 Found 22 hits with Last Name = 'pamment' and Initial = 'm'
Found 22 hits with Last Name = 'pamment' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266775

((2R,4S)-4-(2-Chlorophenyl)-N1-(4-chlorophenyl)-4-h...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccccc1Cl |r| Show InChI InChI=1S/C28H23Cl2N5O4/c29-18-8-10-19(11-9-18)32-27(38)35-17-28(39,21-5-1-2-6-22(21)30)15-23(35)26(37)33-24-13-12-20(16-31-24)34-14-4-3-7-25(34)36/h1-14,16,23,39H,15,17H2,(H,32,38)(H,31,33,37)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266920

((2R,4R)-N1-(4-Chlorophenyl)-4-ethoxy-4-ethyl-N2-(2...)Show SMILES CCO[C@]1(CC)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C27H28ClFN4O4/c1-3-27(37-4-2)16-23(33(17-27)26(36)30-19-10-8-18(28)9-11-19)25(35)31-22-13-12-20(15-21(22)29)32-14-6-5-7-24(32)34/h5-15,23H,3-4,16-17H2,1-2H3,(H,30,36)(H,31,35)/t23-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266921
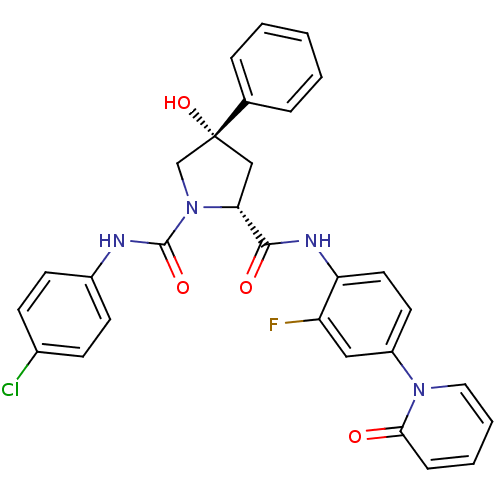
((2R,4S)-N1-(4-Chlorophenyl)-N2-(2-fluoro-4-(2-oxop...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)c1ccccc1 |r| Show InChI InChI=1S/C29H24ClFN4O4/c30-20-9-11-21(12-10-20)32-28(38)35-18-29(39,19-6-2-1-3-7-19)17-25(35)27(37)33-24-14-13-22(16-23(24)31)34-15-5-4-8-26(34)36/h1-16,25,39H,17-18H2,(H,32,38)(H,33,37)/t25-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266923
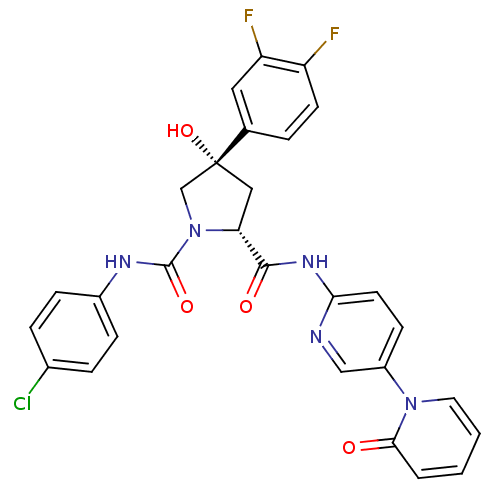
((2R,4S)-N1-(4-Chlorophenyl)-4-(3,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-18-5-7-19(8-6-18)33-27(39)36-16-28(40,17-4-10-21(30)22(31)13-17)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266924
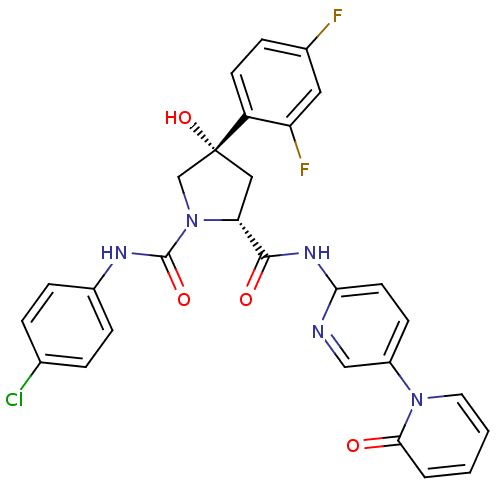
((2R,4S)-N1-(4-Chlorophenyl)-4-(2,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-17-4-7-19(8-5-17)33-27(39)36-16-28(40,21-10-6-18(30)13-22(21)31)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM50266924

((2R,4S)-N1-(4-Chlorophenyl)-4-(2,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-17-4-7-19(8-5-17)33-27(39)36-16-28(40,21-10-6-18(30)13-22(21)31)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.108 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rabbit F10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266773

((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES Cc1ccccc1[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C29H26ClN5O4/c1-19-6-2-3-7-23(19)29(39)16-24(35(18-29)28(38)32-21-11-9-20(30)10-12-21)27(37)33-25-14-13-22(17-31-25)34-15-5-4-8-26(34)36/h2-15,17,24,39H,16,18H2,1H3,(H,32,38)(H,31,33,37)/t24-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266922
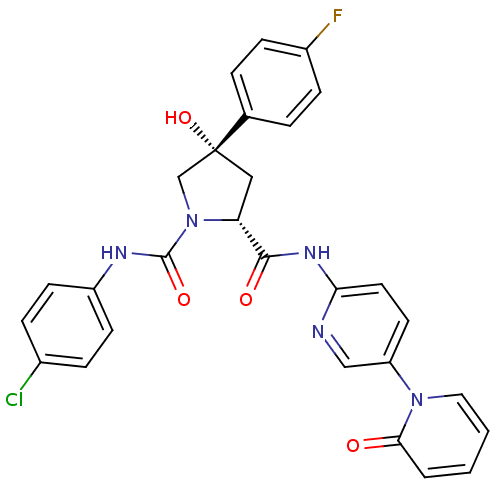
((2R,4S)-N1-(4-Chlorophenyl)-4-(4-fluorophenyl)-4-h...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C28H23ClFN5O4/c29-19-6-10-21(11-7-19)32-27(38)35-17-28(39,18-4-8-20(30)9-5-18)15-23(35)26(37)33-24-13-12-22(16-31-24)34-14-2-1-3-25(34)36/h1-14,16,23,39H,15,17H2,(H,32,38)(H,31,33,37)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266743
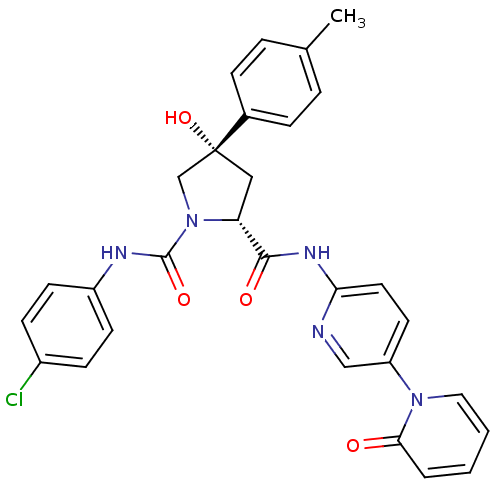
