

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016326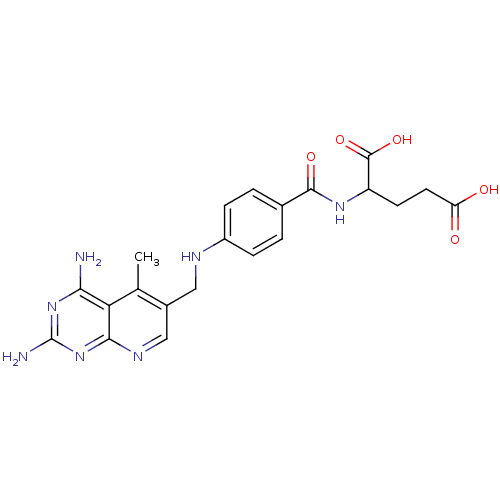 (2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM66082 ((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023681 (2-{4-[(2,4-Diamino-5,7-dimethyl-pyrido[2,3-d]pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043393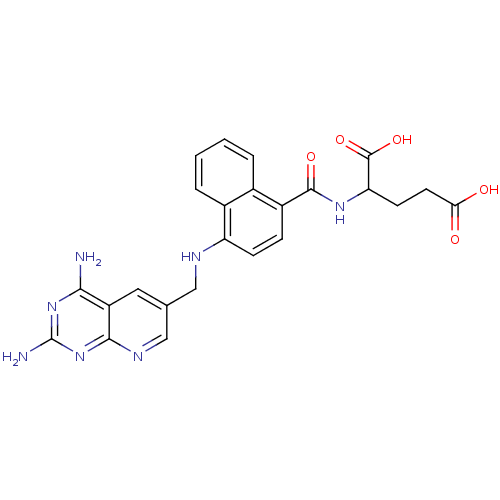 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023682 (2-{4-[(2,4-Diamino-5-methyl-7-phenyl-pyrido[2,3-d]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023684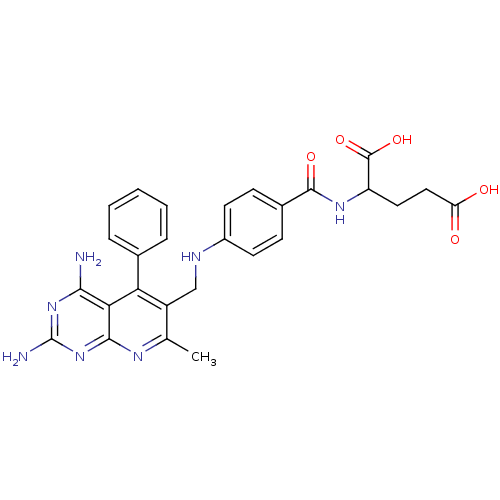 (2-{4-[(2,4-Diamino-7-methyl-5-phenyl-pyrido[2,3-d]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023680 (2-{4-[(2,4-Diamino-7-phenyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023683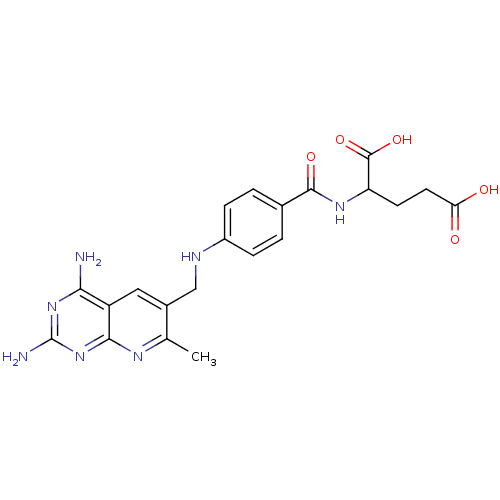 (2-{4-[(2,4-Diamino-7-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043396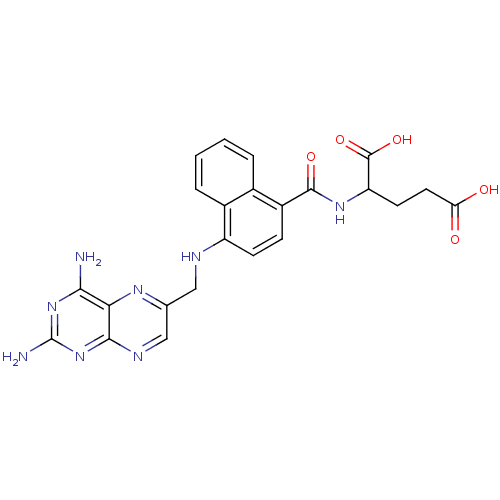 (2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043399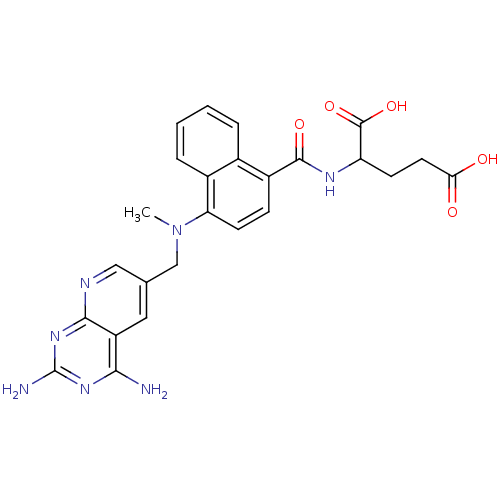 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of dihydrofolate reductase (DHFR) in L1210 cells | J Med