 Found 149 hits with Last Name = 'pang' and Initial = 'r'
Found 149 hits with Last Name = 'pang' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
4,4'-diapophytoene synthase
(Staphylococcus aureus) | BDBM50388903

(CHEMBL2063253)Show InChI InChI=1S/C19H19NO5/c21-17(19(23)24)13-18(22)20-11-5-7-14-6-4-10-16(12-14)25-15-8-2-1-3-9-15/h1-4,6,8-10,12H,5,7,11,13H2,(H,20,22)(H,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus His6-tagged CrtM expressed in Escherichia coli BL21(DE3) using Farnesyl pyrophosphate as substrate incubated for ... |
ACS Med Chem Lett 3: 402-406 (2012)
Article DOI: 10.1021/ml300038t
BindingDB Entry DOI: 10.7270/Q2XW4KVQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
4,4'-diapophytoene synthase
(Staphylococcus aureus) | BDBM50388398
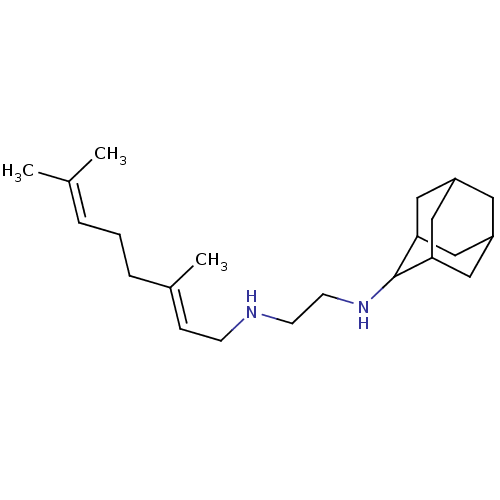
(CHEMBL561057 | SQ-109)Show SMILES CC(C)=CCC\C(C)=C\CNCCNC1C2CC3CC(C2)CC1C3 |TLB:18:19:16.17.23:14,23:22:20:16.17.18,13:14:20:16.17.18,13:14:16.17.23:20.19.21,THB:18:17:14:20.19.21,23:17:20:22.14.21,21:19:16:23.22.14,21:22:16:20.18.19,13:14:16:20.18.19,(-5.14,2.27,;-5.13,1.44,;-5.84,1.02,;-4.41,1.04,;-3.7,1.46,;-2.98,1.06,;-2.28,1.48,;-2.29,2.3,;-1.56,1.08,;-.85,1.5,;-.13,1.09,;.58,1.51,;1.3,1.11,;2.04,1.48,;2.72,1.02,;2.74,.22,;2.11,-.47,;2.91,-.24,;3.67,-.52,;4.2,.17,;3.46,-.03,;4.19,.99,;3.44,1.28,;2.89,.61,)| Show InChI InChI=1S/C22H38N2/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-24H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus ATCC 27659 dehydrosqualene synthase expressed in Escherichia coli BL21(DE3) after 30 mins by spectrophotometric a... |
J Med Chem 55: 4367-72 (2012)
Article DOI: 10.1021/jm300208p
BindingDB Entry DOI: 10.7270/Q2Z320QH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
4,4'-diapophytoene synthase
(Staphylococcus aureus) | BDBM50388906

(CHEMBL2063256)Show InChI InChI=1S/C19H23NO5/c1-2-3-4-5-11-25-15-8-6-7-14(12-15)13-20-10-9-16(21)17(18(20)22)19(23)24/h6-10,12,21H,2-5,11,13H2,1H3,(H,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus His6-tagged CrtM expressed in Escherichia coli BL21(DE3) using Farnesyl pyrophosphate as substrate incubated for ... |
ACS Med Chem Lett 3: 402-406 (2012)
Article DOI: 10.1021/ml300038t
BindingDB Entry DOI: 10.7270/Q2XW4KVQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Squalene synthase
(Homo sapiens (Human)) | BDBM50388398

(CHEMBL561057 | SQ-109)Show SMILES CC(C)=CCC\C(C)=C\CNCCNC1C2CC3CC(C2)CC1C3 |TLB:18:19:16.17.23:14,23:22:20:16.17.18,13:14:20:16.17.18,13:14:16.17.23:20.19.21,THB:18:17:14:20.19.21,23:17:20:22.14.21,21:19:16:23.22.14,21:22:16:20.18.19,13:14:16:20.18.19,(-5.14,2.27,;-5.13,1.44,;-5.84,1.02,;-4.41,1.04,;-3.7,1.46,;-2.98,1.06,;-2.28,1.48,;-2.29,2.3,;-1.56,1.08,;-.85,1.5,;-.13,1.09,;.58,1.51,;1.3,1.11,;2.04,1.48,;2.72,1.02,;2.74,.22,;2.11,-.47,;2.91,-.24,;3.67,-.52,;4.2,.17,;3.46,-.03,;4.19,.99,;3.44,1.28,;2.89,.61,)| Show InChI InChI=1S/C22H38N2/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-24H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human squalene synthase |
J Med Chem 55: 4367-72 (2012)
Article DOI: 10.1021/jm300208p
BindingDB Entry DOI: 10.7270/Q2Z320QH |
More data for this
Ligand-Target Pair | |
4,4'-diapophytoene synthase
(Staphylococcus aureus) | BDBM50388397

(CHEMBL39581)Show InChI InChI=1S/C15H13NO2S/c16-12-19-11-10-17-13-6-8-15(9-7-13)18-14-4-2-1-3-5-14/h1-9H,10-11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus ATCC 27659 dehydrosqualene synthase expressed in Escherichia coli BL21(DE3) after 30 mins by spectrophotometric a... |
J Med Chem 55: 4367-72 (2012)
Article DOI: 10.1021/jm300208p
BindingDB Entry DOI: 10.7270/Q2Z320QH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM2198

