

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM22988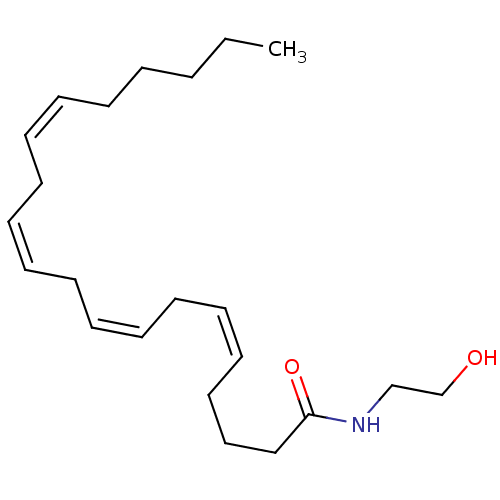 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300767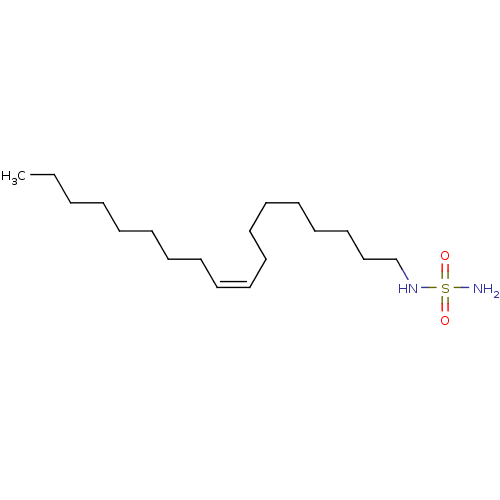 (CHEMBL570566 | {[(9Z)-octadec-9-en-1-yl]sulfamoyl}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300765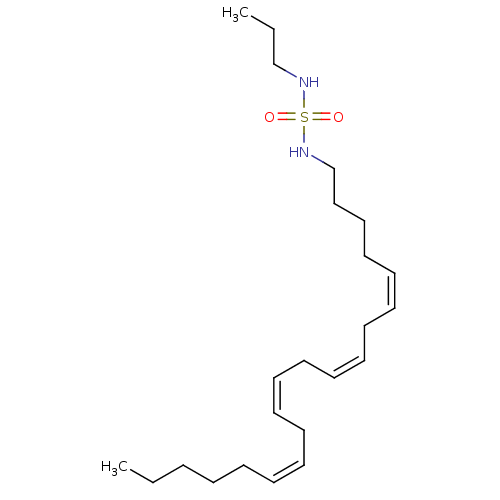 (CHEMBL583108 | N-(cis-5-cis-8-cis-11-cis-14-eicosa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300770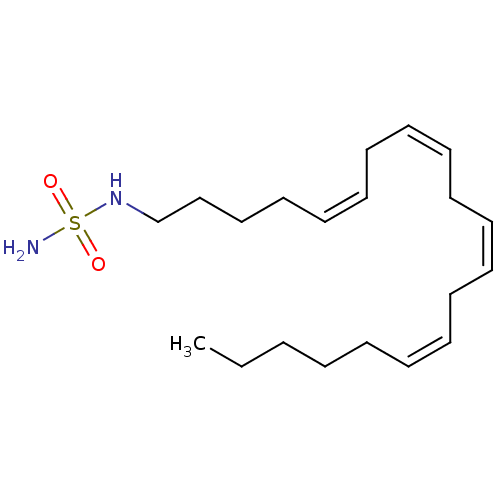 (CHEMBL568489 | N-(cis-5-cis-8-cis-11-cis-14-eicosa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300769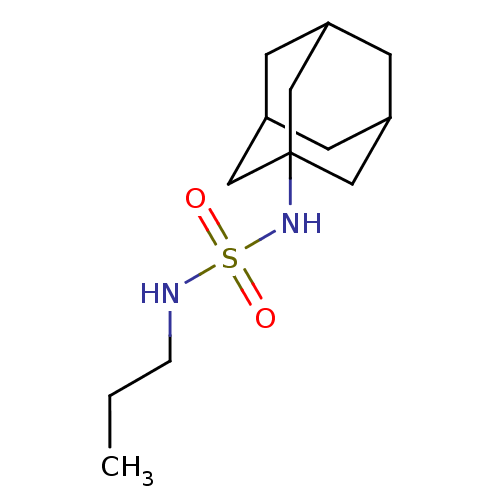 (CHEMBL374746 | N-(1-adamantyl)-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]WIN55212-2 from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300771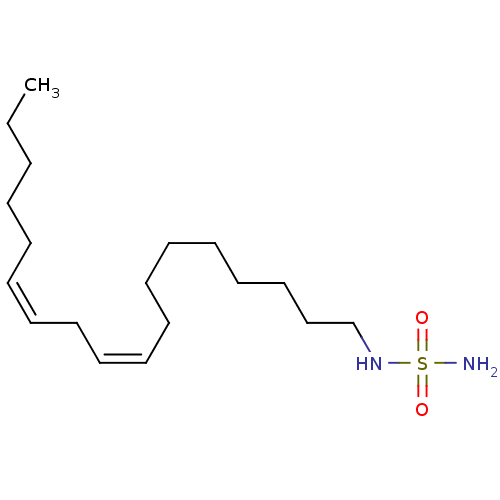 (CHEMBL570988 | N-(cis-9-cis-12-octadecadienyl)sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50202584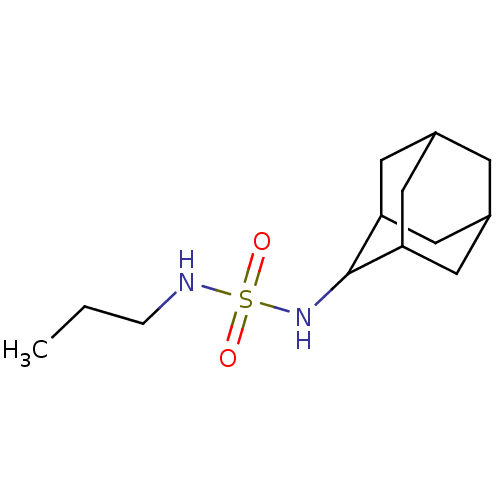 (CHEMBL218643 | N-(2-adamantyl)-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300766 (CHEMBL569658 | N-(cis-9-cis-12-octadecadienyl)-N'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300769 (CHEMBL374746 | N-(1-adamantyl)-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50202583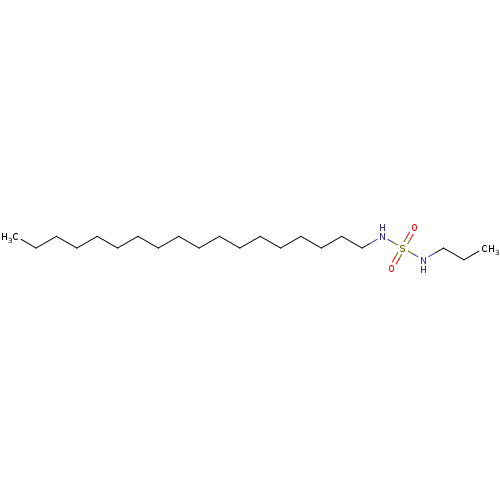 (CHEMBL219156 | N-octadecyl-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50202583 (CHEMBL219156 | N-octadecyl-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]WIN55212-2 from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300764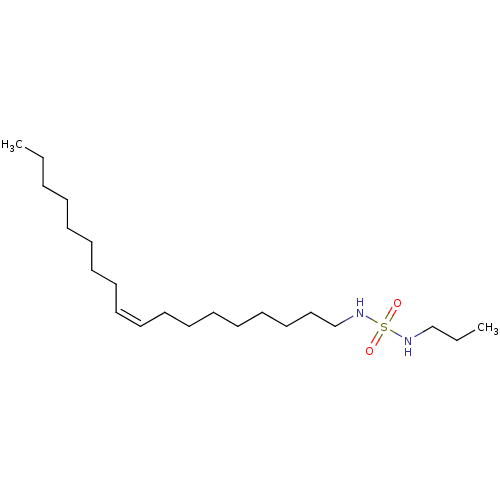 (CHEMBL571044 | N-oleyl-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300768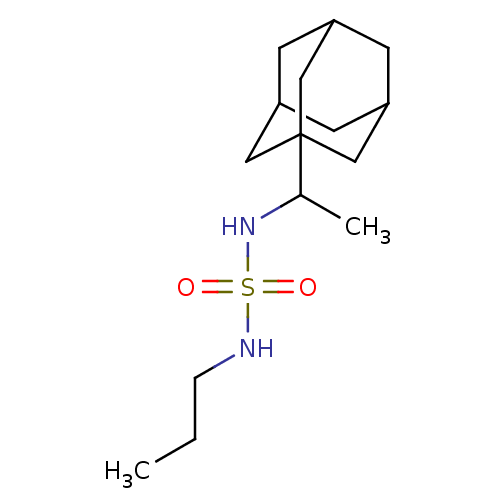 (CHEMBL437157 | N-(1-(1-adamantyl)ethyl)-N'-propyls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50202579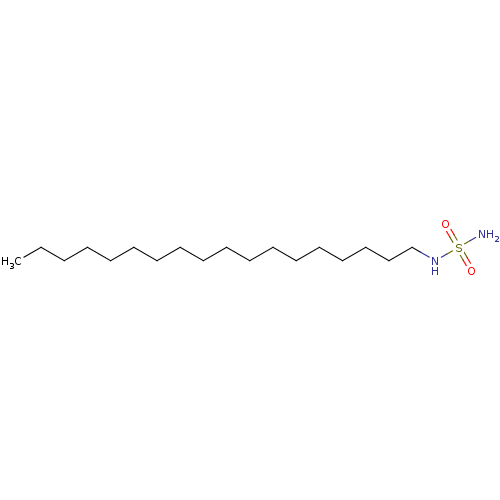 (CHEMBL218794 | N-octadecylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50202584 (CHEMBL218643 | N-(2-adamantyl)-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]WIN55212-2 from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300768 (CHEMBL437157 | N-(1-(1-adamantyl)ethyl)-N'-propyls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]WIN55212-2 from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447027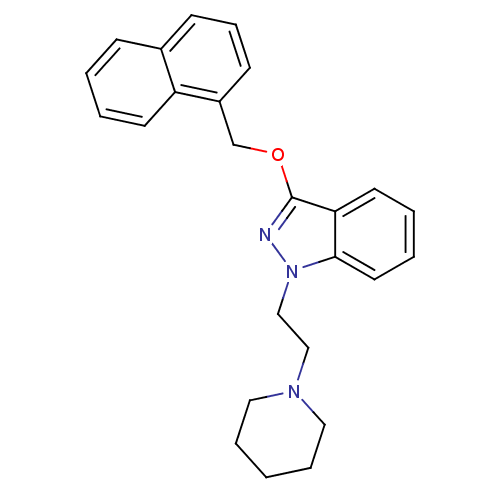 (CHEMBL3116284) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447026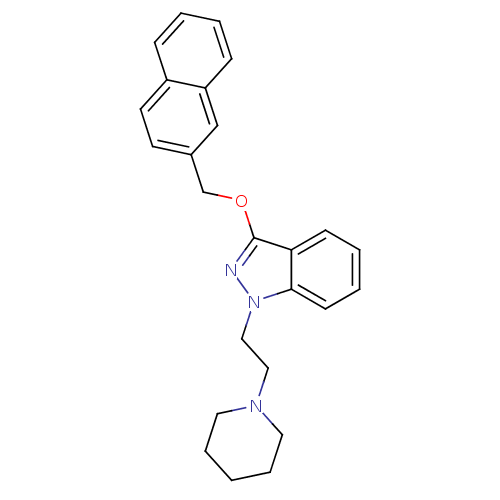 (CHEMBL3116286) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447015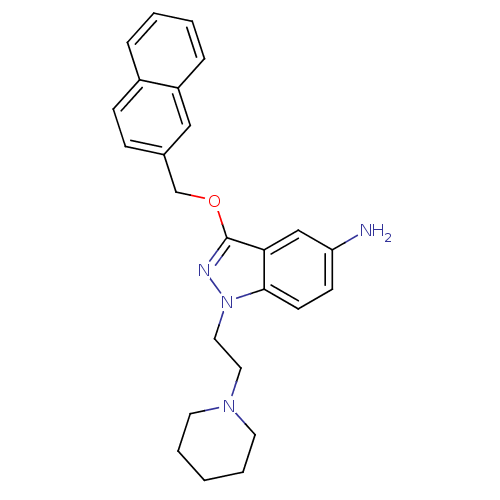 (CHEMBL3116300) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447020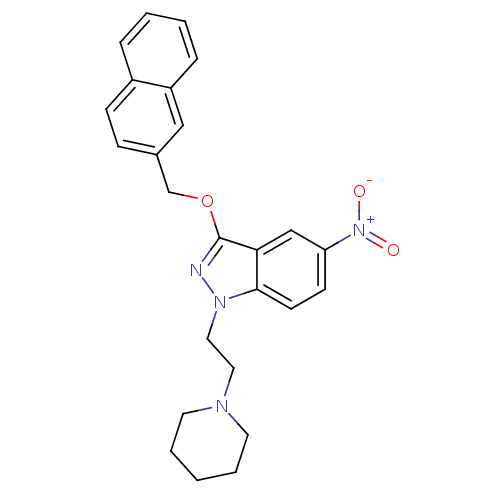 (CHEMBL3116294) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447030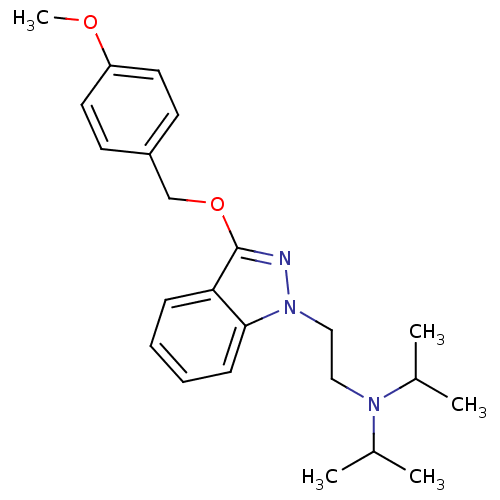 (CHEMBL3116280) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447028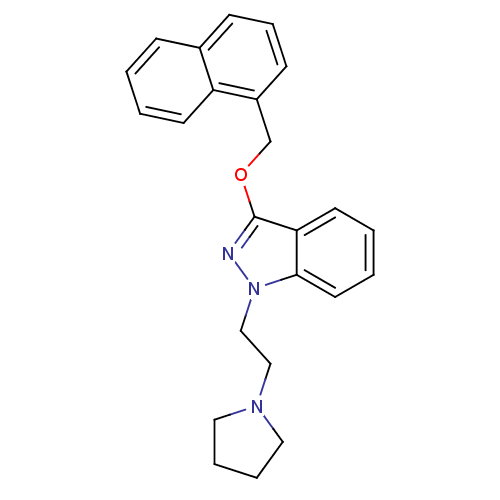 (CHEMBL3116283) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447021 (CHEMBL3116293) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447022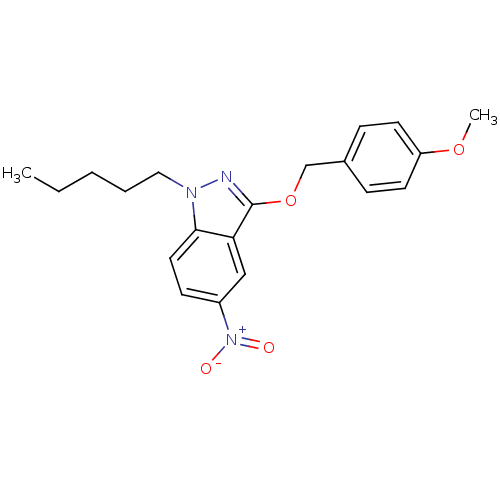 (CHEMBL3116289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447023 (CHEMBL1973869) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447024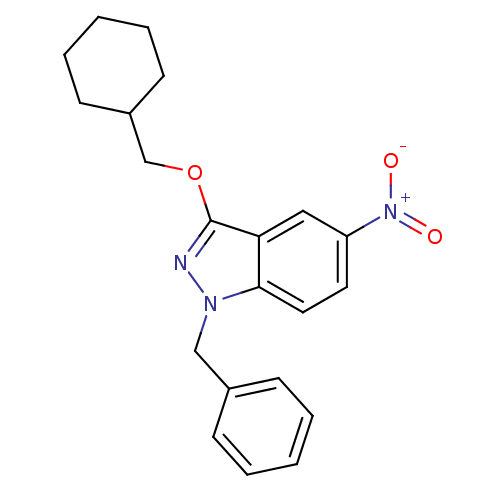 (CHEMBL3116288) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447018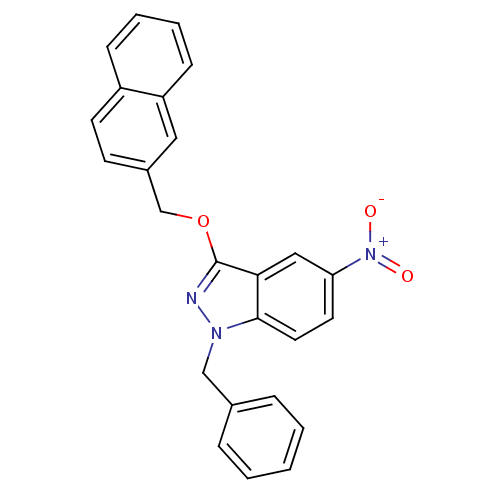 (CHEMBL3116296) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447016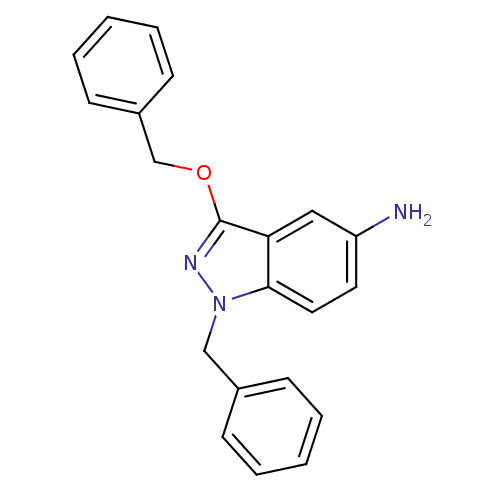 (CHEMBL3116298) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447029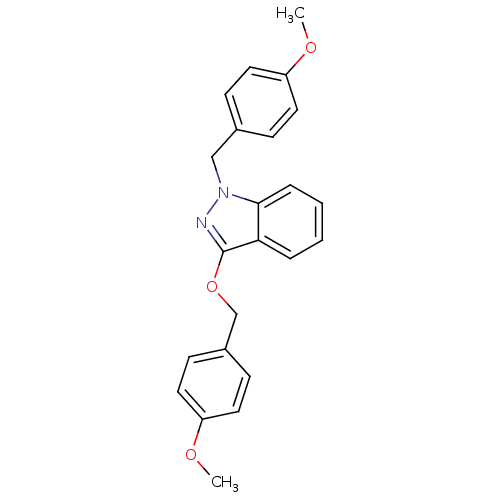 (CHEMBL3116281) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447031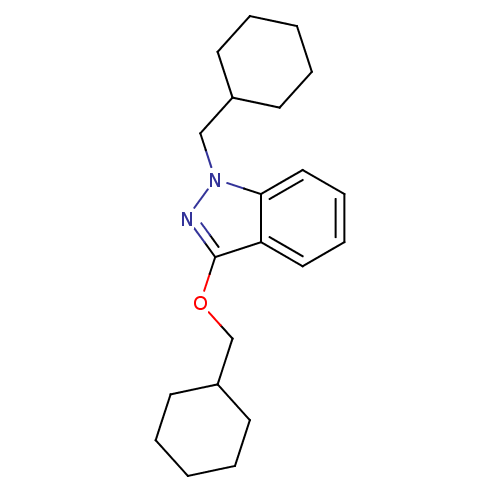 (CHEMBL3116278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447017 (CHEMBL3116297) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447019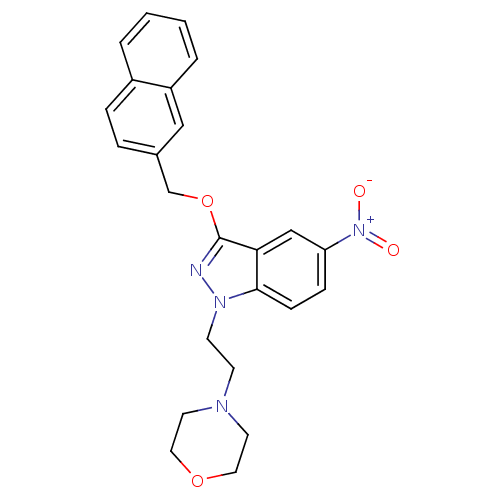 (CHEMBL3116295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447025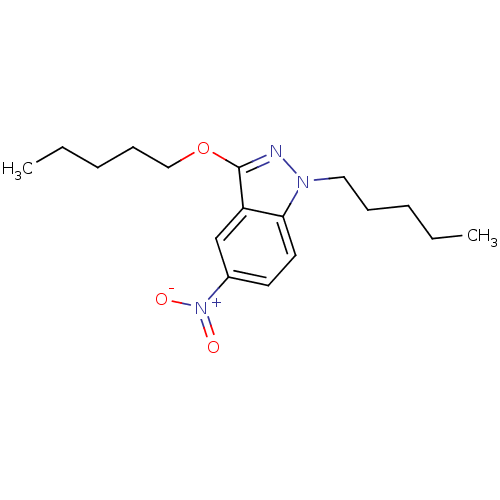 (CHEMBL3116287) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||