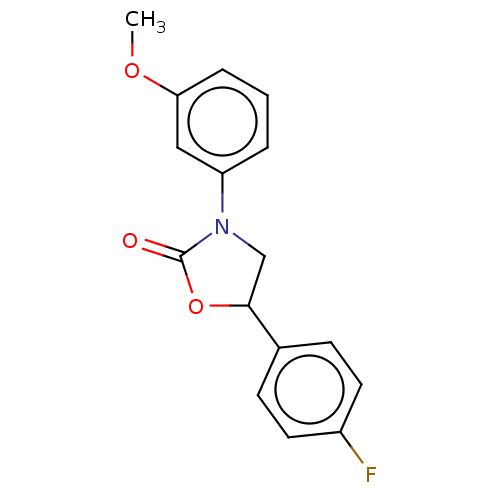 (CHEMBL4060775) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of D5D in human HepG2 cells assessed as [14C]AA formation from [14C]DGLA preincubated for 30 mins followed by [14C]eicosatrienoic acid add... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246000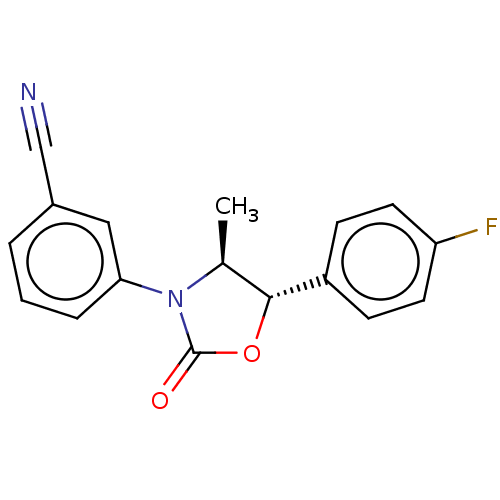 (CHEMBL4070383) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246000 (CHEMBL4070383) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50282224 (CHEMBL4172856) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase obtained from human | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Mus musculus) | BDBM50545122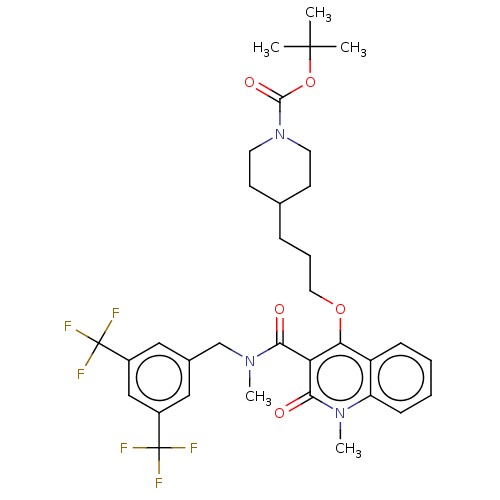 (CHEMBL4646354) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant C-terminal FLAG-tagged SMS2 expressed in mammalian expression system using C14-phosphatidylcholineD72 and C17-ceramid... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246023 (CHEMBL4060775) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Homo sapiens (Human)) | BDBM50246000 (CHEMBL4070383) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of D5D in human HepG2 cells assessed as [14C]AA formation from [14C]DGLA preincubated for 30 mins followed by [14C]eicosatrienoic acid add... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Homo sapiens (Human)) | BDBM50246000 (CHEMBL4070383) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of D5D in human HepG2 cells assessed as [14C]AA formation from [14C]DGLA preincubated for 30 mins followed by [14C]eicosatrienoic acid add... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246076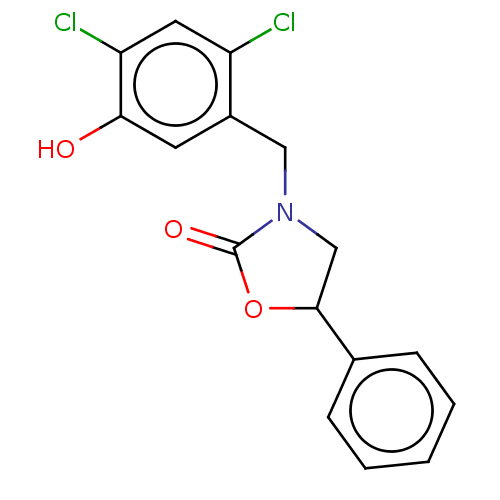 (CHEMBL4063561) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246045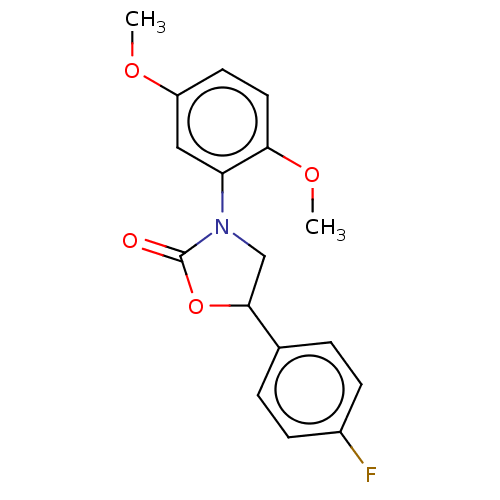 (CHEMBL4090490) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Mus musculus) | BDBM50545127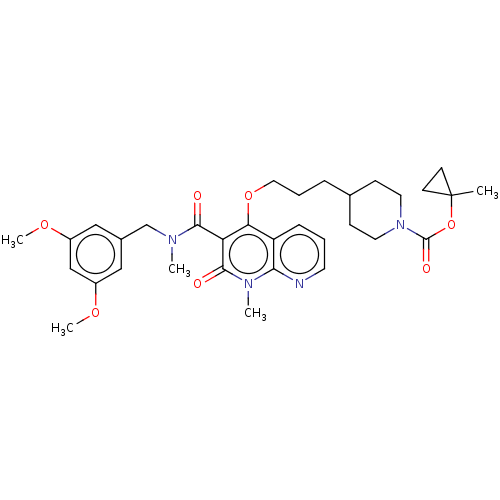 (CHEMBL4644293) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant C-terminal FLAG-tagged SMS2 expressed in mammalian expression system using C14-phosphatidylcholineD72 and C17-ceramid... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50282208 (CHEMBL4177314) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Folyl-polyglutamate synthase obtained from porcine | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM651205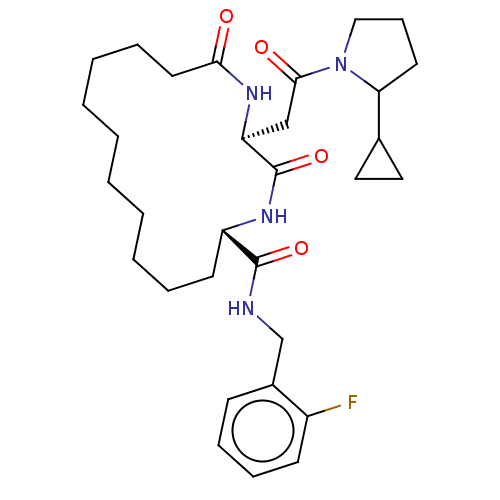 (US20240043470, Compound 4-13) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Homo sapiens (Human)) | BDBM50246045 (CHEMBL4090490) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of D5D in human HepG2 cells assessed as [14C]AA formation from [14C]DGLA preincubated for 30 mins followed by [14C]eicosatrienoic acid add... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Mus musculus) | BDBM50545123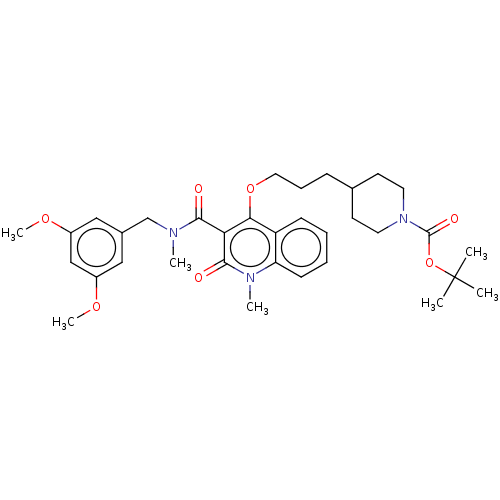 (CHEMBL4638337) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant C-terminal FLAG-tagged SMS2 expressed in mammalian expression system using C14-phosphatidylcholineD72 and C17-ceramid... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246007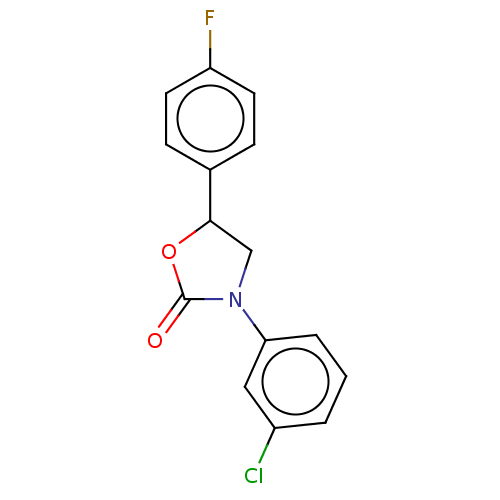 (CHEMBL4104260) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50530609 (CHEMBL4461010) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medicine Curated by ChEMBL | Assay Description Inhibition of human 20S proteasome subunit beta-5i using Ac-ANW-AMC as substrate and measured every 30 secs for 30 mins by microtiter plate assay | J Med Chem 62: 9246-9253 (2019) Article DOI: 10.1021/acs.jmedchem.9b01187 BindingDB Entry DOI: 10.7270/Q25M695M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50530609 (CHEMBL4461010) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medicine Curated by ChEMBL | Assay Description Inhibition of human 20S proteasome subunit beta-5i using Ac-ANW-AMC as substrate and measured every 30 secs for 