

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10439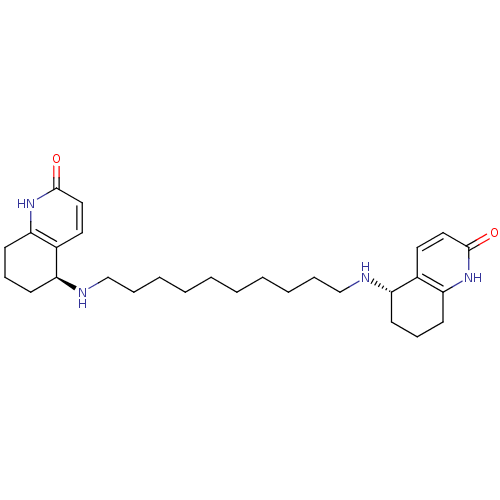 ((5S)-5-[(10-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.800 | -51.4 | 2.40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50007400 (4-Phenyl-1-(4-phenyl-butyl)-piperidine | 4-Phenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]-haloperidol in the presence of 25 nM unlabeled spiperone | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10440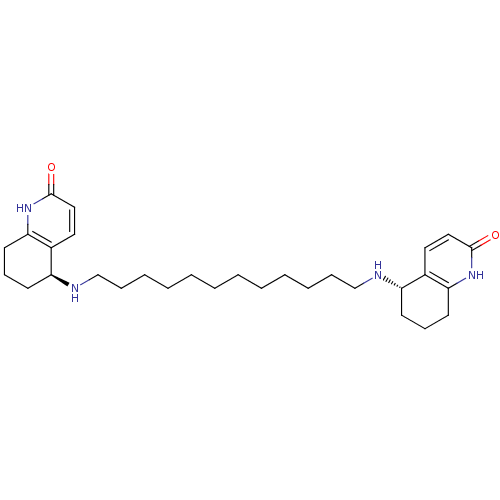 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.5 | -47.2 | 16 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50002245 (4-[2-(1-Cyclopropylmethyl-piperidin-4-yl)-acetyl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]- haloperidol | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50002218 (2-(1-Cyclopropylmethyl-piperidin-4-yl)-1-(4-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]- haloperidol | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10440 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 19.6 | -43.6 | 52 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 47.1 | -41.4 | 114 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 175 | -38.2 | 414 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50002245 (4-[2-(1-Cyclopropylmethyl-piperidin-4-yl)-acetyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against Dopamine receptor D2 in bovine striatal homogenate using 200 pM [3H]- spiperone and 250 nM unlabeled ketanserin | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50002218 (2-(1-Cyclopropylmethyl-piperidin-4-yl)-1-(4-fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against