((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES Cc1ccc(cc1)[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C29H26ClN5O4/c1-19-5-7-20(8-6-19)29(39)16-24(35(18-29)28(38)32-22-11-9-21(30)10-12-22)27(37)33-25-14-13-23(17-31-25)34-15-3-2-4-26(34)36/h2-15,17,24,39H,16,18H2,1H3,(H,32,38)(H,31,33,37)/t24-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266893
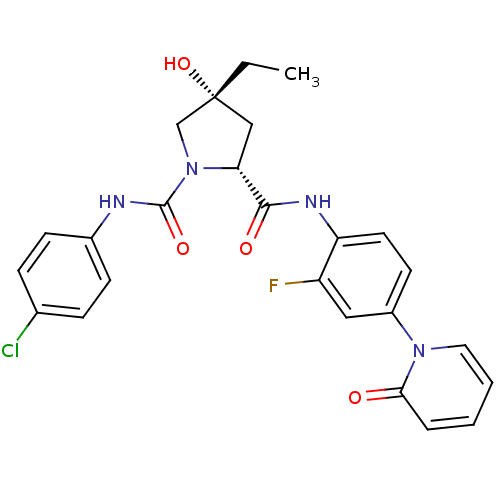
((2R,4R)-N1-(4-Chlorophenyl)-4-ethyl-N2-(2-fluoro-4...)Show SMILES CC[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C25H24ClFN4O4/c1-2-25(35)14-21(31(15-25)24(34)28-17-8-6-16(26)7-9-17)23(33)29-20-11-10-18(13-19(20)27)30-12-4-3-5-22(30)32/h3-13,21,35H,2,14-15H2,1H3,(H,28,34)(H,29,33)/t21-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266919
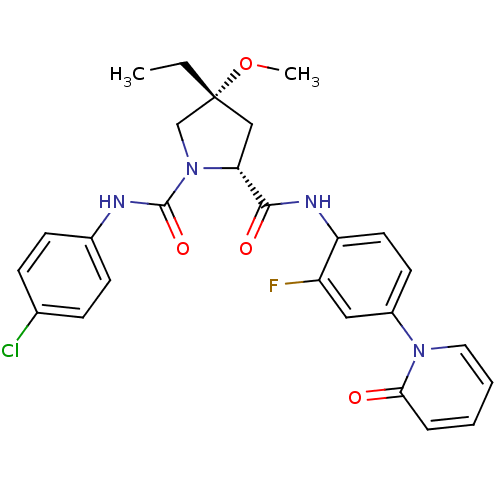
((2R,4R)-N1-(4-Chlorophenyl)-4-ethyl-N2-(2-fluoro-4...)Show SMILES CC[C@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)OC |r| Show InChI InChI=1S/C26H26ClFN4O4/c1-3-26(36-2)15-22(32(16-26)25(35)29-18-9-7-17(27)8-10-18)24(34)30-21-12-11-19(14-20(21)28)31-13-5-4-6-23(31)33/h4-14,22H,3,15-16H2,1-2H3,(H,29,35)(H,30,34)/t22-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266891