30 mins by microtiter plate assay | J Med Chem 62: 9246-9253 (2019) Article DOI: 10.1021/acs.jmedchem.9b01187 BindingDB Entry DOI: 10.7270/Q25M695M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50545122 (CHEMBL4646354) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal FLAG-tagged SMS2 expressed in Freestyle293 cell membrane using C14-phosphatidylcholineD72 and C17-ceramide... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Mus musculus) | BDBM50545126 (CHEMBL4646540) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant C-terminal FLAG-tagged SMS2 expressed in mammalian expression system using C14-phosphatidylcholineD72 and C17-ceramid... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50245997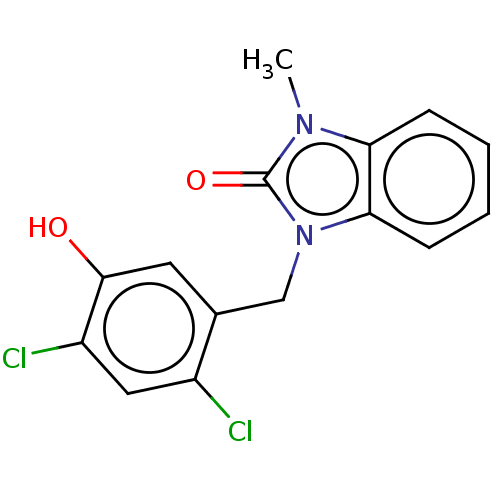 (CHEMBL4072166) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50545129 (CHEMBL4645607) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal FLAG-tagged SMS2 expressed in Freestyle293 cell membrane using C14-phosphatidylcholineD72 and C17-ceramide... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50545128 (CHEMBL4641888) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal FLAG-tagged SMS2 expressed in Freestyle293 cell membrane using C14-phosphatidylcholineD72 and C17-ceramide... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246047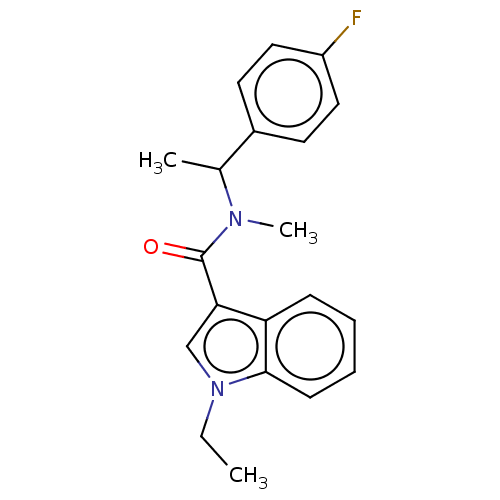 (CHEMBL4097127) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246065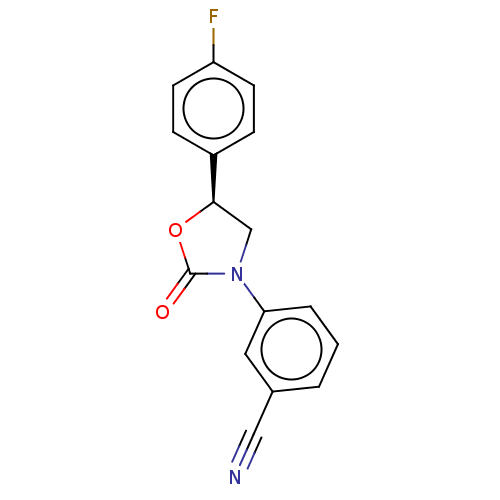 (CHEMBL4066595) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246022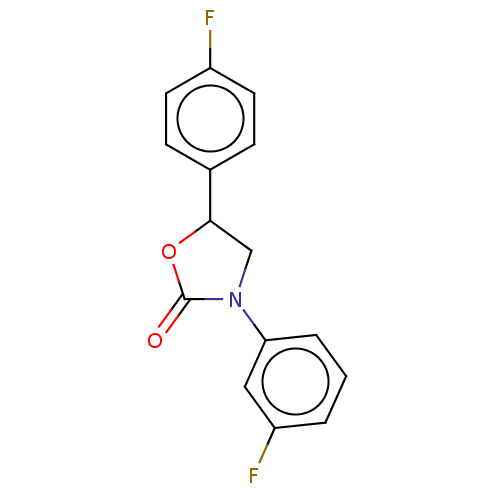 (CHEMBL4080025) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Mus musculus) | BDBM50545124 (CHEMBL4639746) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant C-terminal FLAG-tagged SMS2 expressed in mammalian expression system using C14-phosphatidylcholineD72 and C17-ceramid... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Homo sapiens (Human)) | BDBM50246007 (CHEMBL4104260) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of D5D in human HepG2 cells assessed as [14C]AA formation from [14C]DGLA preincubated for 30 mins followed by [14C]eicosatrienoic acid add... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Homo sapiens (Human)) | BDBM50246065 (CHEMBL4066595) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of D5D in human HepG2 cells assessed as [14C]AA formation from [14C]DGLA preincubated for 30 mins followed by [14C]eicosatrienoic acid add... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM651191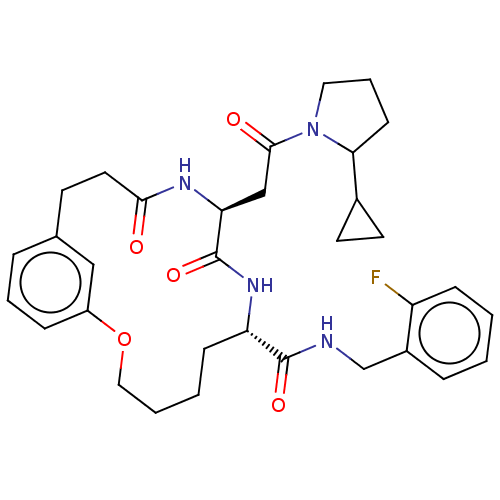 (US20240043470, Compound 3-46-A | US20240043470, Co...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50587109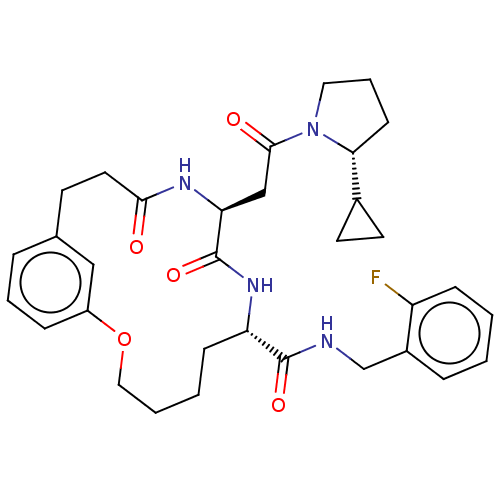 (CHEMBL5079787) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta-5i subunit using Suc-LLVY-AMC as substrate measured over 1.5 to 2 hrs by plate reader assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00296 BindingDB Entry DOI: 10.7270/Q2MG7TFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM651191 (US20240043470, Compound 3-46-A | US20240043470, Co...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 72.3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50587109 (CHEMBL5079787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta-5c subunit using Suc-LLVY-AMC as substrate measured over 1.5 to 2 hrs by plate reader assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00296 BindingDB Entry DOI: 10.7270/Q2MG7TFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246006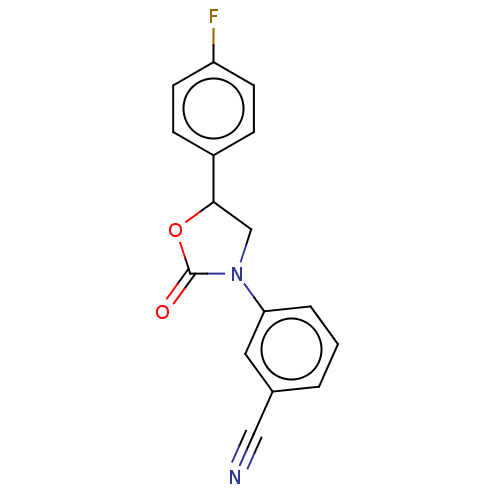 (CHEMBL4087912) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Rattus norvegicus) | BDBM50246046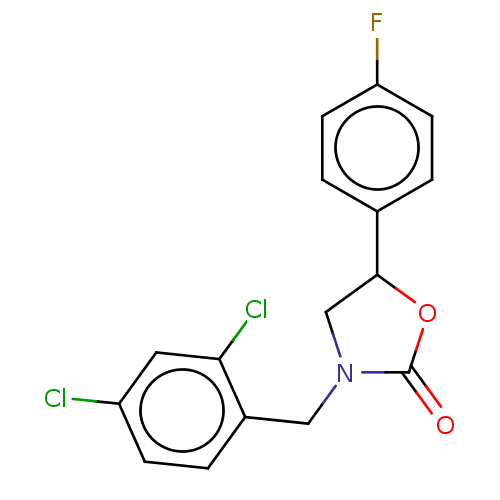 (CHEMBL4069465) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]T-3364366 from D5D in rat liver microsomal membrane preincubated for 15 mins followed by radioligand addition measured after 150 ... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50545123 (CHEMBL4638337) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal FLAG-tagged SMS2 expressed in Freestyle293 cell membrane using C14-phosphatidylcholineD72 and C17-ceramide... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM651209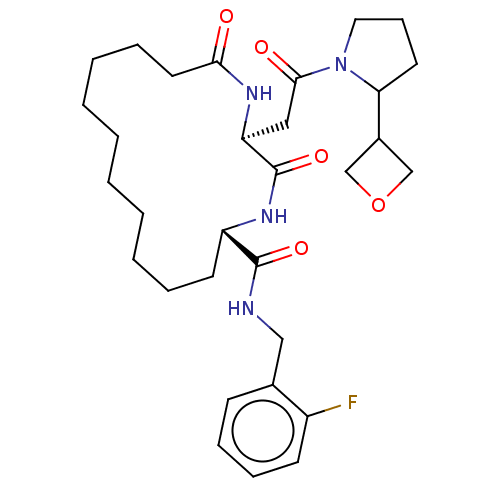 (US20240043470, Compound 4-17) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Mus musculus) | BDBM50545120 (CHEMBL4634697) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant C-terminal FLAG-tagged SMS2 expressed in mammalian expression system using C14-phosphatidylcholineD72 and C17-ceramid... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50545119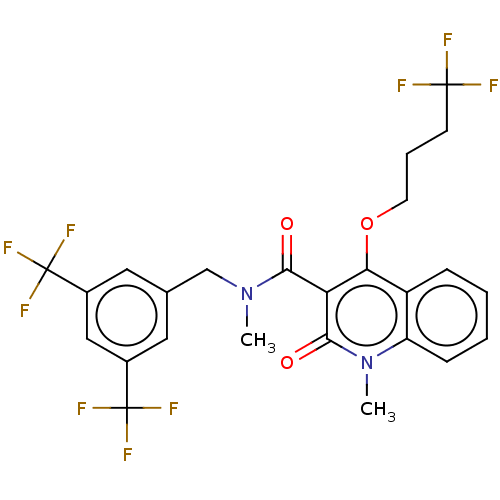 (CHEMBL4634669) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal FLAG-tagged SMS2 expressed in Freestyle293 cell membrane using C14-phosphatidylcholineD72 and C17-ceramide... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50545126 (CHEMBL4646540) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal FLAG-tagged SMS2 expressed in Freestyle293 cell membrane using C14-phosphatidylcholineD72 and C17-ceramide... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115376 BindingDB Entry DOI: 10.7270/Q2J106QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM651113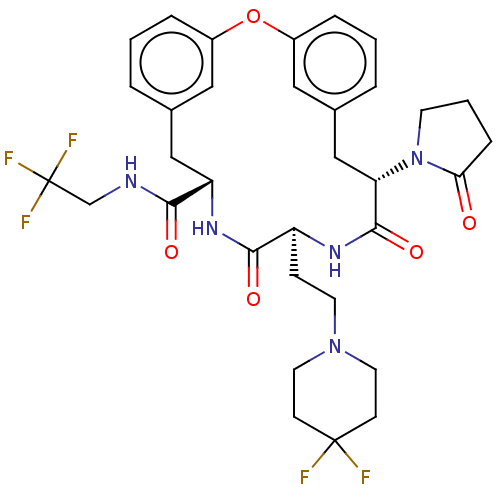 (US20240043470, Compound 1-66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA (8-3)-desaturase (Homo sapiens (Human)) | BDBM50246046 (CHEMBL4069465) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of D5D in human HepG2 cells assessed as [14C]AA formation from [14C]DGLA preincubated for 30 mins followed by [14C]eicosatrienoic acid add... | J Med Chem 60: 8963-8981 (2017) Article DOI: 10.1021/acs.jmedchem.7b01210 BindingDB Entry DOI: 10.7270/Q23N25S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 548 total ) | Next | Last >> |