 Found 217 hits with Last Name = 'oliaro-bosso' and Initial = 's'
Found 217 hits with Last Name = 'oliaro-bosso' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50255431
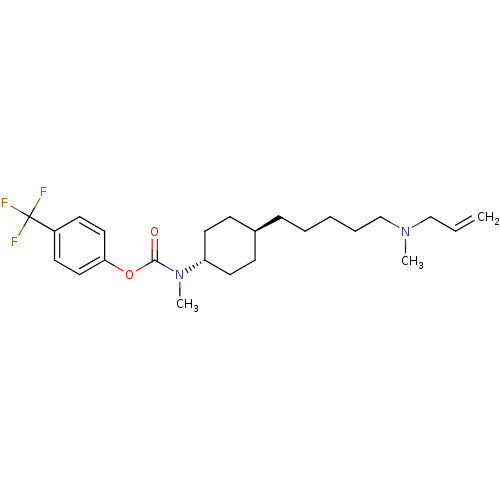
(4-(trifluoromethyl)phenyl 4-(5-(allyl(methyl)amino...)Show SMILES CN(CCCCC[C@H]1CC[C@@H](CC1)N(C)C(=O)Oc1ccc(cc1)C(F)(F)F)CC=C |r,wU:10.13,wD:7.6,(-5.96,-29.96,;-5.96,-31.5,;-4.63,-32.27,;-3.29,-31.5,;-1.96,-32.27,;-.63,-31.5,;.71,-32.27,;2.04,-31.5,;3.36,-32.27,;4.69,-31.51,;4.7,-29.97,;3.37,-29.19,;2.03,-29.96,;6.04,-29.2,;6.04,-27.66,;7.37,-29.98,;7.36,-31.52,;8.71,-29.21,;10.04,-29.99,;10,-31.53,;11.32,-32.32,;12.67,-31.58,;12.7,-30.03,;11.38,-29.24,;13.99,-32.38,;15.32,-33.15,;13.2,-33.7,;14.77,-31.05,;-7.3,-32.27,;-8.63,-31.5,;-9.96,-32.27,)| Show InChI InChI=1S/C24H35F3N2O2/c1-4-17-28(2)18-7-5-6-8-19-9-13-21(14-10-19)29(3)23(30)31-22-15-11-20(12-16-22)24(25,26)27/h4,11-12,15-16,19,21H,1,5-10,13-14,17-18H2,2-3H3/t19-,21- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50593724
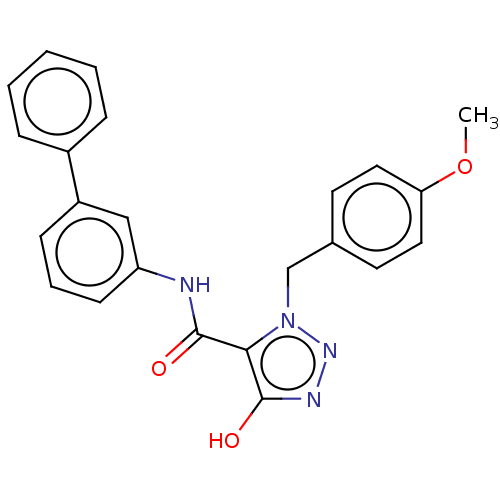
(CHEMBL5194388)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)-c2ccccc2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114366
BindingDB Entry DOI: 10.7270/Q2TB1BWP |
More data for this
Ligand-Target Pair | |
Cycloartenol synthase
(Arabidopsis thaliana) | BDBM50255432

(CHEMBL516921 | N-allyl-4-(3-(4-bromophenyl)benzo[d...)Show InChI InChI=1S/C21H23BrN2OS/c1-3-12-24(2)13-4-5-14-25-18-10-11-19-20(15-18)26-23-21(19)16-6-8-17(22)9-7-16/h3,6-11,15H,1,4-5,12-14H2,2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50128063

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[d]isothiazol-6-...)Show SMILES CN(CCCCCCOc1ccc2c(nsc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C23H27BrN2OS/c1-3-14-26(2)15-6-4-5-7-16-27-20-12-13-21-22(17-20)28-25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50255474
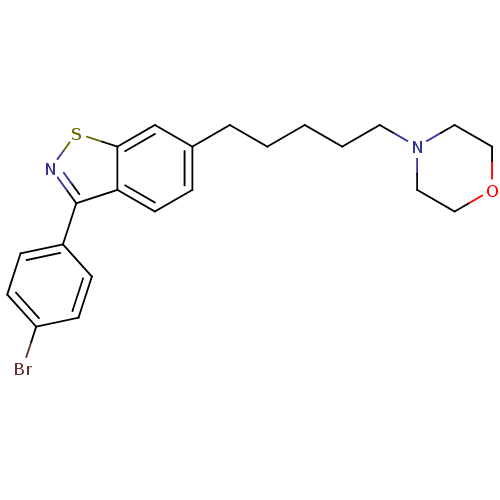
(4-(5-(3-(4-bromophenyl)benzo[d]isothiazol-6-yl)pen...)Show InChI InChI=1S/C22H25BrN2OS/c23-19-8-6-18(7-9-19)22-20-10-5-17(16-21(20)27-24-22)4-2-1-3-11-25-12-14-26-15-13-25/h5-10,16H,1-4,11-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of human OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Antagonistic activity against TP-receptor by inhibition of U 46619-induced contraction of isolated guinea pig trachea |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Turin
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 assessed as reduction in PGF2alpha production by ELISA |
ACS Med Chem Lett 10: 437-443 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00484
BindingDB Entry DOI: 10.7270/Q2HT2SPS |
More data for this
Ligand-Target Pair | |
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50255531