Dopamine receptor D2 in bovine striatal homogenate using 200 pM [3H]- spiperone and 250 nM unlabeled ketanserin | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50036730 (3-[4-(4-Chloro-phenyl)-3,6-dihydro-2H-pyridin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]-haloperidol in the presence of 25 nM unlabeled spiperone | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10469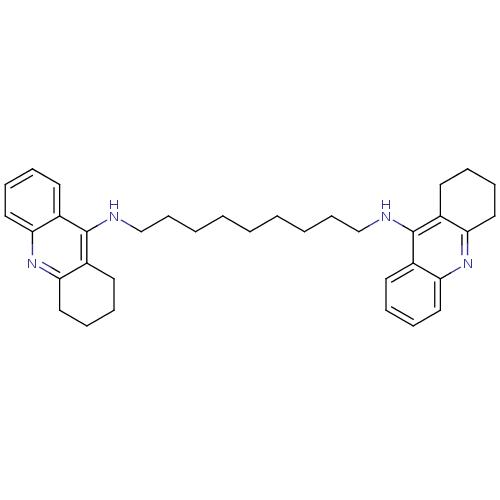 (Bis-THA inhibitor 1c | Bis-THA inhibitor 8 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mayo Foundation for Medical Education and Research | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Biol Chem 271: 23646-9 (1996) Article DOI: 10.1074/jbc.271.39.23646 BindingDB Entry DOI: 10.7270/Q2XW4H0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10470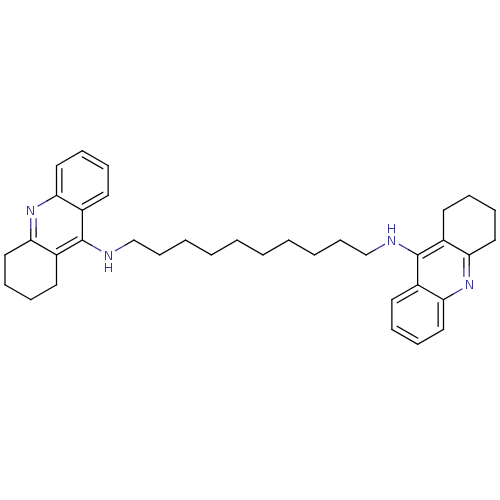 (Bis-THA inhibitor 1d | Bis-THA inhibitor 9 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mayo Foundation for Medical Education and Research | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Biol Chem 271: 23646-9 (1996) Article DOI: 10.1074/jbc.271.39.23646 BindingDB Entry DOI: 10.7270/Q2XW4H0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9047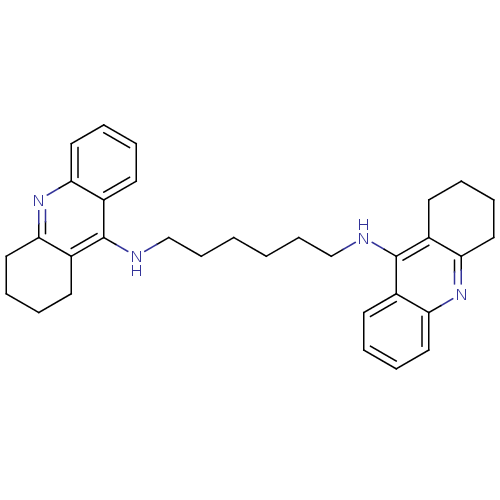 (Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9047 (Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50036722 (3-[4-(4-Fluoro-phenyl)-3,6-dihydro-2H-pyridin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]-haloperidol in the presence of 25 nM unlabeled spiperone | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50036729 (1-Phenyl-3-(4-phenyl-piperidin-1-yl)-propan-1-one ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]-haloperidol in the presence of 25 nM unlabeled spiperone | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8964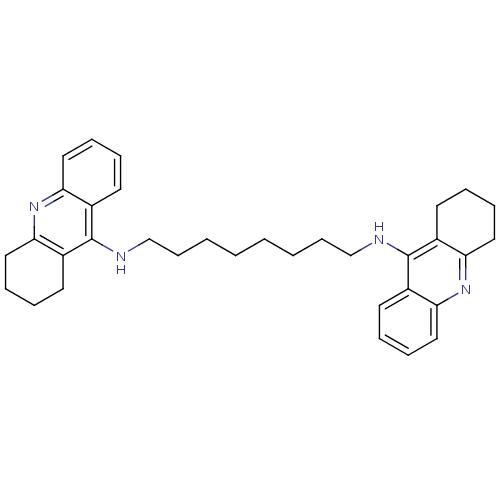 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8964 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50036716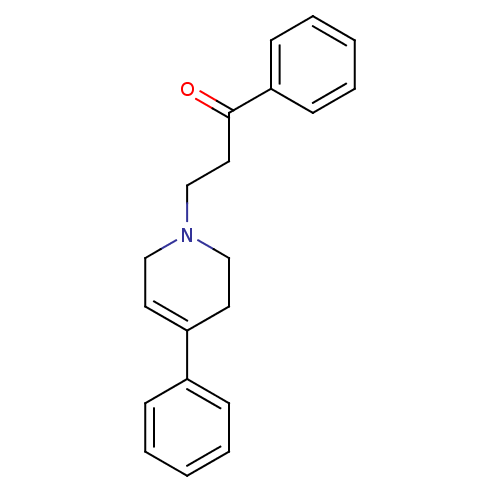 (1-Phenyl-3-(4-phenyl-3,6-dihydro-2H-pyridin-1-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]-haloperidol in the presence of 25 nM unlabeled spiperone | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50080157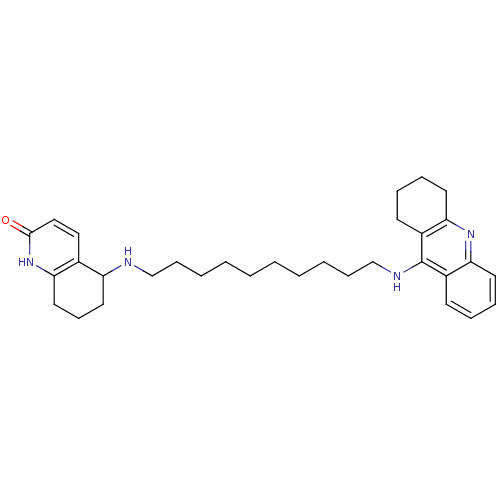 ((RS)-tacrine(10)-hupyridone | 5-[10-(1,2,3,4-Tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9441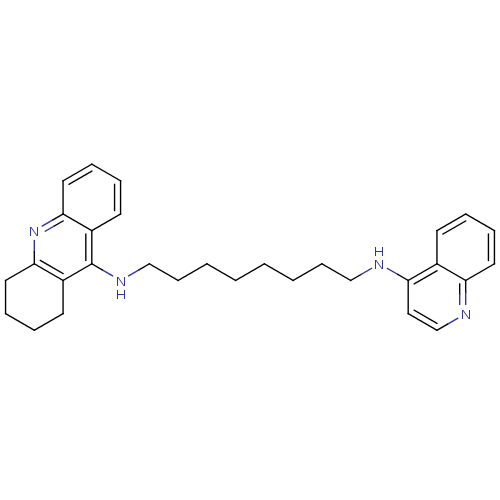 (CHEMBL1082738 | Heterodimeric Tacrine-Based Inhibi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9440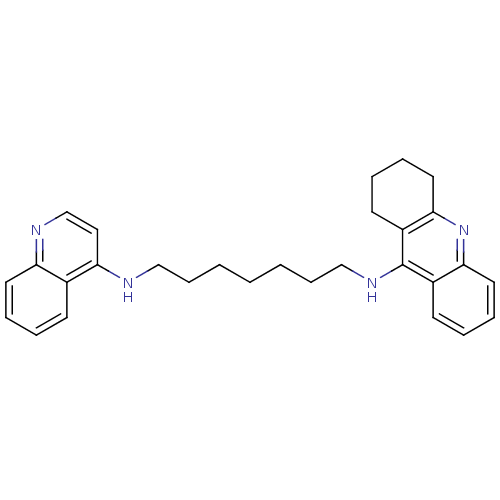 (CHEMBL1084775 | Heterodimeric Tacrine-Based Inhibi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9435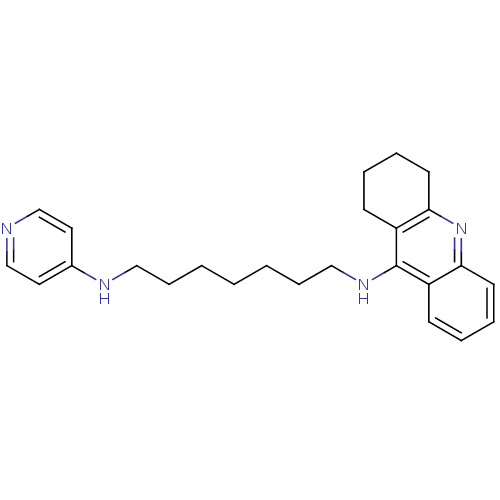 (CHEMBL1084369 | Heterodimeric Tacrine-Based Inhibi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.8 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9436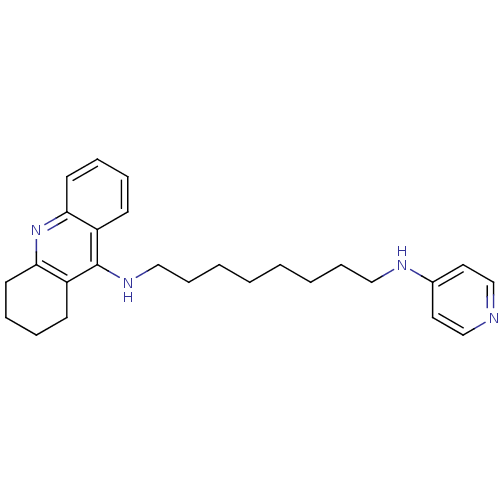 (CHEMBL1084096 | Heterodimeric Tacrine-Based Inhibi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.6 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50036711 (1-(4-Fluoro-phenyl)-3-[4-(4-fluoro-phenyl)-3,6-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]-haloperidol in the presence of 25 nM unlabeled spiperone | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50036724 (1-(4-Chloro-phenyl)-3-[4-(4-fluoro-phenyl)-3,6-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]-haloperidol in the presence of 25 nM unlabeled spiperone | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50080160 (5-[8-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-octyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8962 (Bis-THA inhibitor 4 | CHEMBL179732 | N-[5-(1,2,3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8962 (Bis-THA inhibitor 4 | CHEMBL179732 | N-[5-(1,2,3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10469 (Bis-THA inhibitor 1c | Bis-THA inhibitor 8 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10469 (Bis-THA inhibitor 1c | Bis-THA inhibitor 8 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50080162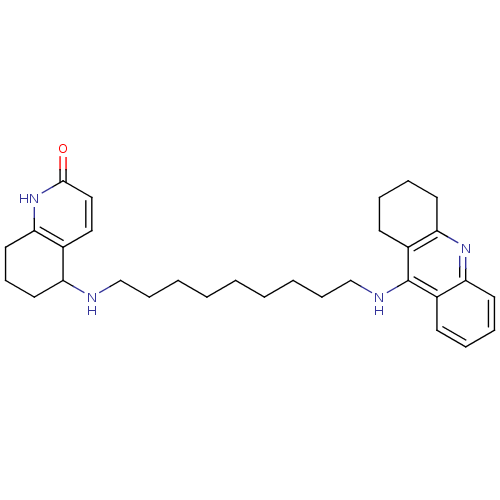 (5-[9-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-nonyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50366608 (CHEMBL1202834) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9442 (CHEMBL1082739 | Heterodimeric Tacrine-Based Inhibi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10470 (Bis-THA inhibitor 1d | Bis-THA inhibitor 9 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10470 (Bis-THA inhibitor 1d | Bis-THA inhibitor 9 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50366606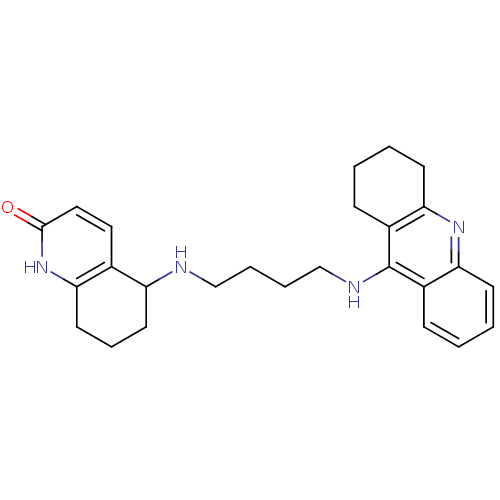 (CHEMBL1202835) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Biol Chem 271: 23646-9 (1996) Article DOI: 10.1074/jbc.271.39.23646 BindingDB Entry DOI: 10.7270/Q2XW4H0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the