((3-(2-tert-Butyl-5-methyl-phenylsulfanyl)-4-hydrox...)Show SMILES CC(C)C1(CCc2ccccc2)CC(=O)C(Sc2cc(C)ccc2C(C)(C)C)C(=O)O1 Show InChI InChI=1S/C27H34O3S/c1-18(2)27(15-14-20-10-8-7-9-11-20)17-22(28)24(25(29)30-27)31-23-16-19(3)12-13-21(23)26(4,5)6/h7-13,16,18,24H,14-15,17H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human immunodeficiency virus 1 protease in MT-4 cells |
Bioorg Med Chem Lett 15: 3257-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.057
BindingDB Entry DOI: 10.7270/Q20V8C9V |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50158830
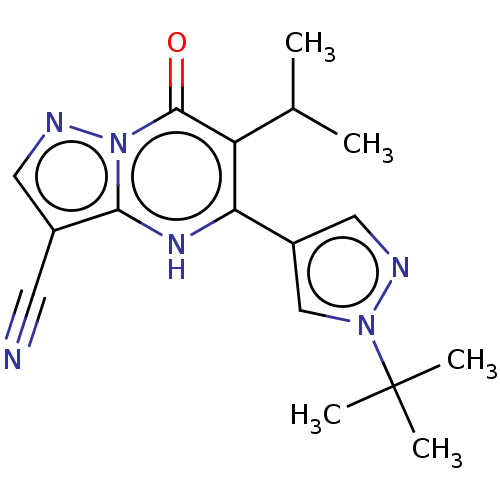
(CHEMBL3785583)Show SMILES CC(C)c1c([nH]c2c(cnn2c1=O)C#N)-c1cnn(c1)C(C)(C)C Show InChI InChI=1S/C17H20N6O/c1-10(2)13-14(12-8-19-22(9-12)17(3,4)5)21-15-11(6-18)7-20-23(15)16(13)24/h7-10,21H,1-5H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of KDM5B (unknown origin) |
Eur J Med Chem 161: 131-140 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.040
BindingDB Entry DOI: 10.7270/Q2RB7853 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM195608
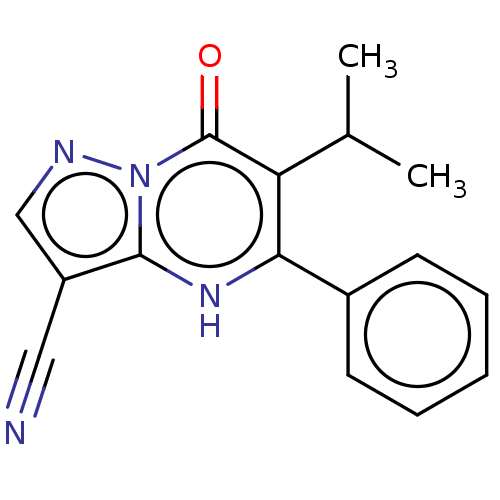
(CPI-455)Show InChI InChI=1S/C16H14N4O/c1-10(2)13-14(11-6-4-3-5-7-11)19-15-12(8-17)9-18-20(15)16(13)21/h3-7,9-10,19H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of KDM5B (unknown origin) |
Eur J Med Chem 161: 131-140 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.040
BindingDB Entry DOI: 10.7270/Q2RB7853 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50168218
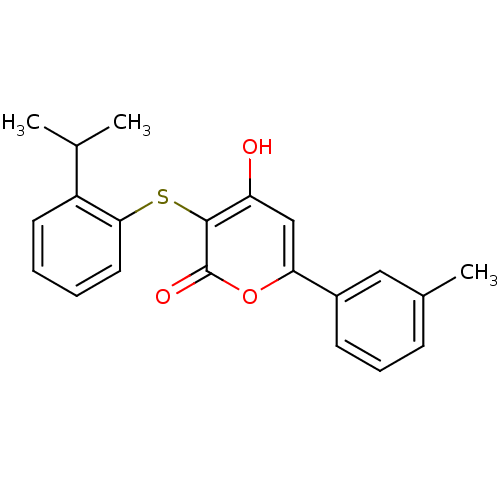
(4-Hydroxy-3-(2-isopropyl-phenylsulfanyl)-6-m-tolyl...)Show InChI InChI=1S/C21H20O3S/c1-13(2)16-9-4-5-10-19(16)25-20-17(22)12-18(24-21(20)23)15-8-6-7-14(3)11-15/h4-13,22H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human immunodeficiency virus 1 protease in MT-4 cells |
Bioorg Med Chem Lett 15: 3257-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.057
BindingDB Entry DOI: 10.7270/Q20V8C9V |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50153092
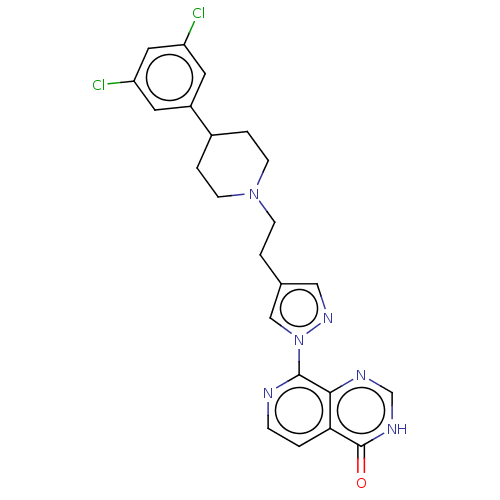
(CHEMBL3775894)Show SMILES Clc1cc(Cl)cc(c1)C1CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1 Show InChI InChI=1S/C23H22Cl2N6O/c24-18-9-17(10-19(25)11-18)16-3-7-30(8-4-16)6-2-15-12-29-31(13-15)22-21-20(1-5-26-22)23(32)28-14-27-21/h1,5,9-14,16H,2-4,6-8H2,(H,27,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of KDM5B (unknown origin) |
Eur J Med Chem 161: 131-140 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.040
BindingDB Entry DOI: 10.7270/Q2RB7853 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM223320
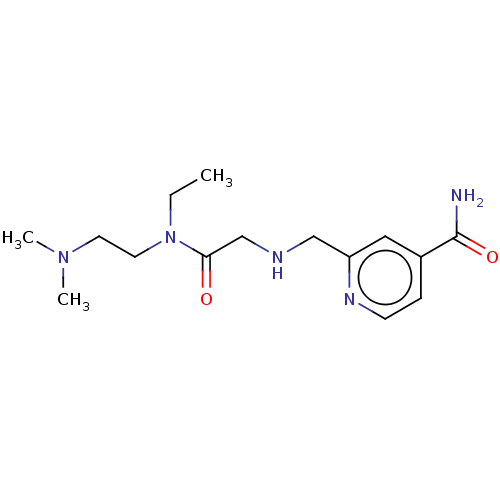
(KDOAM-25)Show InChI InChI=1S/C15H25N5O2/c1-4-20(8-7-19(2)3)14(21)11-17-10-13-9-12(15(16)22)5-6-18-13/h5-6,9,17H,4,7-8,10-11H2,1-3H3,(H2,16,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of KDM5B (unknown origin) |
Eur J Med Chem 161: 131-140 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.040
BindingDB Entry DOI: 10.7270/Q2RB7853 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50540061
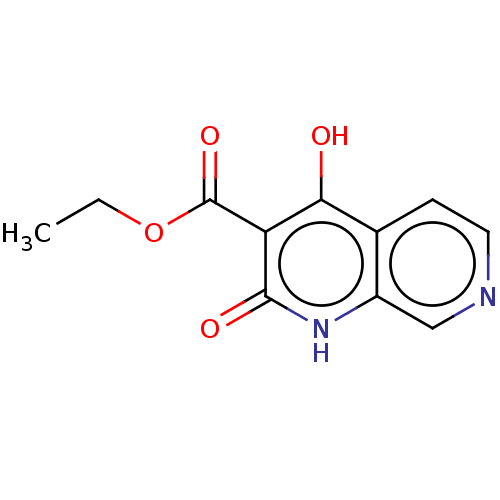
(CHEMBL4637168)Show InChI InChI=1S/C11H10N2O4/c1-2-17-11(16)8-9(14)6-3-4-12-5-7(6)13-10(8)15/h3-5H,2H2,1H3,(H2,13,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of KDM5B (unknown origin) |
Eur J Med Chem 161: 131-140 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.040
BindingDB Entry DOI: 10.7270/Q2RB7853 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428292

(CHEMBL2338356)Show SMILES CCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C9H18N2O7P2/c1-2-3-4-10-5-6-11(8-10)7-9(12,19(13,14)15)20(16,17)18/h5-6,8,12H,2-4,7H2,1H3,(H3-,13,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428292

(CHEMBL2338356)Show SMILES CCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C9H18N2O7P2/c1-2-3-4-10-5-6-11(8-10)7-9(12,19(13,14)15)20(16,17)18/h5-6,8,12H,2-4,7H2,1H3,(H3-,13,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428291
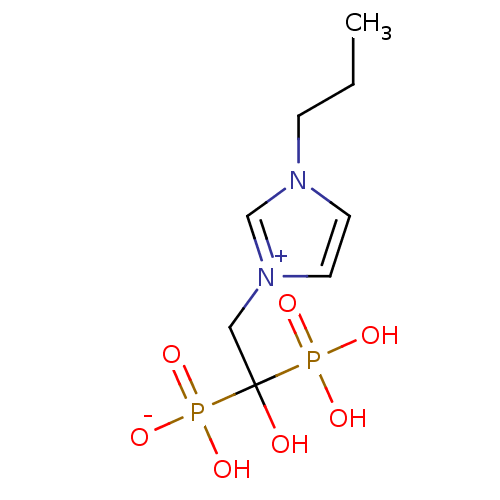
(CHEMBL2338355)Show SMILES CCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C8H16N2O7P2/c1-2-3-9-4-5-10(7-9)6-8(11,18(12,13)14)19(15,16)17/h4-5,7,11H,2-3,6H2,1H3,(H3-,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054619
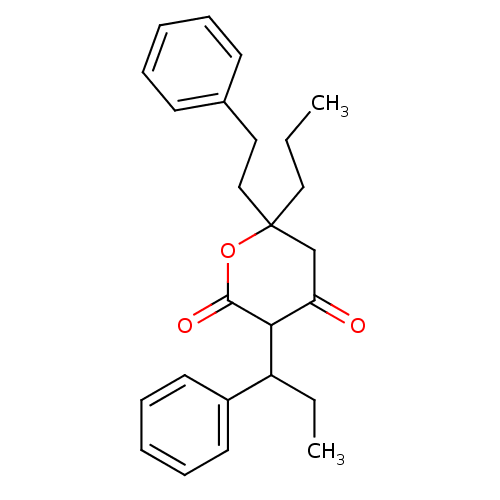
(4-Hydroxy-6-phenethyl-3-(1-phenyl-propyl)-6-propyl...)Show SMILES CCCC1(CCc2ccccc2)CC(=O)C(C(CC)c2ccccc2)C(=O)O1 Show InChI InChI=1S/C25H30O3/c1-3-16-25(17-15-19-11-7-5-8-12-19)18-22(26)23(24(27)28-25)21(4-2)20-13-9-6-10-14-20/h5-14,21,23H,3-4,15-18H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human immunodeficiency virus 1 protease in MT-4 cells |
Bioorg Med Chem Lett 15: 3257-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.057
BindingDB Entry DOI: 10.7270/Q20V8C9V |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428296

(CHEMBL2338360)Show SMILES CCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C13H26N2O7P2/c1-2-3-4-5-6-7-8-14-9-10-15(12-14)11-13(16,23(17,18)19)24(20,21)22/h9-10,12,16H,2-8,11H2,1H3,(H3-,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428295

(CHEMBL2338359)Show SMILES CCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C12H24N2O7P2/c1-2-3-4-5-6-7-13-8-9-14(11-13)10-12(15,22(16,17)18)23(19,20)21/h8-9,11,15H,2-7,10H2,1H3,(H3-,16,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428312
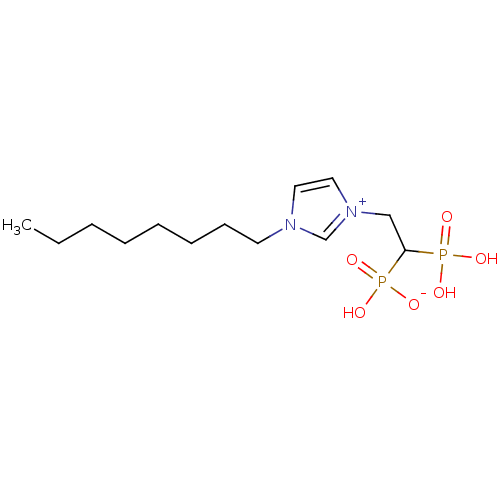
(CHEMBL2338376)Show SMILES CCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C13H26N2O6P2/c1-2-3-4-5-6-7-8-14-9-10-15(12-14)11-13(22(16,17)18)23(19,20)21/h9-10,12-13H,2-8,11H2,1H3,(H3-,16,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428313

(CHEMBL2338377)Show SMILES CCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C14H28N2O6P2/c1-2-3-4-5-6-7-8-9-15-10-11-16(13-15)12-14(23(17,18)19)24(20,21)22/h10-11,13-14H,2-9,12H2,1H3,(H3-,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428311

(CHEMBL2338375)Show SMILES CCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C12H24N2O6P2/c1-2-3-4-5-6-7-13-8-9-14(11-13)10-12(21(15,16)17)22(18,19)20/h8-9,11-12H,2-7,10H2,1H3,(H3-,15,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428294

(CHEMBL2338358)Show SMILES CCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C11H22N2O7P2/c1-2-3-4-5-6-12-7-8-13(10-12)9-11(14,21(15,16)17)22(18,19)20/h7-8,10,14H,2-6,9H2,1H3,(H3-,15,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428290

(CHEMBL2338354)Show InChI InChI=1S/C7H14N2O7P2/c1-2-8-3-4-9(6-8)5-7(10,17(11,12)13)18(14,15)16/h3-4,6,10H,2,5H2,1H3,(H3-,11,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428293
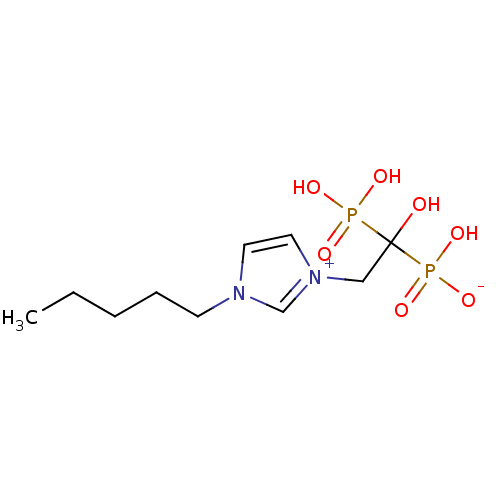
(CHEMBL2338357)Show SMILES CCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C10H20N2O7P2/c1-2-3-4-5-11-6-7-12(9-11)8-10(13,20(14,15)16)21(17,18)19/h6-7,9,13H,2-5,8H2,1H3,(H3-,14,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428310
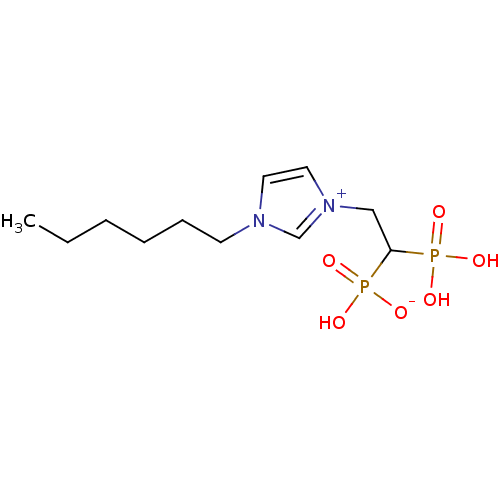
(CHEMBL2338374)Show SMILES CCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C11H22N2O6P2/c1-2-3-4-5-6-12-7-8-13(10-12)9-11(20(14,15)16)21(17,18)19/h7-8,10-11H,2-6,9H2,1H3,(H3-,14,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428307
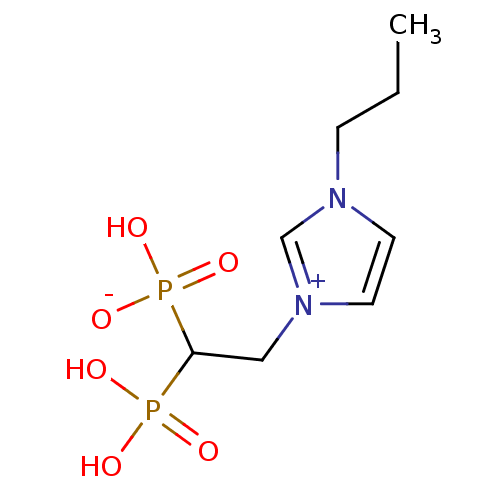
(CHEMBL2338371)Show InChI InChI=1S/C8H16N2O6P2/c1-2-3-9-4-5-10(7-9)6-8(17(11,12)13)18(14,15)16/h4-5,7-8H,2-3,6H2,1H3,(H3-,11,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428308
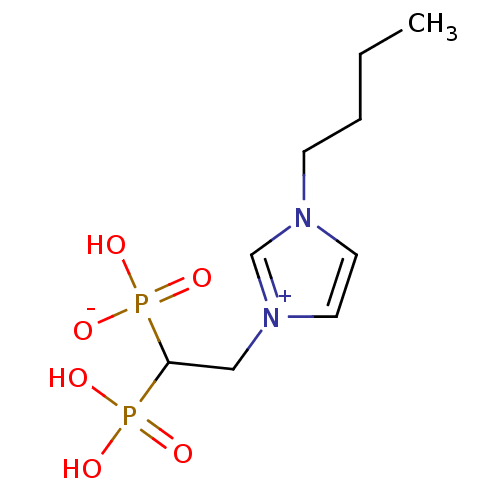
(CHEMBL2338372)Show InChI InChI=1S/C9H18N2O6P2/c1-2-3-4-10-5-6-11(8-10)7-9(18(12,13)14)19(15,16)17/h5-6,8-9H,2-4,7H2,1H3,(H3-,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428306

(CHEMBL2338370)Show InChI InChI=1S/C7H14N2O6P2/c1-2-8-3-4-9(6-8)5-7(16(10,11)12)17(13,14)15/h3-4,6-7H,2,5H2,1H3,(H3-,10,11,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428289
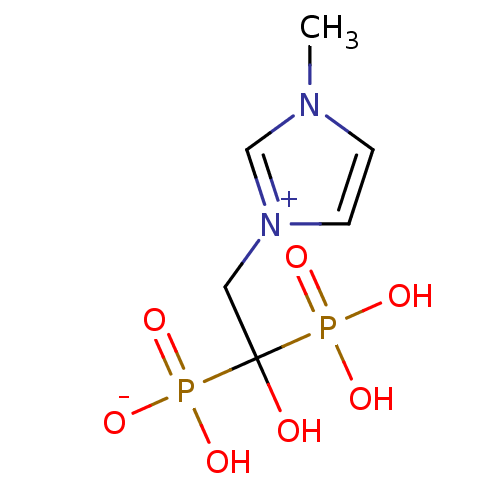
(CHEMBL2338353)Show InChI InChI=1S/C6H12N2O7P2/c1-7-2-3-8(5-7)4-6(9,16(10,11)12)17(13,14)15/h2-3,5,9H,4H2,1H3,(H3-,10,11,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428297
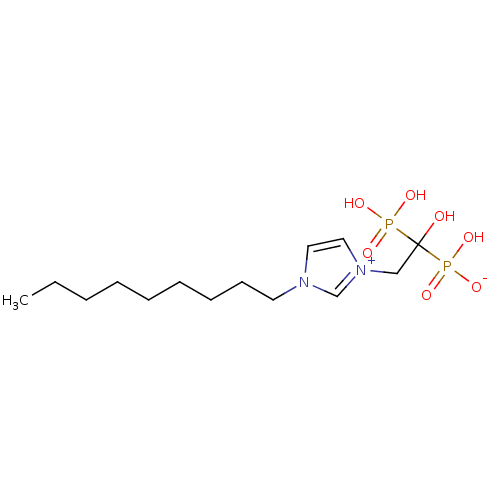
(CHEMBL2338361)Show SMILES CCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C14H28N2O7P2/c1-2-3-4-5-6-7-8-9-15-10-11-16(13-15)12-14(17,24(18,19)20)25(21,22)23/h10-11,13,17H,2-9,12H2,1H3,(H3-,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428313

(CHEMBL2338377)Show SMILES CCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C14H28N2O6P2/c1-2-3-4-5-6-7-8-9-15-10-11-16(13-15)12-14(23(17,18)19)24(20,21)22/h10-11,13-14H,2-9,12H2,1H3,(H3-,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428296

(CHEMBL2338360)Show SMILES CCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C13H26N2O7P2/c1-2-3-4-5-6-7-8-14-9-10-15(12-14)11-13(16,23(17,18)19)24(20,21)22/h9-10,12,16H,2-8,11H2,1H3,(H3-,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428314
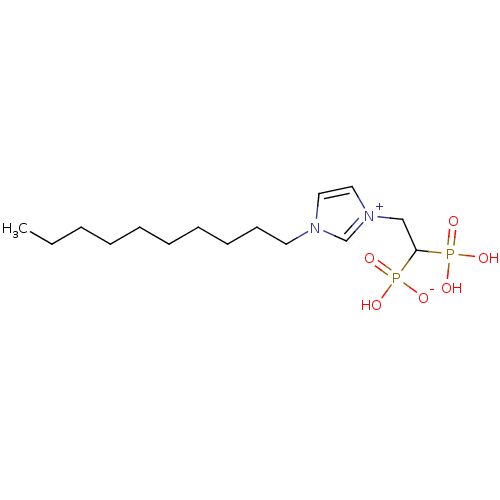
(CHEMBL2338378)Show SMILES CCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C15H30N2O6P2/c1-2-3-4-5-6-7-8-9-10-16-11-12-17(14-16)13-15(24(18,19)20)25(21,22)23/h11-12,14-15H,2-10,13H2,1H3,(H3-,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428309
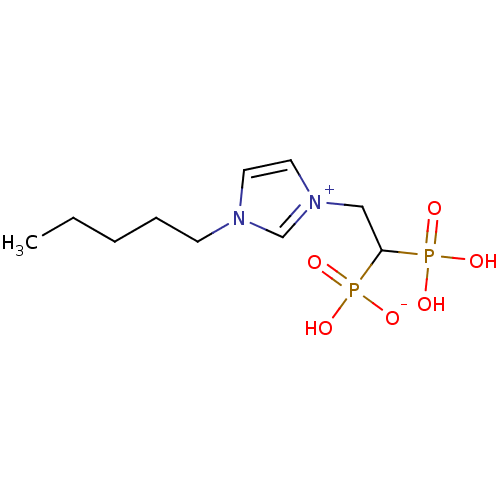
(CHEMBL2338373)Show InChI InChI=1S/C10H20N2O6P2/c1-2-3-4-5-11-6-7-12(9-11)8-10(19(13,14)15)20(16,17)18/h6-7,9-10H,2-5,8H2,1H3,(H3-,13,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428314

(CHEMBL2338378)Show SMILES CCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C15H30N2O6P2/c1-2-3-4-5-6-7-8-9-10-16-11-12-17(14-16)13-15(24(18,19)20)25(21,22)23/h11-12,14-15H,2-10,13H2,1H3,(H3-,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428295

(CHEMBL2338359)Show SMILES CCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C12H24N2O7P2/c1-2-3-4-5-6-7-13-8-9-14(11-13)10-12(15,22(16,17)18)23(19,20)21/h8-9,11,15H,2-7,10H2,1H3,(H3-,16,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428297

(CHEMBL2338361)Show SMILES CCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C14H28N2O7P2/c1-2-3-4-5-6-7-8-9-15-10-11-16(13-15)12-14(17,24(18,19)20)25(21,22)23/h10-11,13,17H,2-9,12H2,1H3,(H3-,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428293

(CHEMBL2338357)Show SMILES CCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C10H20N2O7P2/c1-2-3-4-5-11-6-7-12(9-11)8-10(13,20(14,15)16)21(17,18)19/h6-7,9,13H,2-5,8H2,1H3,(H3-,14,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428290

(CHEMBL2338354)Show InChI InChI=1S/C7H14N2O7P2/c1-2-8-3-4-9(6-8)5-7(10,17(11,12)13)18(14,15)16/h3-4,6,10H,2,5H2,1H3,(H3-,11,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428294