(4-chlorophenyl 4-(5-(ethyl(2-hydroxyethyl)amino)pe...)Show SMILES CCN(CCO)CCCCC[C@H]1CC[C@@H](CC1)N(C)C(=O)Oc1ccc(Cl)cc1 |r,wU:14.17,wD:11.10,(19.37,-12.82,;18.05,-13.6,;18.08,-15.14,;16.75,-15.92,;15.41,-15.17,;14.08,-15.96,;19.42,-15.89,;20.74,-15.09,;22.1,-15.85,;23.42,-15.06,;24.77,-15.83,;26.11,-15.04,;27.44,-15.8,;28.77,-15.03,;28.77,-13.49,;27.44,-12.73,;26.11,-13.5,;30.11,-12.71,;30.1,-11.17,;31.45,-13.48,;31.47,-15.01,;32.78,-12.71,;32.78,-11.17,;34.11,-10.4,;34.12,-8.85,;32.78,-8.09,;32.78,-6.55,;31.46,-8.86,;31.46,-10.4,)| Show InChI InChI=1S/C23H37ClN2O3/c1-3-26(17-18-27)16-6-4-5-7-19-8-12-21(13-9-19)25(2)23(28)29-22-14-10-20(24)11-15-22/h10-11,14-15,19,21,27H,3-9,12-13,16-18H2,1-2H3/t19-,21- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50255532
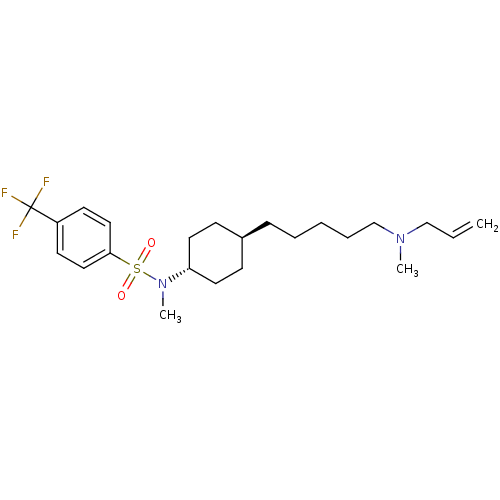
(CHEMBL481199 | N-(4-(5-(allyl(methyl)amino)pentyl)...)Show SMILES CN(CCCCC[C@H]1CC[C@@H](CC1)N(C)S(=O)(=O)c1ccc(cc1)C(F)(F)F)CC=C |r,wU:10.13,wD:7.6,(1.8,-10.87,;1.88,-12.4,;3.25,-13.1,;4.53,-12.26,;5.9,-12.96,;7.19,-12.12,;8.57,-12.82,;9.86,-11.98,;11.23,-12.68,;12.52,-11.84,;12.43,-10.3,;11.06,-9.6,;9.78,-10.44,;13.72,-9.46,;13.63,-7.93,;15.09,-10.15,;16.46,-10.86,;14.39,-11.53,;15.78,-8.78,;15.03,-7.43,;15.82,-6.1,;17.37,-6.13,;18.11,-7.47,;17.31,-8.8,;18.16,-4.82,;18.94,-3.51,;19.47,-5.61,;16.85,-4.03,;.59,-13.24,;-.78,-12.54,;-2.06,-13.38,)| Show InChI InChI=1S/C23H35F3N2O2S/c1-4-17-27(2)18-7-5-6-8-19-9-13-21(14-10-19)28(3)31(29,30)22-15-11-20(12-16-22)23(24,25)26/h4,11-12,15-16,19,21H,1,5-10,13-14,17-18H2,2-3H3/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of human OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50593730
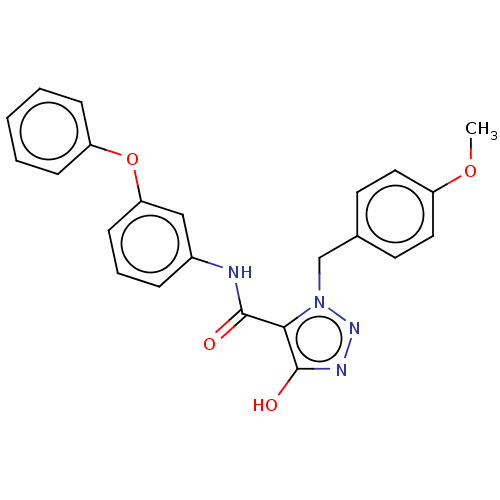
(CHEMBL5172658)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(Oc3ccccc3)c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114366
BindingDB Entry DOI: 10.7270/Q2TB1BWP |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50509767
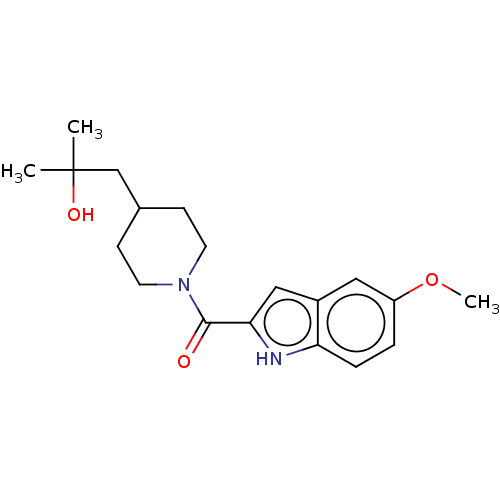
(CHEMBL4513510)Show SMILES COc1ccc2[nH]c(cc2c1)C(=O)N1CCC(CC(C)(C)O)CC1 Show InChI InChI=1S/C19H26N2O3/c1-19(2,23)12-13-6-8-21(9-7-13)18(22)17-11-14-10-15(24-3)4-5-16(14)20-17/h4-5,10-11,13,20,23H,6-9,12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114366
BindingDB Entry DOI: 10.7270/Q2TB1BWP |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50255433
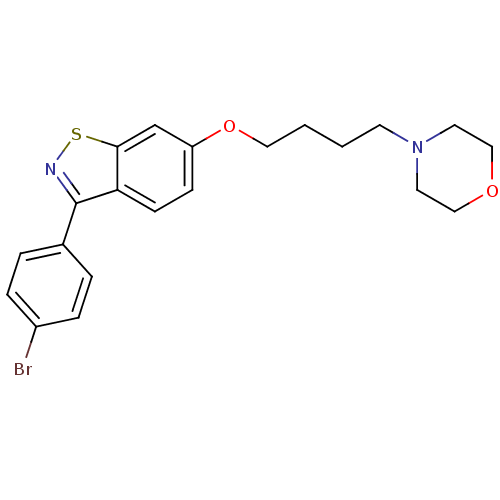
(4-(4-(3-(4-bromophenyl)benzo[d]isothiazol-6-yloxy)...)Show InChI InChI=1S/C21H23BrN2O2S/c22-17-5-3-16(4-6-17)21-19-8-7-18(15-20(19)27-23-21)26-12-2-1-9-24-10-13-25-14-11-24/h3-8,15H,1-2,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of human OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50255431