Chem 35: 3002-6 (1992) BindingDB Entry DOI: 10.7270/Q2MP527Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00482 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043395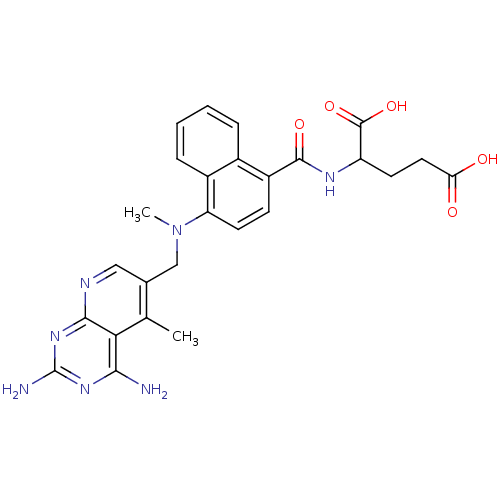 (2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00484 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043400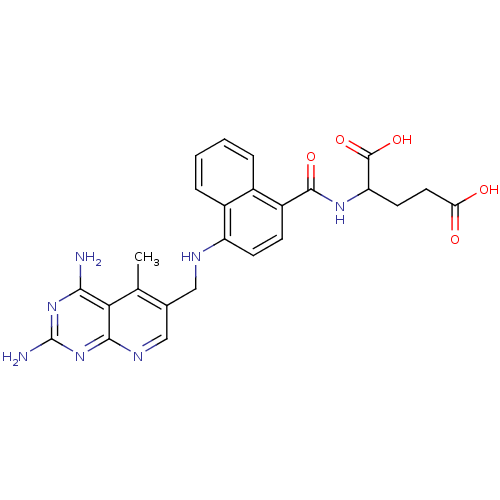 (2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00508 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043398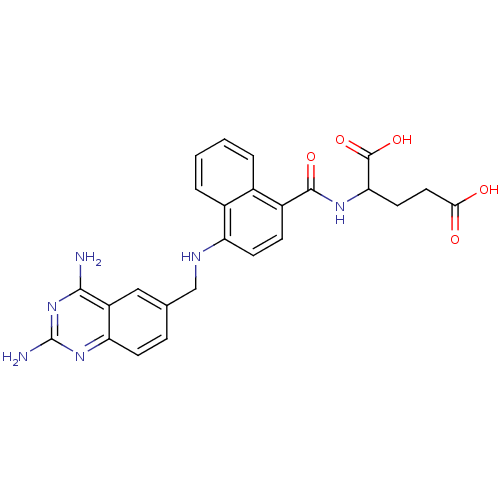 (2-({4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043394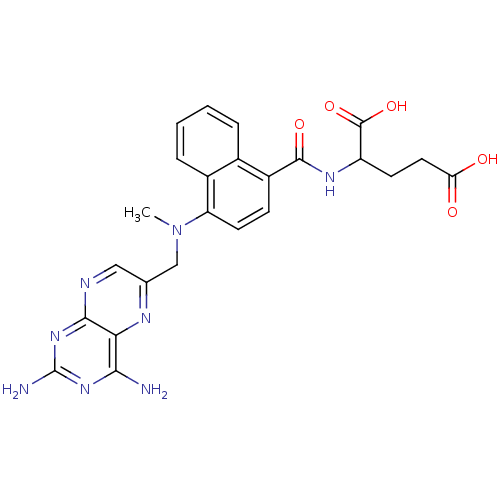 (2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043397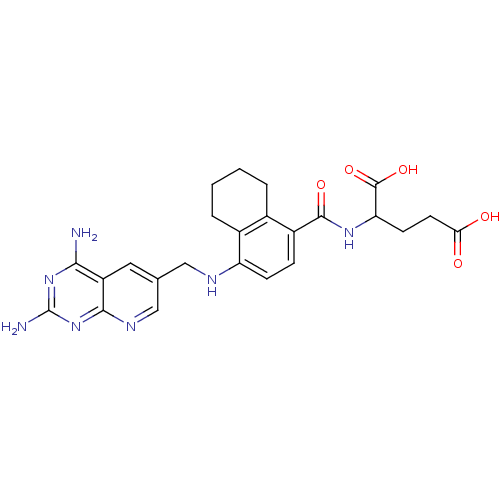 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50004544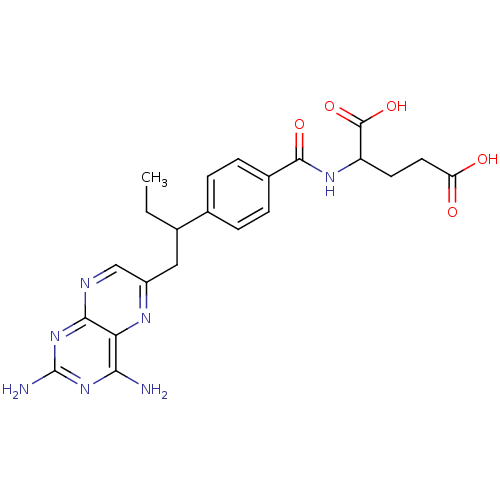 (2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-propyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of dihydrofolate reductase (DHFR) in L1210 cells | J Med Chem 35: 3002-6 (1992) BindingDB Entry DOI: 10.7270/Q2MP527Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM50043394 (2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-am...