(CHEMBL2338358)Show SMILES CCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C11H22N2O7P2/c1-2-3-4-5-6-12-7-8-13(10-12)9-11(14,21(15,16)17)22(18,19)20/h7-8,10,14H,2-6,9H2,1H3,(H3-,15,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428298
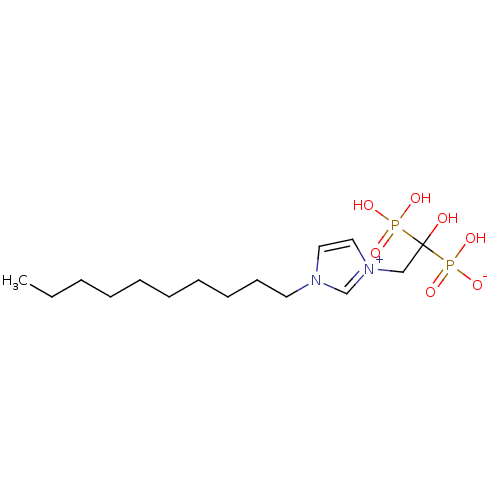
(CHEMBL2338362)Show SMILES CCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C15H30N2O7P2/c1-2-3-4-5-6-7-8-9-10-16-11-12-17(14-16)13-15(18,25(19,20)21)26(22,23)24/h11-12,14,18H,2-10,13H2,1H3,(H3-,19,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50267744
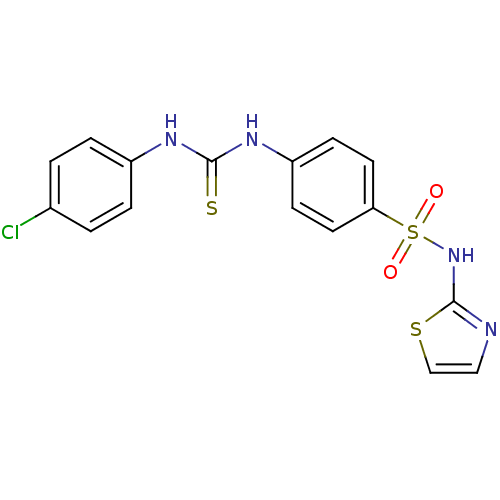
(4-(3-(4-Chlorophenyl)thioureido)-N-(thiazol-2-yl)b...)Show SMILES Clc1ccc(NC(=S)Nc2ccc(cc2)S(=O)(=O)Nc2nccs2)cc1 Show InChI InChI=1S/C16H13ClN4O2S3/c17-11-1-3-12(4-2-11)19-15(24)20-13-5-7-14(8-6-13)26(22,23)21-16-18-9-10-25-16/h1-10H,(H,18,21)(H2,19,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human cyclophilin A at 6 degC |
Bioorg Med Chem 17: 3177-88 (2009)
Article DOI: 10.1016/j.bmc.2009.02.051
BindingDB Entry DOI: 10.7270/Q2Z31ZJG |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428308

(CHEMBL2338372)Show InChI InChI=1S/C9H18N2O6P2/c1-2-3-4-10-5-6-11(8-10)7-9(18(12,13)14)19(15,16)17/h5-6,8-9H,2-4,7H2,1H3,(H3-,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428304

(CHEMBL2338368)Show InChI InChI=1S/C5H10N2O6P2/c8-14(9,10)5(15(11,12)13)3-7-2-1-6-4-7/h1-2,4-5H,3H2,(H4,8,9,10,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428315

(CHEMBL2338379)Show SMILES CCCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C16H32N2O6P2/c1-2-3-4-5-6-7-8-9-10-11-17-12-13-18(15-17)14-16(25(19,20)21)26(22,23)24/h12-13,15-16H,2-11,14H2,1H3,(H3-,19,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428307

(CHEMBL2338371)Show InChI InChI=1S/C8H16N2O6P2/c1-2-3-9-4-5-10(7-9)6-8(17(11,12)13)18(14,15)16/h4-5,7-8H,2-3,6H2,1H3,(H3-,11,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428289

(CHEMBL2338353)Show InChI InChI=1S/C6H12N2O7P2/c1-7-2-3-8(5-7)4-6(9,16(10,11)12)17(13,14)15/h2-3,5,9H,4H2,1H3,(H3-,10,11,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428312

(CHEMBL2338376)Show SMILES CCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C13H26N2O6P2/c1-2-3-4-5-6-7-8-14-9-10-15(12-14)11-13(22(16,17)18)23(19,20)21/h9-10,12-13H,2-8,11H2,1H3,(H3-,16,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data