(4-(trifluoromethyl)phenyl 4-(5-(allyl(methyl)amino...)Show SMILES CN(CCCCC[C@H]1CC[C@@H](CC1)N(C)C(=O)Oc1ccc(cc1)C(F)(F)F)CC=C |r,wU:10.13,wD:7.6,(-5.96,-29.96,;-5.96,-31.5,;-4.63,-32.27,;-3.29,-31.5,;-1.96,-32.27,;-.63,-31.5,;.71,-32.27,;2.04,-31.5,;3.36,-32.27,;4.69,-31.51,;4.7,-29.97,;3.37,-29.19,;2.03,-29.96,;6.04,-29.2,;6.04,-27.66,;7.37,-29.98,;7.36,-31.52,;8.71,-29.21,;10.04,-29.99,;10,-31.53,;11.32,-32.32,;12.67,-31.58,;12.7,-30.03,;11.38,-29.24,;13.99,-32.38,;15.32,-33.15,;13.2,-33.7,;14.77,-31.05,;-7.3,-32.27,;-8.63,-31.5,;-9.96,-32.27,)| Show InChI InChI=1S/C24H35F3N2O2/c1-4-17-28(2)18-7-5-6-8-19-9-13-21(14-10-19)29(3)23(30)31-22-15-11-20(12-16-22)24(25,26)27/h4,11-12,15-16,19,21H,1,5-10,13-14,17-18H2,2-3H3/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of human OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128063

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[d]isothiazol-6-...)Show SMILES CN(CCCCCCOc1ccc2c(nsc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C23H27BrN2OS/c1-3-14-26(2)15-6-4-5-7-16-27-20-12-13-21-22(17-20)28-25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of human OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128065

(CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of human OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50128062
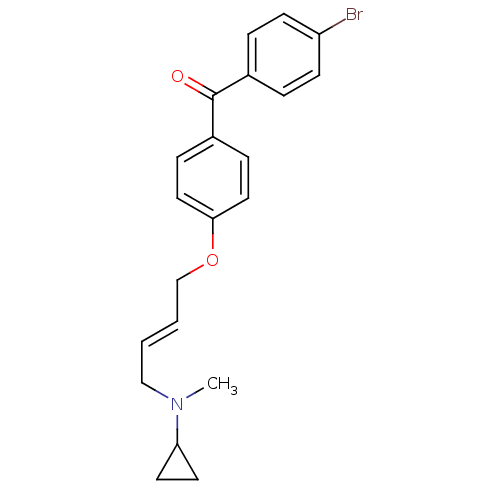
((4-BROMOPHENYL)[4-({(2E)-4-[CYCLOPROPYL(METHYL)AMI...)Show SMILES CN(C\C=C\COc1ccc(cc1)C(=O)c1ccc(Br)cc1)C1CC1 Show InChI InChI=1S/C21H22BrNO2/c1-23(19-10-11-19)14-2-3-15-25-20-12-6-17(7-13-20)21(24)16-4-8-18(22)9-5-16/h2-9,12-13,19H,10-11,14-15H2,1H3/b3-2+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50593732

(CHEMBL5174744)Show SMILES COc1ccc(Cc2onc(O)c2C(=O)Nc2cccc(c2)-c2ccccc2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114366
BindingDB Entry DOI: 10.7270/Q2TB1BWP |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50462392
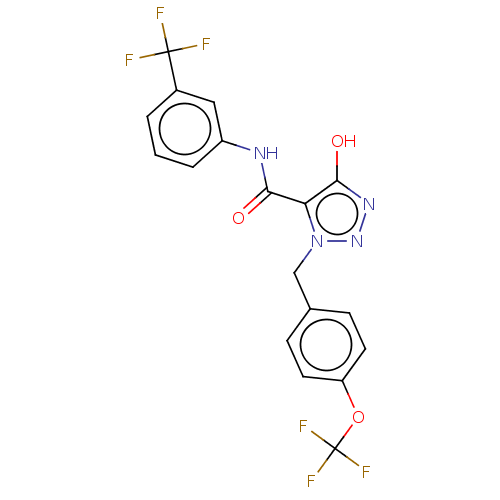
(CHEMBL4248154)Show SMILES Oc1nnn(Cc2ccc(OC(F)(F)F)cc2)c1C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C18H12F6N4O3/c19-17(20,21)11-2-1-3-12(8-11)25-15(29)14-16(30)26-27-28(14)9-10-4-6-13(7-5-10)31-18(22,23)24/h1-8,30H,9H2,(H,25,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Cycloartenol synthase
(Arabidopsis thaliana) | BDBM50433360

(CHEMBL2375380)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CCC2=C(CCC[n+]3ccccc3)CCC[C@]12C |r,c:11| Show InChI InChI=1S/C26H42N/c1-21(2)11-8-12-22(3)24-15-16-25-23(13-9-17-26(24,25)4)14-10-20-27-18-6-5-7-19-27/h5-7,18-19,21-22,24H,8-17,20H2,1-4H3/q+1/t22-,24-,26-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8[pSM60.21] using [14C]-(3S)-2,3-oxidosqualene as s... |
Eur J Med Chem 63: 758-64 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.002
BindingDB Entry DOI: 10.7270/Q2FB54B4 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50593726
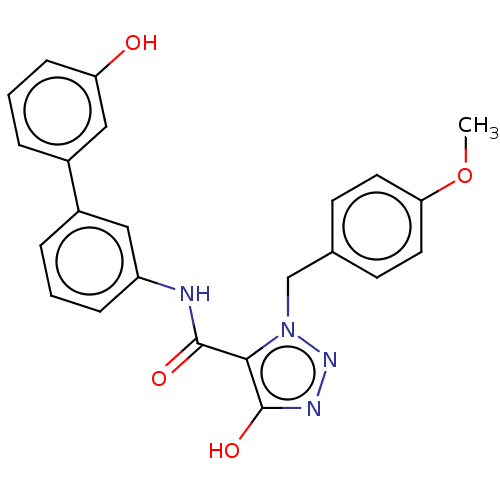
(CHEMBL5204156)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)-c2cccc(O)c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114366
BindingDB Entry DOI: 10.7270/Q2TB1BWP |
More data for this
Ligand-Target Pair | |
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50128065

(CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50128056
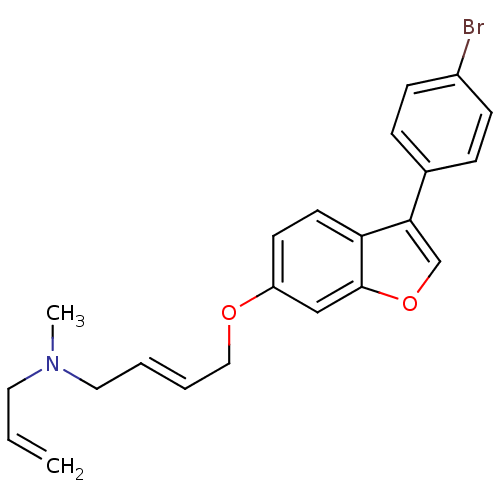
((E)-N-allyl-4-(3-(4-bromophenyl)benzofuran-6-yloxy...)Show SMILES CN(CC=C)C\C=C\COc1ccc2c(coc2c1)-c1ccc(Br)cc1 Show InChI InChI=1S/C22H22BrNO2/c1-3-12-24(2)13-4-5-14-25-19-10-11-20-21(16-26-22(20)15-19)17-6-8-18(23)9-7-17/h3-11,15-16H,1,12-14H2,2H3/b5-4+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50593727
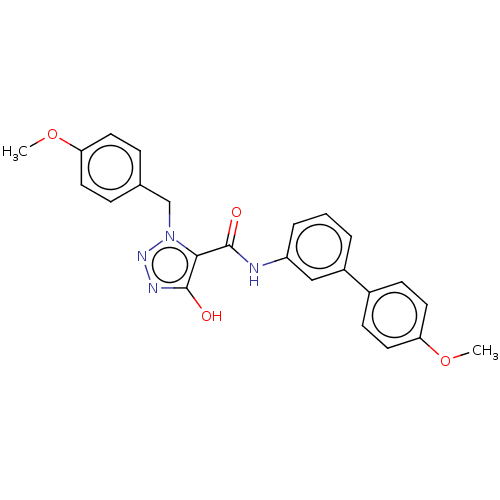
(CHEMBL5170149)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)-c2ccc(OC)cc2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114366
BindingDB Entry DOI: 10.7270/Q2TB1BWP |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50255475
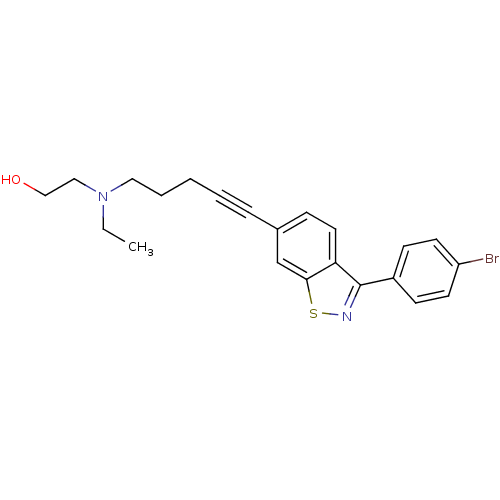
(2-((5-(3-(4-bromophenyl)benzo[d]isothiazol-6-yl)pe...)Show InChI InChI=1S/C22H23BrN2OS/c1-2-25(14-15-26)13-5-3-4-6-17-7-12-20-21(16-17)27-24-22(20)18-8-10-19(23)11-9-18/h7-12,16,26H,2-3,5,13-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of human OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Cycloartenol synthase
(Arabidopsis thaliana) | BDBM50128070
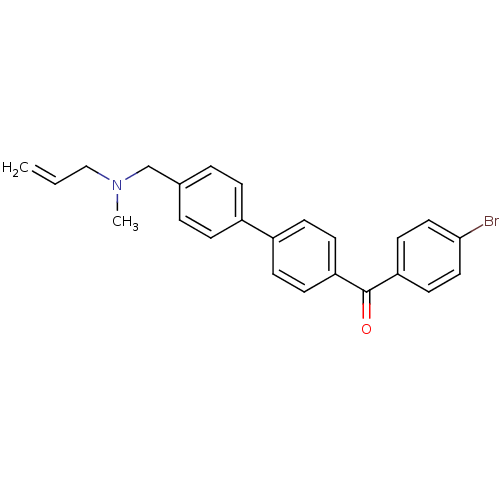
((4''-{[ALLYL(METHYL)AMINO]METHYL}-1,1''-BIPHENYL-4...)Show SMILES CN(CC=C)Cc1ccc(cc1)-c1ccc(cc1)C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H22BrNO/c1-3-16-26(2)17-18-4-6-19(7-5-18)20-8-10-21(11-9-20)24(27)22-12-14-23(25)15-13-22/h3-15H,1,16-17H2,2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128070

((4''-{[ALLYL(METHYL)AMINO]METHYL}-1,1''-BIPHENYL-4...)Show SMILES CN(CC=C)Cc1ccc(cc1)-c1ccc(cc1)C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H22BrNO/c1-3-16-26(2)17-18-4-6-19(7-5-18)20-8-10-21(11-9-20)24(27)22-12-14-23(25)15-13-22/h3-15H,1,16-17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of human OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50593728
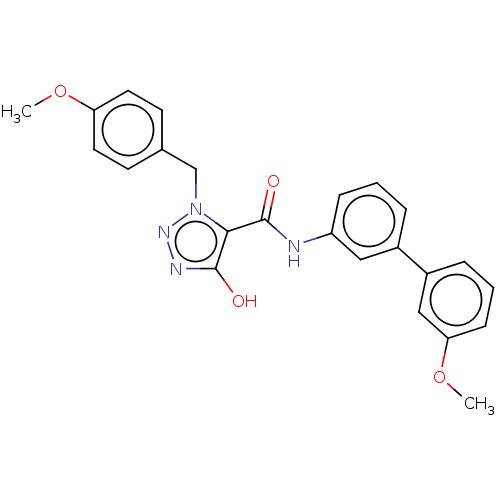
(CHEMBL5170401)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)-c2cccc(OC)c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114366
BindingDB Entry DOI: 10.7270/Q2TB1BWP |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50462395
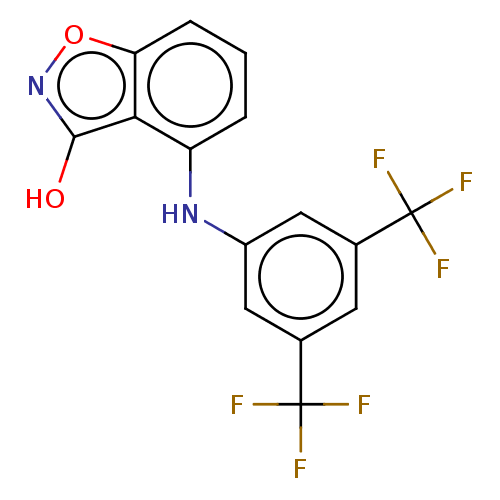
(CHEMBL4238437)Show SMILES Oc1noc2cccc(Nc3cc(cc(c3)C(F)(F)F)C(F)(F)F)c12 Show InChI InChI=1S/C15H8F6N2O2/c16-14(17,18)7-4-8(15(19,20)21)6-9(5-7)22-10-2-1-3-11-12(10)13(24)23-25-11/h1-6,22H,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50255432

(CHEMBL516921 | N-allyl-4-(3-(4-bromophenyl)benzo[d...)Show InChI InChI=1S/C21H23BrN2OS/c1-3-12-24(2)13-4-5-14-25-18-10-11-19-20(15-18)26-23-21(19)16-6-8-17(22)9-7-16/h3,6-11,15H,1,4-5,12-14H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of human OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Cycloartenol synthase
(Arabidopsis thaliana) | BDBM50128062

((4-BROMOPHENYL)[4-({(2E)-4-[CYCLOPROPYL(METHYL)AMI...)Show SMILES CN(C\C=C\COc1ccc(cc1)C(=O)c1ccc(Br)cc1)C1CC1 Show InChI InChI=1S/C21H22BrNO2/c1-23(19-10-11-19)14-2-3-15-25-20-12-6-17(7-13-20)21(24)16-4-8-18(22)9-5-16/h2-9,12-13,19H,10-11,14-15H2,1H3/b3-2+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50593729
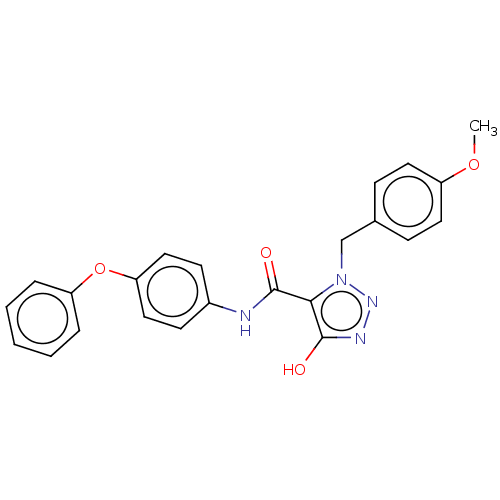
(CHEMBL5175151)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2ccc(Oc3ccccc3)cc2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114366
BindingDB Entry DOI: 10.7270/Q2TB1BWP |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128062

((4-BROMOPHENYL)[4-({(2E)-4-[CYCLOPROPYL(METHYL)AMI...)Show SMILES CN(C\C=C\COc1ccc(cc1)C(=O)c1ccc(Br)cc1)C1CC1 Show InChI InChI=1S/C21H22BrNO2/c1-23(19-10-11-19)14-2-3-15-25-20-12-6-17(7-13-20)21(24)16-4-8-18(22)9-5-16/h2-9,12-13,19H,10-11,14-15H2,1H3/b3-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of human OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50255430

(CHEMBL473035 | N-(4-(5-(ethyl(2-hydroxyethyl)amino...)Show SMILES CCN(CCO)CCCCC[C@H]1CC[C@@H](CC1)N(C)S(=O)(=O)c1ccc(cc1)C(F)(F)F |r,wU:14.17,wD:11.10,(2.96,-12.19,;4.3,-12.96,;4.3,-14.5,;2.96,-15.27,;1.63,-14.5,;.3,-15.27,;5.63,-15.27,;6.96,-14.5,;8.3,-15.27,;9.63,-14.5,;10.96,-15.27,;12.3,-14.5,;13.62,-15.27,;14.95,-14.51,;14.96,-12.97,;13.63,-12.19,;12.29,-12.96,;16.3,-12.2,;16.3,-10.66,;17.63,-12.98,;18.95,-13.74,;16.86,-14.31,;18.41,-11.65,;19.95,-11.66,;20.73,-10.34,;19.97,-9,;18.42,-8.99,;17.65,-10.32,;20.74,-7.67,;21.51,-6.32,;22.08,-8.44,;19.41,-6.9,)| Show InChI InChI=1S/C23H37F3N2O3S/c1-3-28(17-18-29)16-6-4-5-7-19-8-12-21(13-9-19)27(2)32(30,31)22-14-10-20(11-15-22)23(24,25)26/h10-11,14-15,19,21,29H,3-9,12-13,16-18H2,1-2H3/t19-,21- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50128052
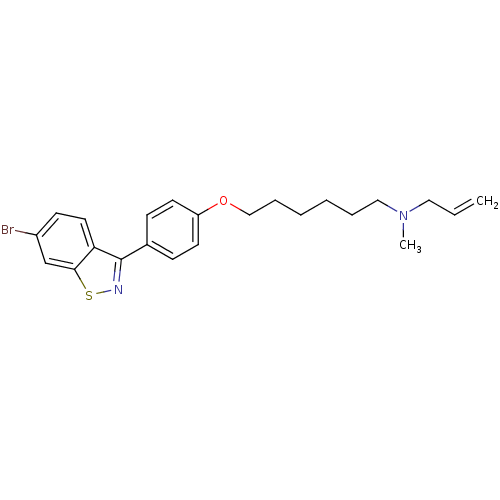
(Allyl-{6-[4-(6-bromo-benzo[d]isothiazol-3-yl)-phen...)Show SMILES CN(CCCCCCOc1ccc(cc1)-c1nsc2cc(Br)ccc12)CC=C Show InChI InChI=1S/C23H27BrN2OS/c1-3-14-26(2)15-6-4-5-7-16-27-20-11-8-18(9-12-20)23-21-13-10-19(24)17-22(21)28-25-23/h3,8-13,17H,1,4-7,14-16H2,2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50128058
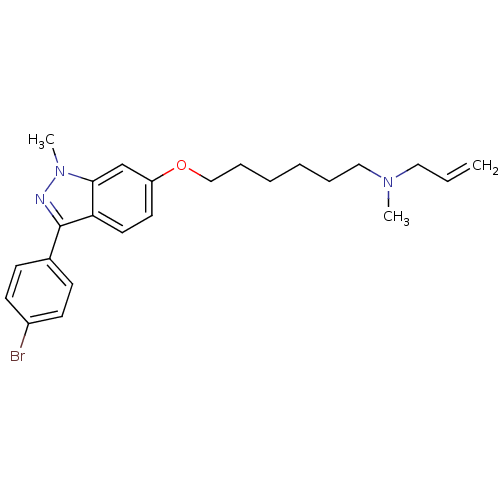
(ALLYL-{6-[3-(4-BROMO-PHENYL)-1-METHYL-1H-INDAZOL-6...)Show SMILES CN(CCCCCCOc1ccc2c(nn(C)c2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C24H30BrN3O/c1-4-15-27(2)16-7-5-6-8-17-29-21-13-14-22-23(18-21)28(3)26-24(22)19-9-11-20(25)12-10-19/h4,9-14,18H,1,5-8,15-17H2,2-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50255476

(2-((3-(3-(4-bromophenyl)benzo[d]isothiazol-6-yl)pr...)Show InChI InChI=1S/C20H19BrN2OS/c1-2-23(12-13-24)11-3-4-15-5-10-18-19(14-15)25-22-20(18)16-6-8-17(21)9-7-16/h5-10,14,24H,2,11-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50509974

(CHEMBL4434843)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(Cc3nonc3O)c2c1 Show InChI InChI=1S/C20H16ClN3O4/c1-11-15(10-17-19(25)23-28-22-17)16-9-14(27-2)7-8-18(16)24(11)20(26)12-3-5-13(21)6-4-12/h3-9H,10H2,1-2H3,(H,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Turin
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged recombinant human AKR1C3 His5Gln mutant expressed in Escherichia coli BL21 (DE) Codon Plus RP cells assessed as r... |
ACS Med Chem Lett 10: 437-443 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00484
BindingDB Entry DOI: 10.7270/Q2HT2SPS |
More data for this
Ligand-Target Pair | |
Cycloartenol synthase
(Arabidopsis thaliana) | BDBM50433362

(CHEMBL2377451)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CCC2=C(CCCN(C)C)CCC[C@]12C |r,c:11| Show InChI InChI=1S/C23H43N/c1-18(2)10-7-11-19(3)21-14-15-22-20(13-9-17-24(5)6)12-8-16-23(21,22)4/h18-19,21H,7-17H2,1-6H3/t19-,21-,23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8[pSM60.21] using [14C]-(3S)-2,3-oxidosqualene as s... |
Eur J Med Chem 63: 758-64 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.002
BindingDB Entry DOI: 10.7270/Q2FB54B4 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50255532

(CHEMBL481199 | N-(4-(5-(allyl(methyl)amino)pentyl)...)Show SMILES CN(CCCCC[C@H]1CC[C@@H](CC1)N(C)S(=O)(=O)c1ccc(cc1)C(F)(F)F)CC=C |r,wU:10.13,wD:7.6,(1.8,-10.87,;1.88,-12.4,;3.25,-13.1,;4.53,-12.26,;5.9,-12.96,;7.19,-12.12,;8.57,-12.82,;9.86,-11.98,;11.23,-12.68,;12.52,-11.84,;12.43,-10.3,;11.06,-9.6,;9.78,-10.44,;13.72,-9.46,;13.63,-7.93,;15.09,-10.15,;16.46,-10.86,;14.39,-11.53,;15.78,-8.78,;15.03,-7.43,;15.82,-6.1,;17.37,-6.13,;18.11,-7.47,;17.31,-8.8,;18.16,-4.82,;18.94,-3.51,;19.47,-5.61,;16.85,-4.03,;.59,-13.24,;-.78,-12.54,;-2.06,-13.38,)| Show InChI InChI=1S/C23H35F3N2O2S/c1-4-17-27(2)18-7-5-6-8-19-9-13-21(14-10-19)28(3)31(29,30)22-15-11-20(12-16-22)23(24,25)26/h4,11-12,15-16,19,21H,1,5-10,13-14,17-18H2,2-3H3/t19-,21- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50593725
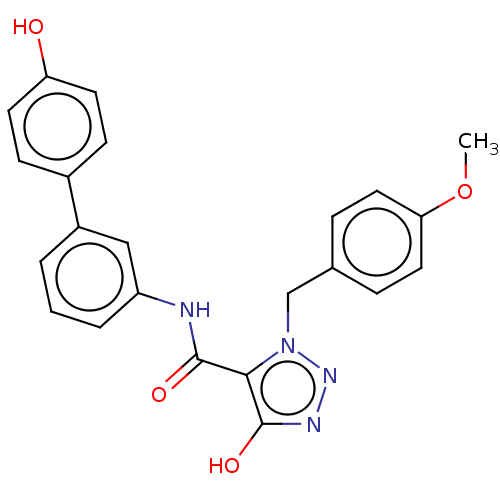
(CHEMBL5206583)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)-c2ccc(O)cc2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114366
BindingDB Entry DOI: 10.7270/Q2TB1BWP |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50277998
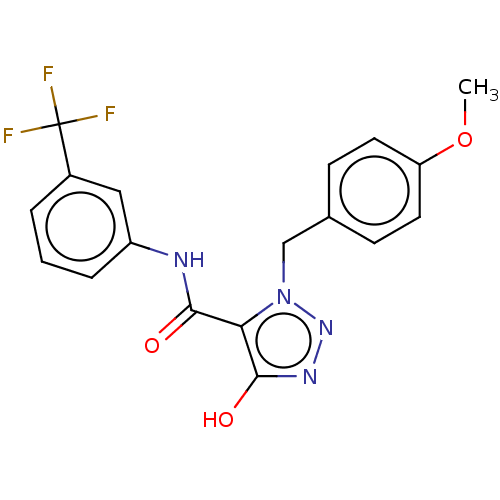
(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114366
BindingDB Entry DOI: 10.7270/Q2TB1BWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50462394

(CHEMBL4249650)Show InChI InChI=1S/C13H9F5N2O2S/c14-23(15,16,17,18)9-4-1-3-8(7-9)19-10-5-2-6-11-12(10)13(21)20-22-11/h1-7,19H,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50277998

(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50277998

(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) using S-tetralol as substrate in presence of NADP+ by fluorimtery |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lanosterol synthase ERG7
(Saccharomyces cerevisiae) | BDBM50128070

((4''-{[ALLYL(METHYL)AMINO]METHYL}-1,1''-BIPHENYL-4...)Show SMILES CN(CC=C)Cc1ccc(cc1)-c1ccc(cc1)C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H22BrNO/c1-3-16-26(2)17-18-4-6-19(7-5-18)20-8-10-21(11-9-20)24(27)22-12-14-23(25)15-13-22/h3-15H,1,16-17H2,2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Cycloartenol synthase
(Arabidopsis thaliana) | BDBM50255602

(CHEMBL520926 | N-(4-(4-(1-(diethylamino)cyclopropy...)Show SMILES CCN(CC)C1(CCCC[C@H]2CC[C@@H](CC2)N(C)S(=O)(=O)c2ccc(cc2)C(F)(F)F)CC1 |r,wU:13.16,wD:10.9,(4.17,-7.27,;2.84,-8.04,;2.84,-9.57,;1.51,-10.35,;.18,-9.57,;4.17,-10.35,;5.5,-9.57,;6.83,-10.35,;8.16,-9.57,;9.5,-10.35,;10.83,-9.57,;12.16,-10.35,;13.5,-9.57,;13.49,-8.03,;12.16,-7.27,;10.83,-8.03,;14.82,-7.26,;14.82,-5.73,;16.15,-8.03,;17.48,-8.8,;15.38,-9.36,;16.91,-6.69,;16.14,-5.37,;16.9,-4.02,;18.45,-4.02,;19.22,-5.34,;18.45,-6.68,;19.21,-2.69,;19.97,-1.36,;20.54,-3.45,;17.88,-1.92,;3.4,-11.66,;4.93,-11.66,)| Show InChI InChI=1S/C25H39F3N2O2S/c1-4-30(5-2)24(18-19-24)17-7-6-8-20-9-13-22(14-10-20)29(3)33(31,32)23-15-11-21(12-16-23)25(26,27)28/h11-12,15-16,20,22H,4-10,13-14,17-19H2,1-3H3/t20-,22- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8 |
Bioorg Med Chem Lett 19: 718-23 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.040
BindingDB Entry DOI: 10.7270/Q2SF2W2N |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Pneumocystis carinii) | BDBM50195963
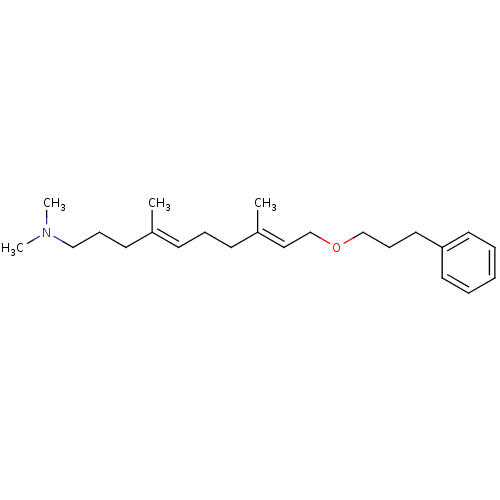
((4E,8E)-N,N,4,8-tetramethyl-10-(3-phenylpropoxy)de...)Show InChI InChI=1S/C23H37NO/c1-21(13-9-18-24(3)4)11-8-12-22(2)17-20-25-19-10-16-23-14-6-5-7-15-23/h5-7,11,14-15,17H,8-10,12-13,16,18-20H2,1-4H3/b21-11+,22-17+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi del Piemonte Orientale A. Avogadro
Curated by ChEMBL
| Assay Description
Inhibition of Pneumocystis carinii oxidosqualene cyclase |
Bioorg Med Chem Lett 17: 220-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.058
BindingDB Entry DOI: 10.7270/Q27M08SM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Pneumocystis carinii) | BDBM50195962
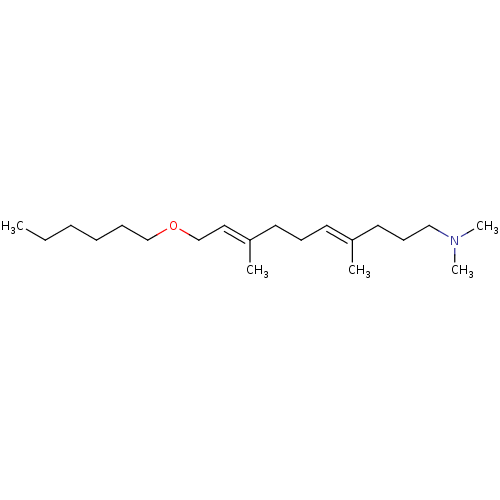
((4E,8E)-10-(hexyloxy)-N,N,4,8-tetramethyldeca-4,8-...)Show InChI InChI=1S/C20H39NO/c1-6-7-8-9-17-22-18-15-20(3)13-10-12-19(2)14-11-16-21(4)5/h12,15H,6-11,13-14,16-18H2,1-5H3/b19-12+,20-15+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi del Piemonte Orientale A. Avogadro
Curated by ChEMBL
| Assay Description
Inhibition of Pneumocystis carinii oxidosqualene cyclase |
Bioorg Med Chem Lett 17: 220-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.058
BindingDB Entry DOI: 10.7270/Q27M08SM |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50593722
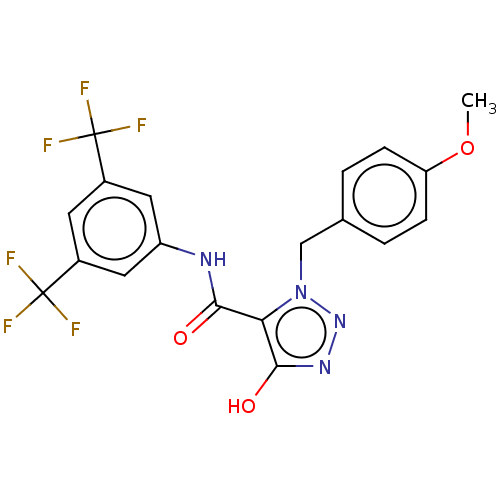
(CHEMBL5191752)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cc(cc(c2)C(F)(F)F)C(F)(F)F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114366
BindingDB Entry DOI: 10.7270/Q2TB1BWP |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50462391
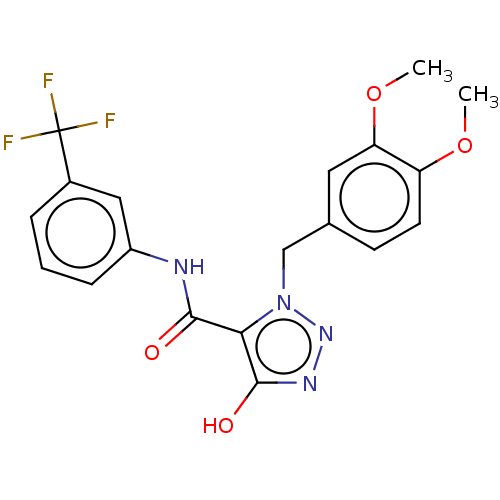
(CHEMBL4238142)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1OC Show InChI InChI=1S/C19H17F3N4O4/c1-29-14-7-6-11(8-15(14)30-2)10-26-16(18(28)24-25-26)17(27)23-13-5-3-4-12(9-13)19(20,21)22/h3-9,28H,10H2,1-2H3,(H,23,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data