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.000190 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Tested for inhibition against P. carinii DHFR (dihydrofolate reductase) | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM50016325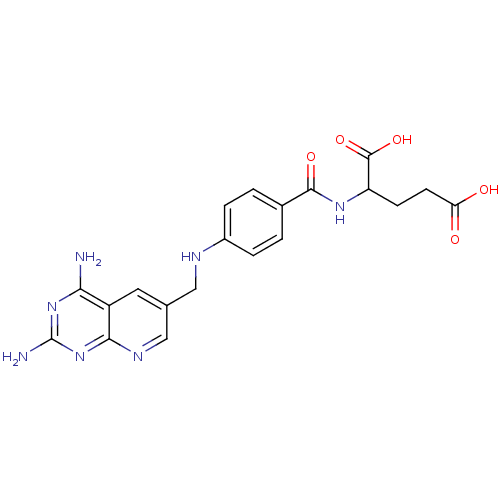 (2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.000530 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition against Pneumocystis carinii Dihydrofolate reductase | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM50043393 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.000530 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Tested for inhibition against Dihydrofolate reductase from P. carinii | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM50043393 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.000530 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Tested for inhibition against Dihydrofolate reductase from P. carinii | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50043393 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00160 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Tested for inhibition against Dihydrofolate reductase from T. gondii | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50016325 (2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00160 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition against rat liver Dihydrofolate reductase | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50016325 (2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00210 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition against Toxoplasma gondii Dihydrofolate reductase | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50043394 (2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Tested for inhibition against rat liver DHFR (dihydrofolate reductase) | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470378 (CHEMBL108283) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470365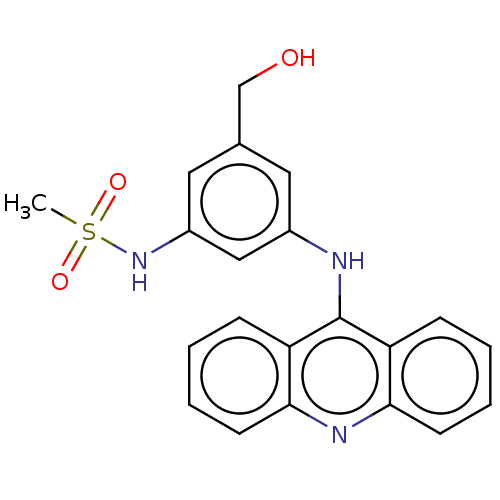 (CHEMBL326664) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470374 (CHEMBL322325) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470371 (CHEMBL325543) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470361 (CHEMBL108404) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470369 (CHEMBL325588) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470357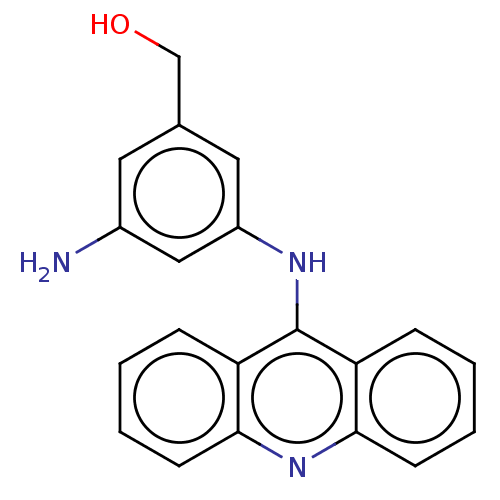 (CHEMBL321473) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470362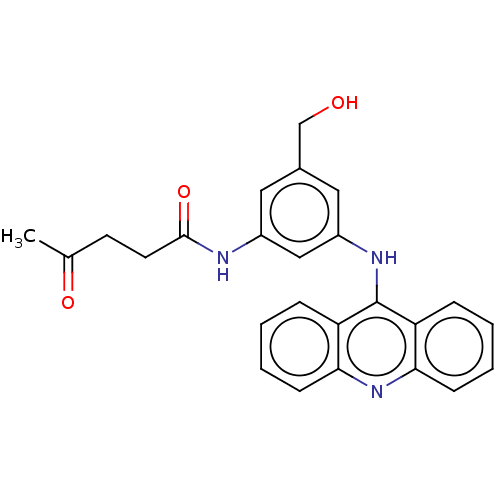 (CHEMBL444329) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM87351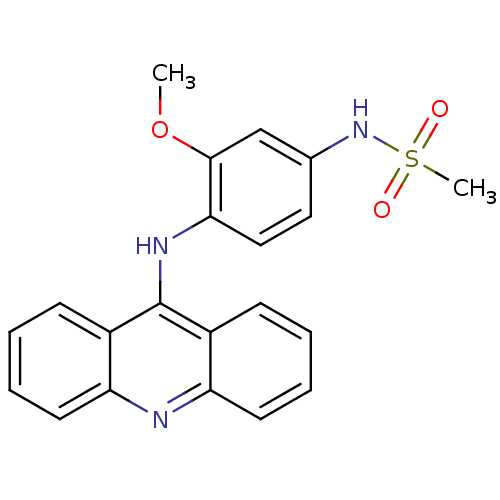 (Amsacrine hydrochloride | CHEMBL43 | MLS002153376 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470360 (CHEMBL109928) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470364 (CHEMBL111592) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470367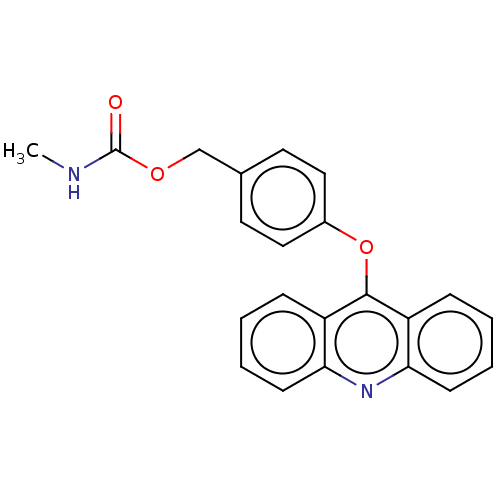 (CHEMBL109942) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470355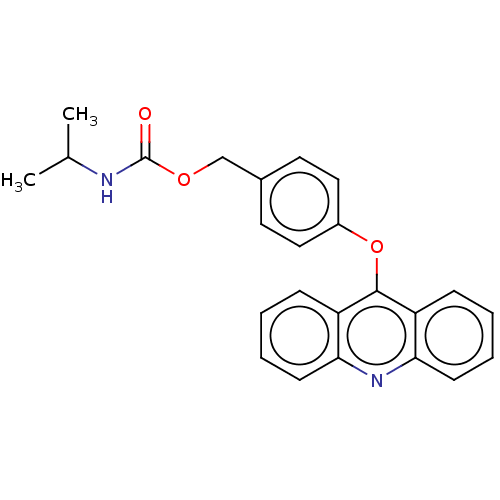 (CHEMBL320354) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470373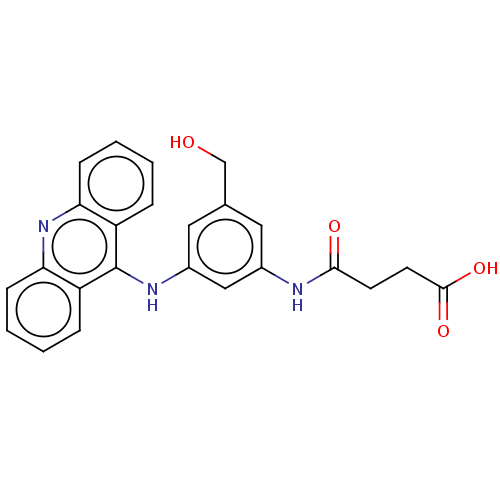 (CHEMBL326274) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470372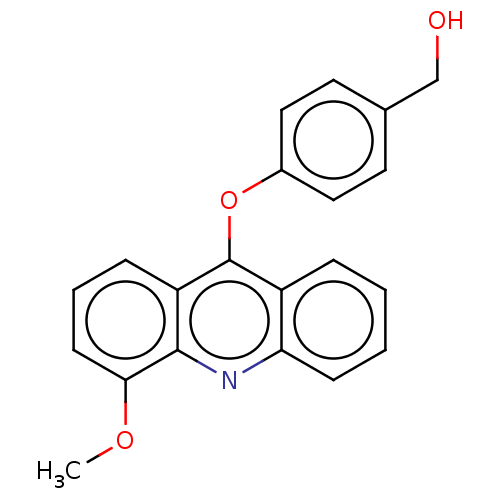 (CHEMBL322491) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470375 (CHEMBL111261) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470376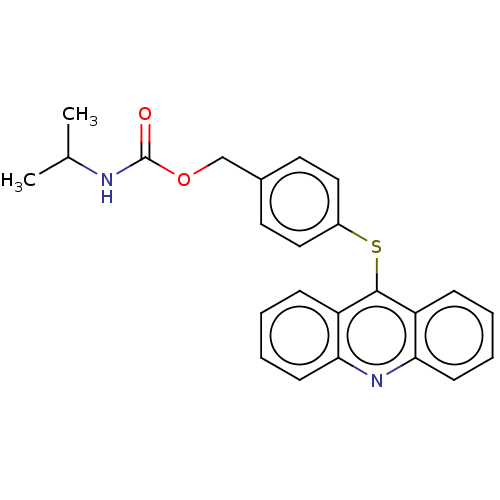 (CHEMBL109880) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470359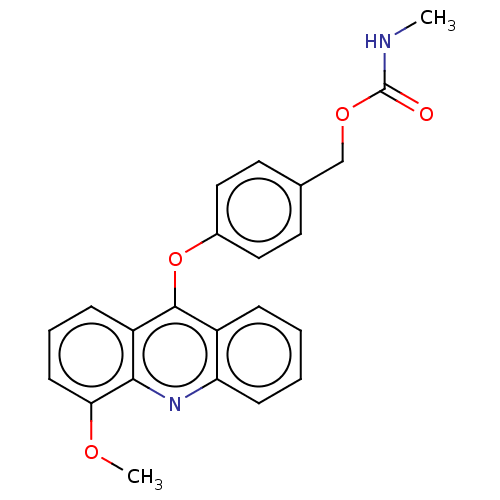 (CHEMBL320495) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470363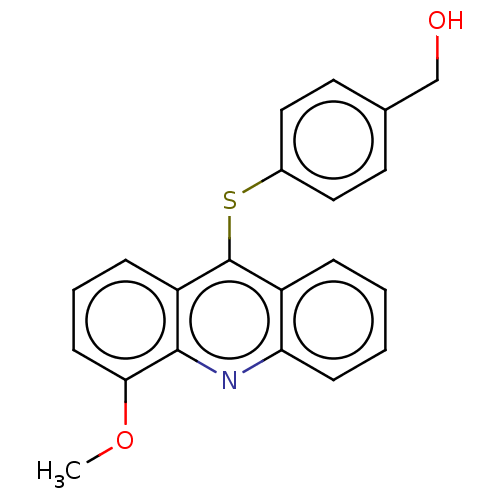 (CHEMBL111735) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470356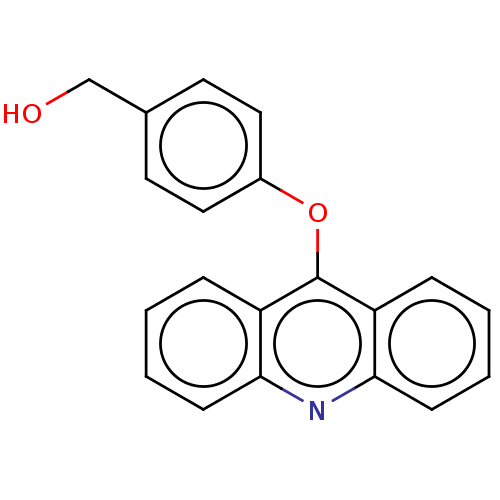 (CHEMBL109743) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470368 (CHEMBL111502) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470370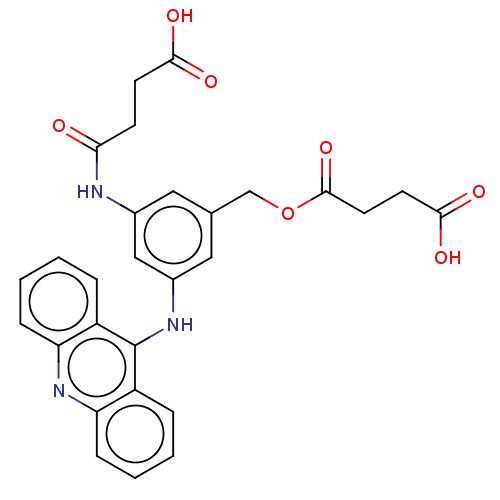 (CHEMBL109468) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470358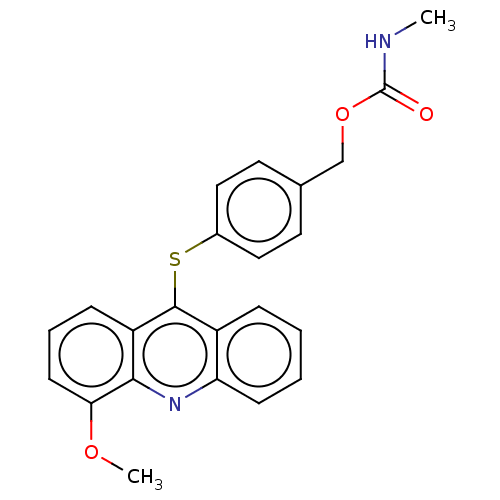 (CHEMBL109922) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50470366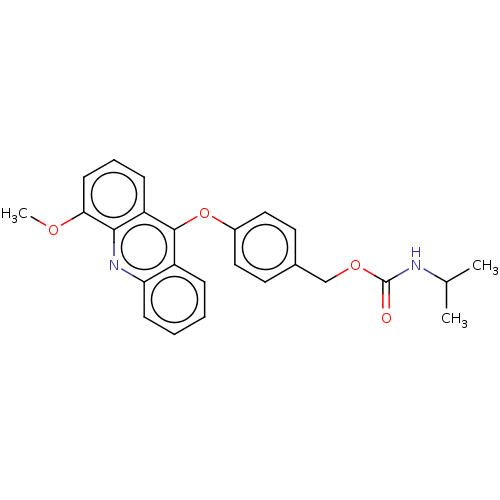 (CHEMBL109534) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research Curated by ChEMBL | Assay Description In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation | J Med Chem 38: 3226-35 (1995) Article DOI: 10.1021/jm00017a006 BindingDB Entry DOI: 10.7270/Q2XS5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |