 Found 641 hits with Last Name = 'ong' and Initial = 'eh'
Found 641 hits with Last Name = 'ong' and Initial = 'eh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82505
(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
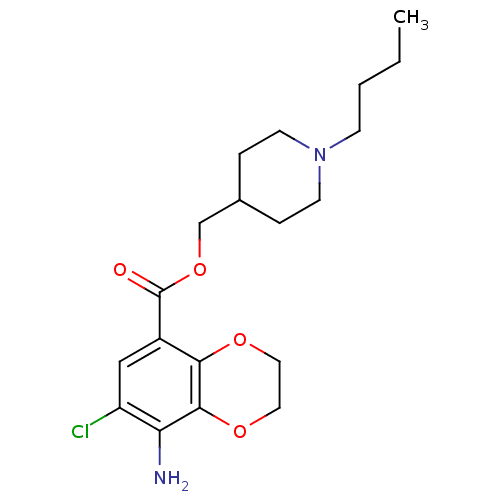
(RAT) | BDBM50054820
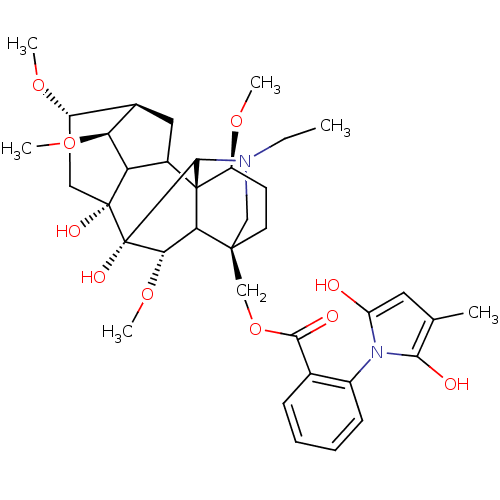
(Methyllycaconitine | [(1S,4S,5R,6S,8R,9R,13S,16S,1...)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34C5C[C@H]6[C@H](OC)C5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |wU:4.4,42.46,36.39,44.48,39.43,wD:28.55,32.34,25.27,31.32,TLB:28:29:32:36.38.39,1:2:47:25.23.24,25:28:42.44:4.2.3,THB:42:48:47:25.23.24,29:28:42.44:4.2.3,2:48:36.35.29:47.44,(3.83,-6.7,;5.61,-4.92,;8.1,-4.92,;7.12,-7.5,;8.22,-6.42,;8.22,-7.94,;7.82,-9.45,;8.61,-10.78,;9.94,-10.01,;8.62,-12.33,;7.29,-13.1,;7.29,-14.64,;8.62,-15.41,;9.95,-14.64,;9.95,-13.1,;11.49,-13.09,;12.9,-13.7,;13.3,-15.19,;13.93,-12.55,;13.14,-11.22,;13.91,-9.88,;11.64,-11.55,;10.86,-10.2,;6.88,-5.65,;6.87,-4.11,;8.21,-3.34,;8.2,-1.8,;6.87,-1.03,;9.54,-4.11,;10.5,-3.18,;10.3,-1.71,;11.64,-1.06,;12.67,-2.15,;14.21,-2.15,;14.96,-3.46,;11.97,-3.45,;12.65,-4.9,;13.98,-5.67,;16.6,-3.1,;16.6,-1.56,;17.69,-.47,;19.02,-1.24,;11.93,-6.28,;12.7,-7.59,;10.46,-6.53,;10.06,-8.01,;11.39,-8.78,;9.55,-5.63,;9.53,-2.57,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22?,24+,25+,27?,28+,29?,30+,33?,34+,35-,36+,37+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50007872
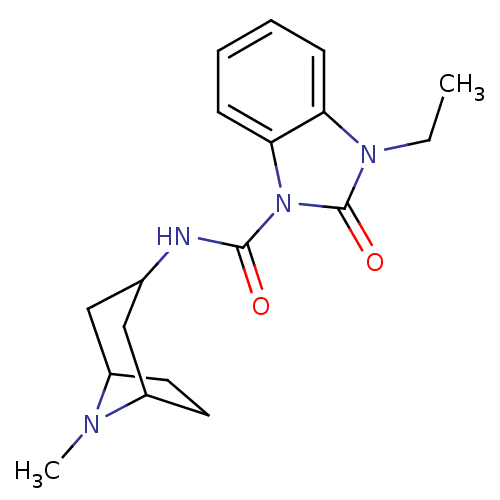
(3-Ethyl-2-oxo-2,3-dihydro-benzoimidazole-1-carboxy...)Show SMILES CCn1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:12:13:20:16.17| Show InChI InChI=1S/C18H24N4O2/c1-3-21-15-6-4-5-7-16(15)22(18(21)24)17(23)19-12-10-13-8-9-14(11-12)20(13)2/h4-7,12-14H,3,8-11H2,1-2H3,(H,19,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50056404
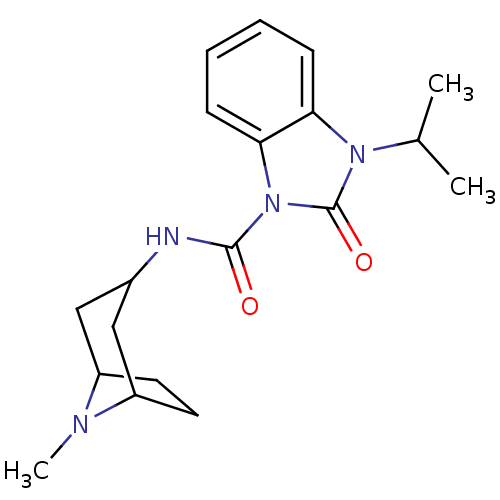
(3-Isopropyl-2-oxo-2,3-dihydro-benzoimidazole-1-car...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:13:14:21:17.18| Show InChI InChI=1S/C19H26N4O2/c1-12(2)22-16-6-4-5-7-17(16)23(19(22)25)18(24)20-13-10-14-8-9-15(11-13)21(14)3/h4-7,12-15H,8-11H2,1-3H3,(H,20,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50056404

(3-Isopropyl-2-oxo-2,3-dihydro-benzoimidazole-1-car...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:13:14:21:17.18| Show InChI InChI=1S/C19H26N4O2/c1-12(2)22-16-6-4-5-7-17(16)23(19(22)25)18(24)20-13-10-14-8-9-15(11-13)21(14)3/h4-7,12-15H,8-11H2,1-3H3,(H,20,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50107863
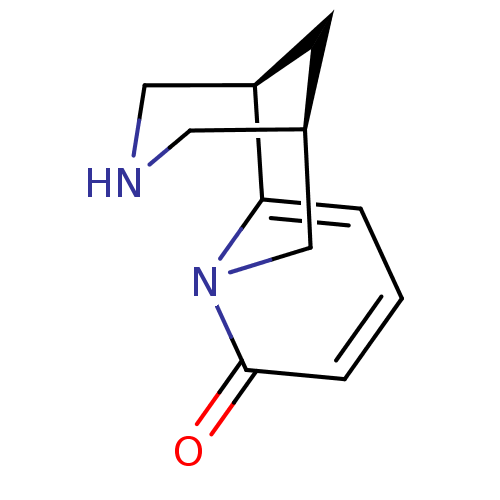
((-)-cytisine | (1R,9R)-7,11-diazatricyclo[7.3.1.0~...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85135
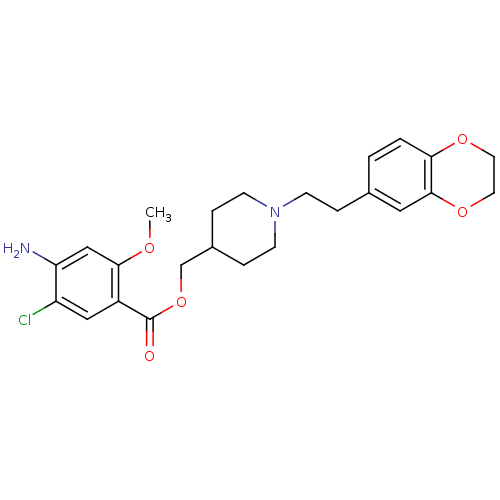
(RS 57639)Show SMILES COc1cc(N)c(Cl)cc1C(=O)OCC1CCN(CCc2ccc3OCCOc3c2)CC1 Show InChI InChI=1S/C24H29ClN2O5/c1-29-22-14-20(26)19(25)13-18(22)24(28)32-15-17-5-8-27(9-6-17)7-4-16-2-3-21-23(12-16)31-11-10-30-21/h2-3,12-14,17H,4-11,15,26H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85135