((2R,4R)-N1-(4-Chlorophenyl)-N2-(2-fluoro-4-(2-oxop...)Show SMILES CO[C@]1(C)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C25H24ClFN4O4/c1-25(35-2)14-21(31(15-25)24(34)28-17-8-6-16(26)7-9-17)23(33)29-20-11-10-18(13-19(20)27)30-12-4-3-5-22(30)32/h3-13,21H,14-15H2,1-2H3,(H,28,34)(H,29,33)/t21-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266774

((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C29H23ClF3N5O4/c30-18-8-10-19(11-9-18)35-27(41)38-17-28(42,21-5-1-2-6-22(21)29(31,32)33)15-23(38)26(40)36-24-13-12-20(16-34-24)37-14-4-3-7-25(37)39/h1-14,16,23,42H,15,17H2,(H,35,41)(H,34,36,40)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266742
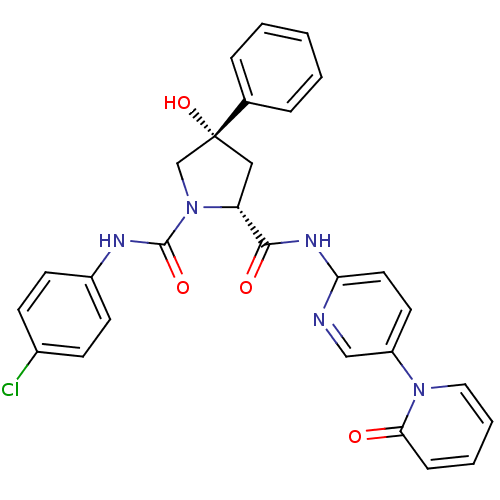
((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccccc1 |r| Show InChI InChI=1S/C28H24ClN5O4/c29-20-9-11-21(12-10-20)31-27(37)34-18-28(38,19-6-2-1-3-7-19)16-23(34)26(36)32-24-14-13-22(17-30-24)33-15-5-4-8-25(33)35/h1-15,17,23,38H,16,18H2,(H,31,37)(H,30,32,36)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266892
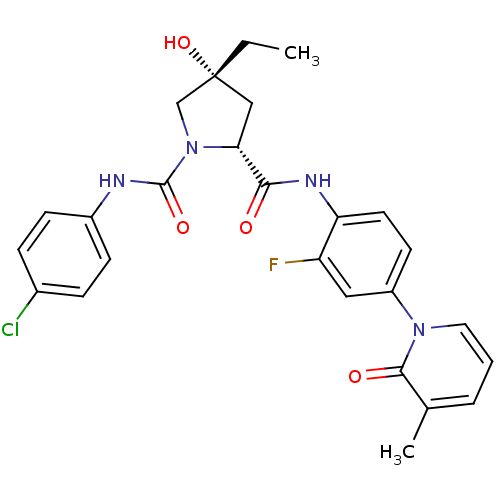
((2R,4R)-N1-(4-Chlorophenyl)-4-ethyl-N2-(2-fluoro-4...)Show SMILES CC[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1cccc(C)c1=O |r| Show InChI InChI=1S/C26H26ClFN4O4/c1-3-26(36)14-22(32(15-26)25(35)29-18-8-6-17(27)7-9-18)23(33)30-21-11-10-19(13-20(21)28)31-12-4-5-16(2)24(31)34/h4-13,22,36H,3,14-15H2,1-2H3,(H,29,35)(H,30,33)/t22-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266890

((2R,4R)-N1-(4-Chlorophenyl)-N2-(2-fluoro-4-(3-meth...)Show SMILES Cc1cccn(-c2ccc(NC(=O)[C@H]3C[C@@](C)(O)CN3C(=O)Nc3ccc(Cl)cc3)c(F)c2)c1=O |r| Show InChI InChI=1S/C25H24ClFN4O4/c1-15-4-3-11-30(23(15)33)18-9-10-20(19(27)12-18)29-22(32)21-13-25(2,35)14-31(21)24(34)28-17-7-5-16(26)6-8-17/h3-12,21,35H,13-14H2,1-2H3,(H,28,34)(H,29,32)/t21-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266770

((2R,4R)-N~1~-(4-CHLOROPHENYL)-N~2~-[2-FLUORO-4-(2-...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C24H22ClFN4O4/c1-34-18-13-21(30(14-18)24(33)27-16-7-5-15(25)6-8-16)23(32)28-20-10-9-17(12-19(20)26)29-11-3-2-4-22(29)31/h2-12,18,21H,13-14H2,1H3,(H,27,33)(H,28,32)/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266772

((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES Cc1cccc(c1)[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C29H26ClN5O4/c1-19-5-4-6-20(15-19)29(39)16-24(35(18-29)28(38)32-22-10-8-21(30)9-11-22)27(37)33-25-13-12-23(17-31-25)34-14-3-2-7-26(34)36/h2-15,17,24,39H,16,18H2,1H3,(H,32,38)(H,31,33,37)/t24-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Rattus norvegicus (rat)) | BDBM50266924

((2R,4S)-N1-(4-Chlorophenyl)-4-(2,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-17-4-7-19(8-5-17)33-27(39)36-16-28(40,21-10-6-18(30)13-22(21)31)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rat F10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266771
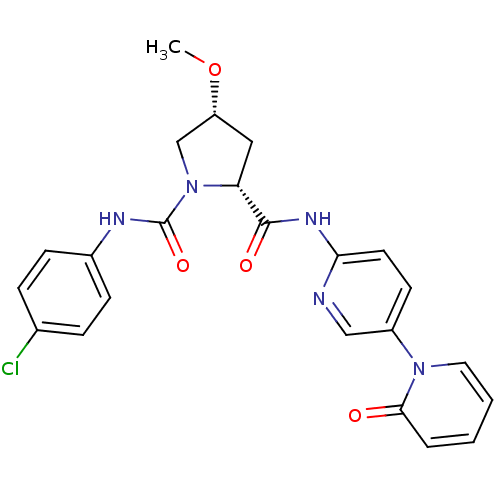
((2R,4R)-N1-(4-chlorophenyl)-4-methoxy-N2-(5-(2-oxo...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C23H22ClN5O4/c1-33-18-12-19(29(14-18)23(32)26-16-7-5-15(24)6-8-16)22(31)27-20-10-9-17(13-25-20)28-11-3-2-4-21(28)30/h2-11,13,18-19H,12,14H2,1H3,(H,26,32)(H,25,27,31)/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50425732
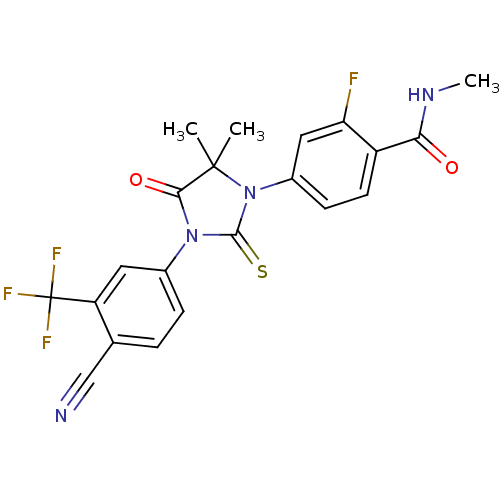
(ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...)Show SMILES CNC(=O)c1ccc(cc1F)N1C(=S)N(C(=O)C1(C)C)c1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C21H16F4N4O2S/c1-20(2)18(31)28(12-5-4-11(10-26)15(8-12)21(23,24)25)19(32)29(20)13-6-7-14(16(22)9-13)17(30)27-3/h4-9H,1-3H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC Frederick, Inc.
Curated by ChEMBL
| Assay Description
Displacement of Fluormone AL Green from androgen receptor ligand binding domain (unknown origin) after 4 hrs by fluorescence polarization assay |
J Med Chem 56: 8280-97 (2013)
Article DOI: 10.1021/jm301714s
BindingDB Entry DOI: 10.7270/Q2FF3TTZ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50441938

(CHEMBL2440233)Show SMILES Fc1ccc(C(=O)O[C@@H]2[C@H](N(C=CC2=O)C(=O)C=Cc2ccccc2)c2ccccc2)c(c1)C(F)(F)F |r,w:17.17,c:11| Show InChI InChI=1S/C28H19F4NO4/c29-20-12-13-21(22(17-20)28(30,31)32)27(36)37-26-23(34)15-16-33(25(26)19-9-5-2-6-10-19)24(35)14-11-18-7-3-1-4-8-18/h1-17,25-26H/t25-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC Frederick, Inc.
Curated by ChEMBL
| Assay Description
Displacement of Fluormone AL Green from androgen receptor ligand binding domain (unknown origin) after 4 hrs by fluorescence polarization assay |
J Med Chem 56: 8280-97 (2013)
Article DOI: 10.1021/jm301714s
BindingDB Entry DOI: 10.7270/Q2FF3TTZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data