action of acetylcholinesterase isolated from rat brain cortex | J Med Chem 34: 3399-402 (1992) BindingDB Entry DOI: 10.7270/Q2TB17HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the action of acetylcholinesterase isolated from rat brain cortex | J Med Chem 34: 3399-402 (1992) BindingDB Entry DOI: 10.7270/Q2TB17HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9433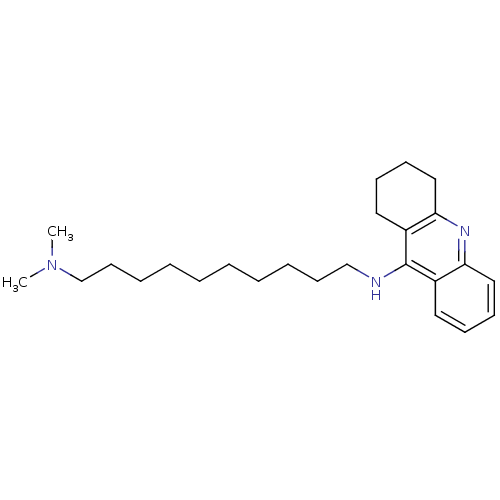 (CHEMBL1084367 | Heterodimeric Tacrine-Based Inhibi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9431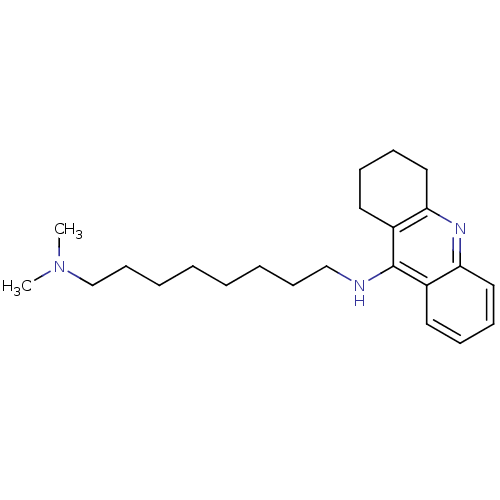 (CHEMBL1084094 | Heterodimeric Tacrine-Based Inhibi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50036712 (4-(4-Chloro-phenyl)-1,2,3,6-tetrahydro-pyridine | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]-haloperidol in the presence of 25 nM unlabeled spiperone | J Med Chem 36: 3923-8 (1994) BindingDB Entry DOI: 10.7270/Q2H13122 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9430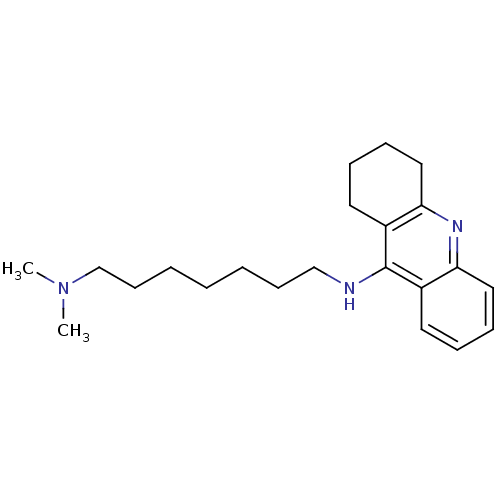 (CHEMBL1083791 | Heterodimeric Tacrine-Based Inhibi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50366605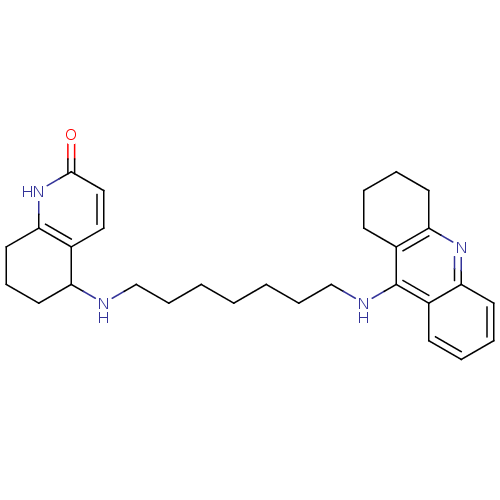 (CHEMBL1202836) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10446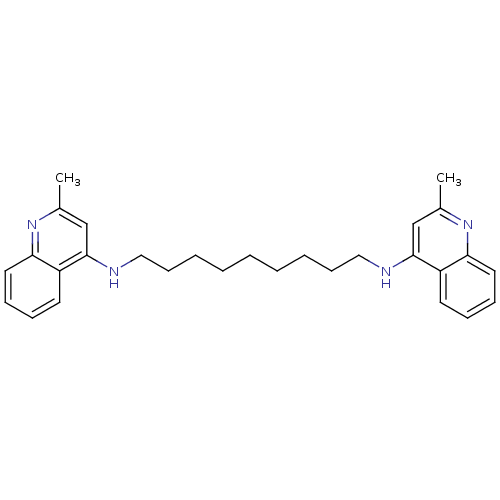 (2-methyl-N-{9-[(2-methylquinolin-4-yl)amino]nonyl}...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54.1 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 2569-75 (1999) Article DOI: 10.1016/s0968-0896(99)00178-9 BindingDB Entry DOI: 10.7270/Q22N50GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 235 total ) | Next | Last >> |