(RS 57639)Show SMILES COc1cc(N)c(Cl)cc1C(=O)OCC1CCN(CCc2ccc3OCCOc3c2)CC1 Show InChI InChI=1S/C24H29ClN2O5/c1-29-22-14-20(26)19(25)13-18(22)24(28)32-15-17-5-8-27(9-6-17)7-4-16-2-3-21-23(12-16)31-11-10-30-21/h2-3,12-14,17H,4-11,15,26H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50007871
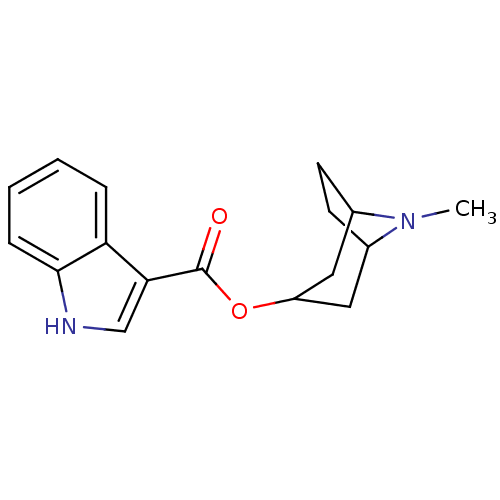
(CHEMBL8197 | ICS 205-930 | TROPISETRON)Show SMILES CN1C2CCC1CC(C2)OC(=O)c1c[nH]c2ccccc12 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190788
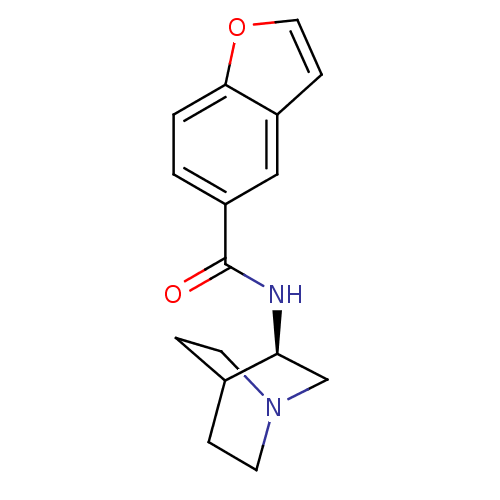
(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50007872

(3-Ethyl-2-oxo-2,3-dihydro-benzoimidazole-1-carboxy...)Show SMILES CCn1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:12:13:20:16.17| Show InChI InChI=1S/C18H24N4O2/c1-3-21-15-6-4-5-7-16(15)22(18(21)24)17(23)19-12-10-13-8-9-14(11-12)20(13)2/h4-7,12-14H,3,8-11H2,1-2H3,(H,19,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190785
(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM81924
((R)-3-(1-Propyl-piperidin-3-yl)-phenol | 3PPP(+/-)...)Show InChI InChI=1S/C14H21NO/c1-2-8-15-9-4-6-13(11-15)12-5-3-7-14(16)10-12/h3,5,7,10,13,16H,2,4,6,8-9,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85135

(RS 57639)Show SMILES COc1cc(N)c(Cl)cc1C(=O)OCC1CCN(CCc2ccc3OCCOc3c2)CC1 Show InChI InChI=1S/C24H29ClN2O5/c1-29-22-14-20(26)19(25)13-18(22)24(28)32-15-17-5-8-27(9-6-17)7-4-16-2-3-21-23(12-16)31-11-10-30-21/h2-3,12-14,17H,4-11,15,26H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM85330

(CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...)Show InChI InChI=1S/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190793
(CHEMBL268939 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2sccc2cn1 |wU:3.2,(26.77,-30.5,;26.77,-32.04,;28.11,-32.81,;29.44,-32.03,;29.43,-30.5,;30.77,-29.72,;32.11,-30.49,;32.11,-32.03,;30.78,-32.8,;29.9,-31.66,;30.72,-30.86,;25.44,-32.82,;24.1,-32.06,;22.78,-32.83,;21.31,-32.36,;20.4,-33.6,;21.31,-34.85,;22.77,-34.37,;24.1,-35.14,;25.45,-34.37,)| Show InChI InChI=1S/C15H17N3OS/c19-15(12-7-14-11(8-16-12)3-6-20-14)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190791
(CHEMBL210865 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccsc2cn1 |wU:3.2,(11.37,-32.25,;11.38,-33.79,;12.72,-34.55,;14.05,-33.78,;14.04,-32.24,;15.38,-31.47,;16.72,-32.24,;16.71,-33.78,;15.39,-34.55,;14.51,-33.4,;15.33,-32.61,;10.05,-34.57,;8.71,-33.8,;7.38,-34.58,;5.92,-34.1,;5.01,-35.35,;5.92,-36.59,;7.38,-36.12,;8.71,-36.89,;10.06,-36.12,)| Show InChI InChI=1S/C15H17N3OS/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190789
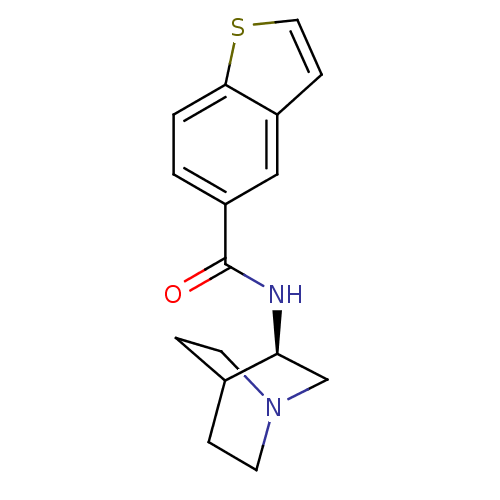
(CHEMBL208565 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2sccc2c1 |wU:3.2,(11.98,-5.28,;11.99,-6.82,;13.33,-7.58,;14.66,-6.8,;14.65,-5.27,;15.99,-4.5,;17.32,-5.26,;17.32,-6.8,;16,-7.58,;15.12,-6.43,;15.94,-5.63,;10.66,-7.59,;10.66,-9.15,;9.32,-9.92,;7.99,-9.15,;6.52,-9.62,;5.62,-8.37,;6.53,-7.13,;7.99,-7.61,;9.32,-6.83,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM94507
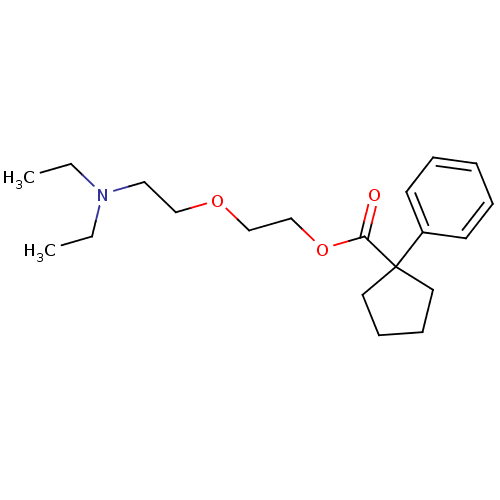
(2-[2-(diethylamino)ethoxy]ethyl 1-phenylcyclopenta...)Show InChI InChI=1S/C20H31NO3/c1-3-21(4-2)14-15-23-16-17-24-19(22)20(12-8-9-13-20)18-10-6-5-7-11-18/h5-7,10-11H,3-4,8-9,12-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50007871

(CHEMBL8197 | ICS 205-930 | TROPISETRON)Show SMILES CN1C2CCC1CC(C2)OC(=O)c1c[nH]c2ccccc12 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 8.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190786
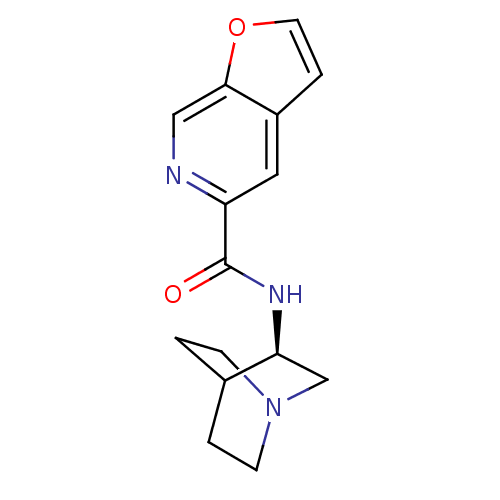
((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM85330

(CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...)Show InChI InChI=1S/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 9.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190783
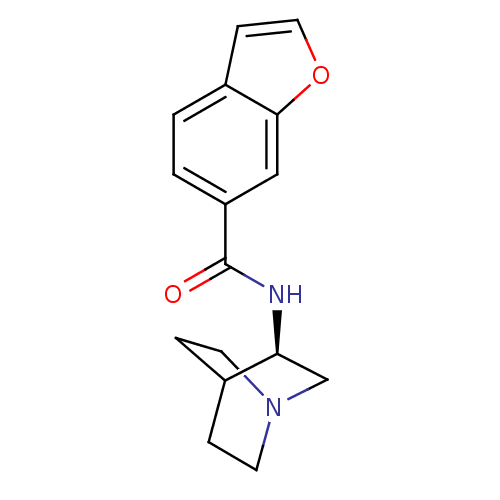
(CHEMBL379302 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccoc2c1 |wU:3.2,(27.6,-12.86,;27.61,-14.4,;28.95,-15.16,;30.28,-14.39,;30.27,-12.85,;31.61,-12.08,;32.95,-12.85,;32.94,-14.39,;31.62,-15.16,;30.74,-14.01,;31.56,-13.22,;26.28,-15.18,;26.29,-16.73,;24.94,-17.5,;23.61,-16.73,;22.15,-17.2,;21.24,-15.96,;22.15,-14.71,;23.61,-15.19,;24.94,-14.41,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190784
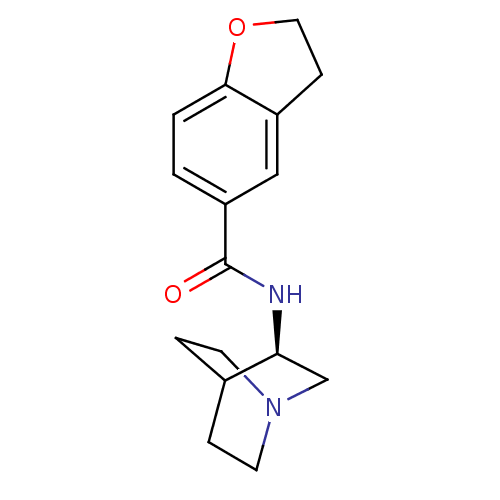
(CHEMBL378496 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2OCCc2c1 |wU:3.2,(11.67,-14.82,;11.67,-16.36,;13.01,-17.12,;14.34,-16.35,;14.33,-14.81,;15.67,-14.04,;17.01,-14.81,;17.01,-16.35,;15.68,-17.12,;14.8,-15.97,;15.62,-15.18,;10.34,-17.14,;10.35,-18.69,;9,-19.46,;7.67,-18.69,;6.21,-19.16,;5.3,-17.92,;6.21,-16.67,;7.68,-17.15,;9,-16.37,)| Show InChI InChI=1S/C16H20N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,9,11,14H,3-8,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 15.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50001028
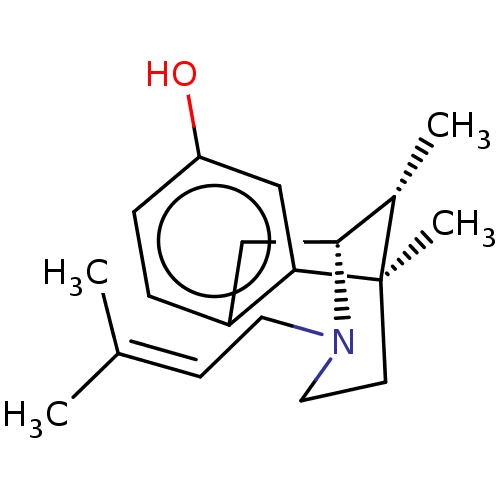
((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...)Show SMILES [#6]-[#6@H]1-[#6@H]-2-[#6]-c3ccc(-[#8])cc3[C@]1([#6])[#6]-[#6]-[#7]-2-[#6]\[#6]=[#6](\[#6])-[#6] |r,TLB:16:15:1:3.4.10| Show InChI InChI=1S/C19H27NO/c1-13(2)7-9-20-10-8-19(4)14(3)18(20)11-15-5-6-16(21)12-17(15)19/h5-7,12,14,18,21H,8-11H2,1-4H3/t14-,18+,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50056404

(3-Isopropyl-2-oxo-2,3-dihydro-benzoimidazole-1-car...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:13:14:21:17.18| Show InChI InChI=1S/C19H26N4O2/c1-12(2)22-16-6-4-5-7-17(16)23(19(22)25)18(24)20-13-10-14-8-9-15(11-13)21(14)3/h4-7,12-15H,8-11H2,1-3H3,(H,20,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50056401
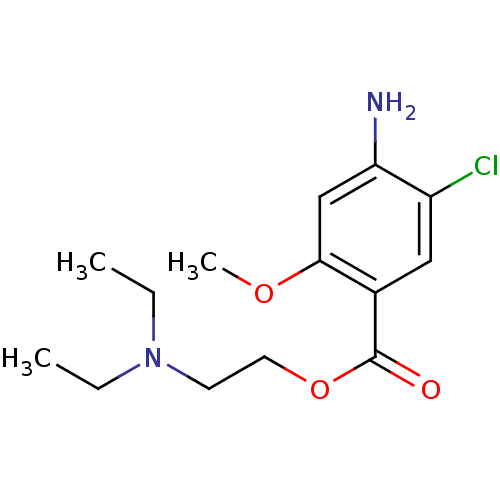
(2-diethylaminoethyl [2-methoxy-4-amino-5-chloro]be...)Show InChI InChI=1S/C14H21ClN2O3/c1-4-17(5-2)6-7-20-14(18)10-8-11(15)12(16)9-13(10)19-3/h8-9H,4-7,16H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50056401

(2-diethylaminoethyl [2-methoxy-4-amino-5-chloro]be...)Show InChI InChI=1S/C14H21ClN2O3/c1-4-17(5-2)6-7-20-14(18)10-8-11(15)12(16)9-13(10)19-3/h8-9H,4-7,16H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50056404

(3-Isopropyl-2-oxo-2,3-dihydro-benzoimidazole-1-car...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:13:14:21:17.18| Show InChI InChI=1S/C19H26N4O2/c1-12(2)22-16-6-4-5-7-17(16)23(19(22)25)18(24)20-13-10-14-8-9-15(11-13)21(14)3/h4-7,12-15H,8-11H2,1-3H3,(H,20,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50056404

(3-Isopropyl-2-oxo-2,3-dihydro-benzoimidazole-1-car...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:13:14:21:17.18| Show InChI InChI=1S/C19H26N4O2/c1-12(2)22-16-6-4-5-7-17(16)23(19(22)25)18(24)20-13-10-14-8-9-15(11-13)21(14)3/h4-7,12-15H,8-11H2,1-3H3,(H,20,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50161764
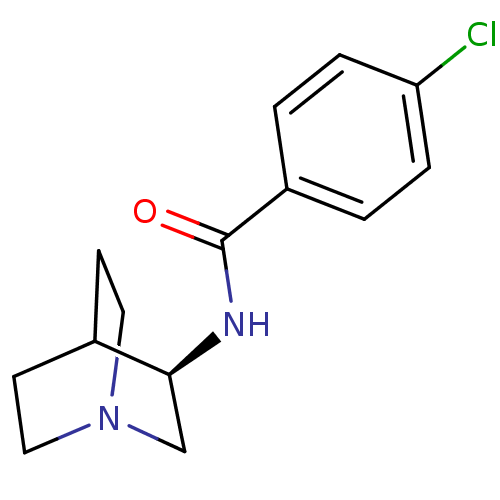
((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50056401

(2-diethylaminoethyl [2-methoxy-4-amino-5-chloro]be...)Show InChI InChI=1S/C14H21ClN2O3/c1-4-17(5-2)6-7-20-14(18)10-8-11(15)12(16)9-13(10)19-3/h8-9H,4-7,16H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82422
(CAS_109872-41-5 | NSC_3035240 | RENZAPRIDE)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CCN2CCCC1C2 |TLB:12:13:17.18.19:21| Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-14-4-6-20-5-2-3-10(14)9-20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data