

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277781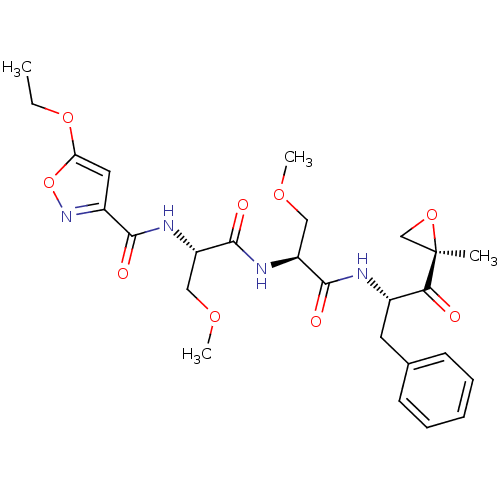 ((2S)-2-[(2S)-2-[(5-ethoxy-1,2-oxazol-3-yl)formamid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500324 (CHEMBL3747448) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500325 (Cb 5083 | Cb-5083) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383200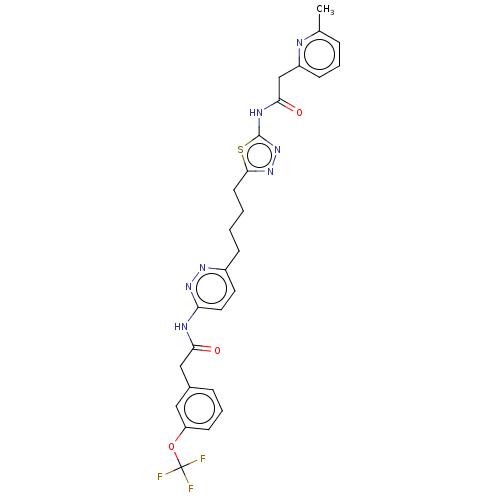 (US10278968, Compound 728 | US10793535, Cmpd ID 728) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383202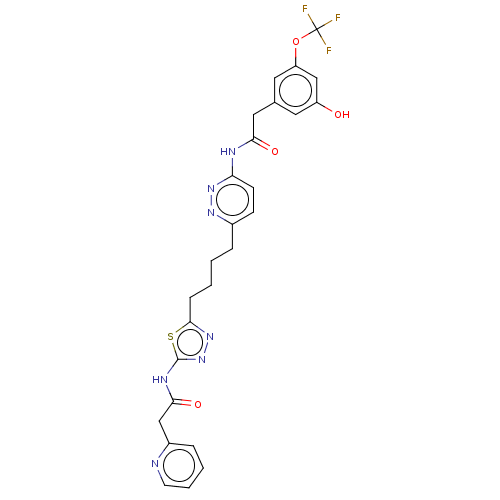 (US10278968, Compound 730 | US10793535, Cmpd ID 730) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277889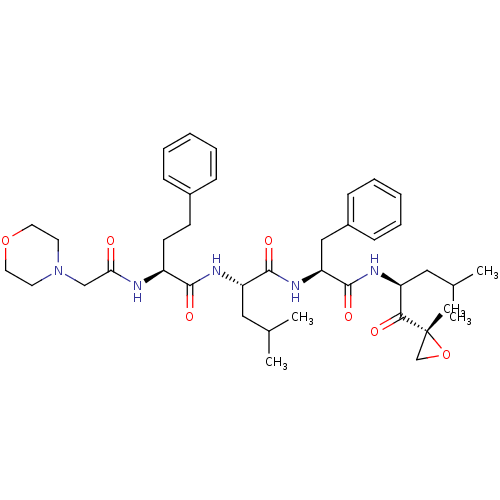 (CARFILZOMIB | CHEMBL451887) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500319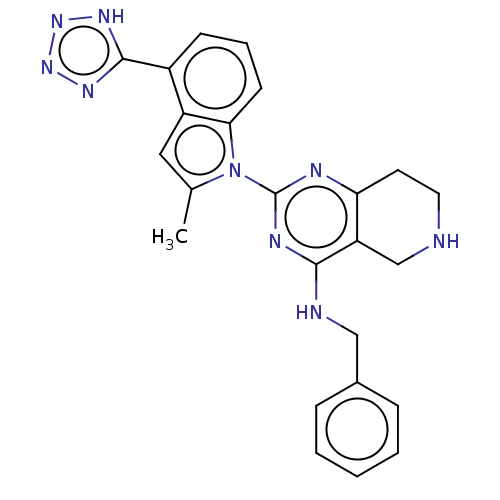 (CHEMBL3746179) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383201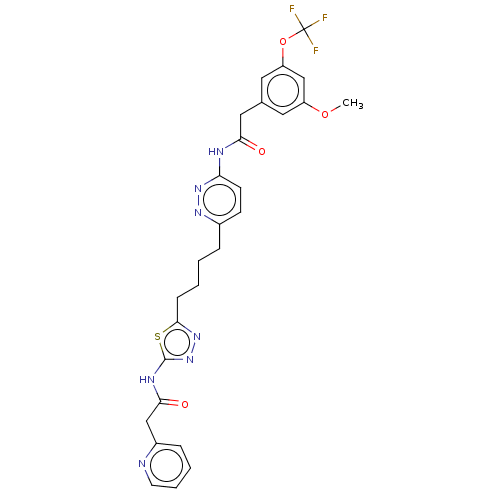 (US10278968, Compound 729 | US10793535, Cmpd ID 729) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383193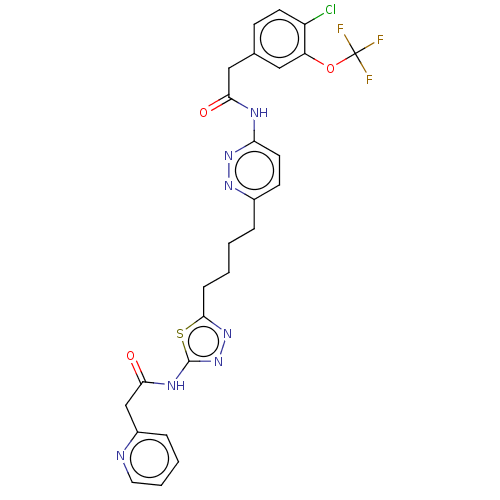 (US10278968, Compound 721 | US10793535, Cmpd ID 721) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383192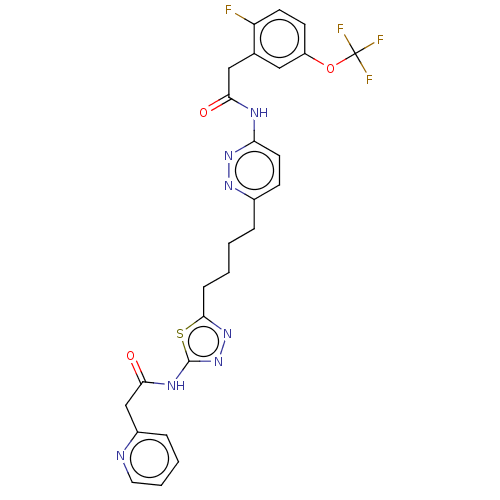 (US10278968, Compound 720 | US10793535, Cmpd ID 720) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277779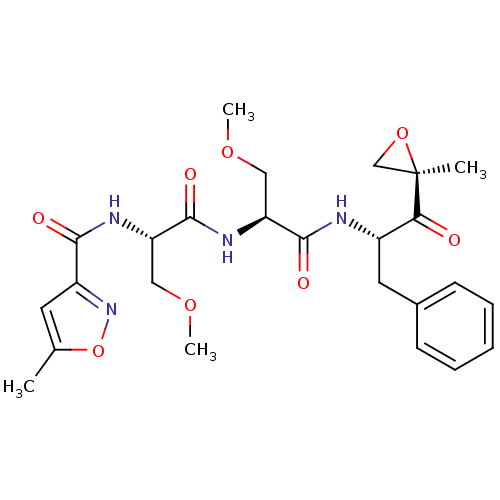 (CHEMBL484003 | N-((S)-3-methoxy-1-((S)-3-methoxy-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277815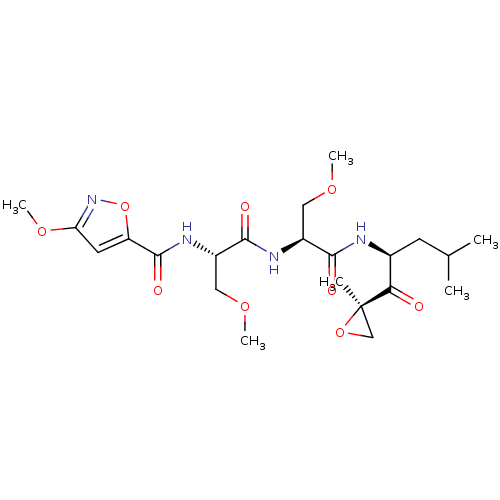 (3-methoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM109086 (US10793535, Cmpd ID 727 | US8604016, 670 | US99382...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500347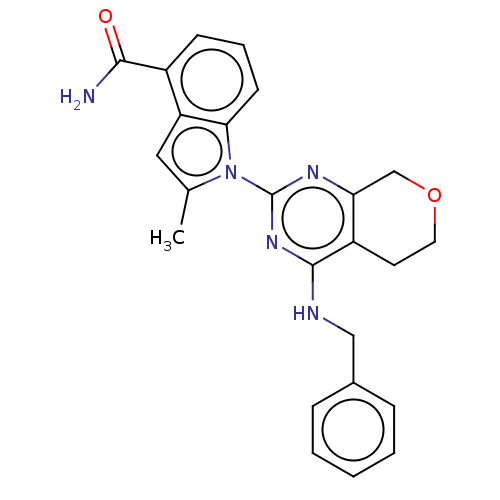 (CHEMBL3747049) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383194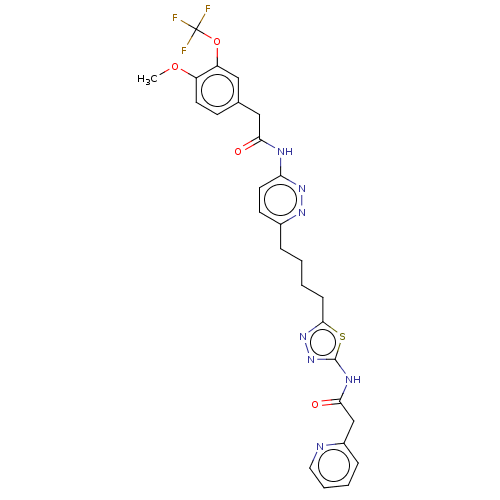 (US10278968, Compound 722 | US10793535, Cmpd ID 722) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383190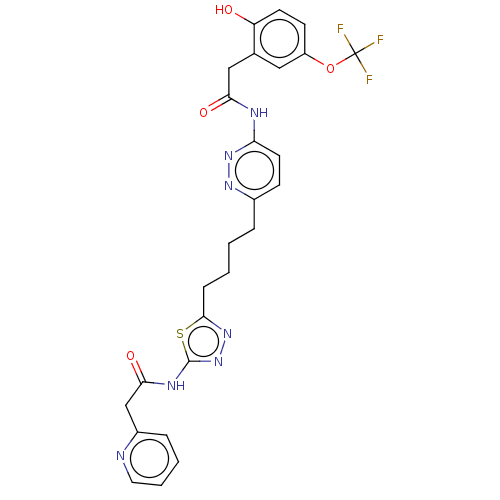 (US10278968, Compound 718 | US10793535, Cmpd ID 718) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383191 (US10278968, Compound 719 | US10793535, Cmpd ID 719) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383189 (US10278968, Compound 717 | US10793535, Cmpd ID 717) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277816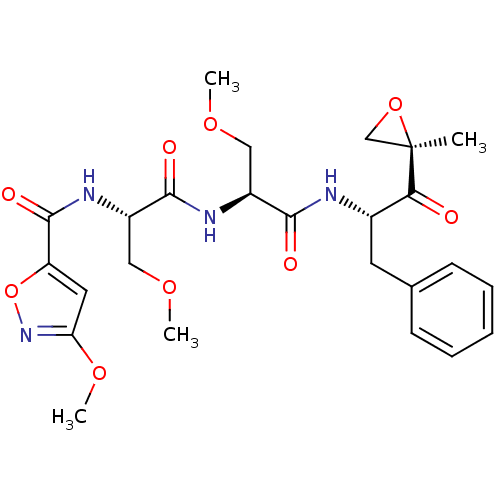 (3-methoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500310 (CHEMBL3747647) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277780 (5-ethoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383195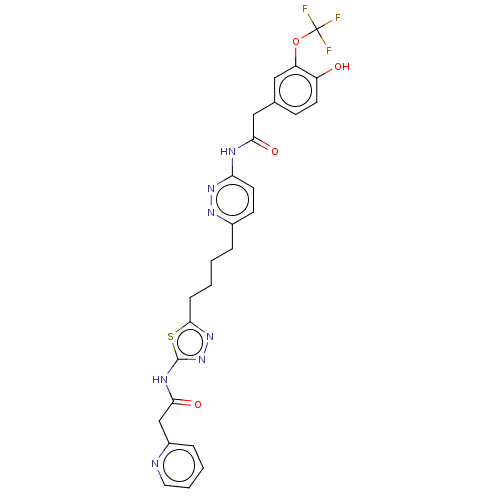 (US10278968, Compound 723 | US10793535, Cmpd ID 723) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM163143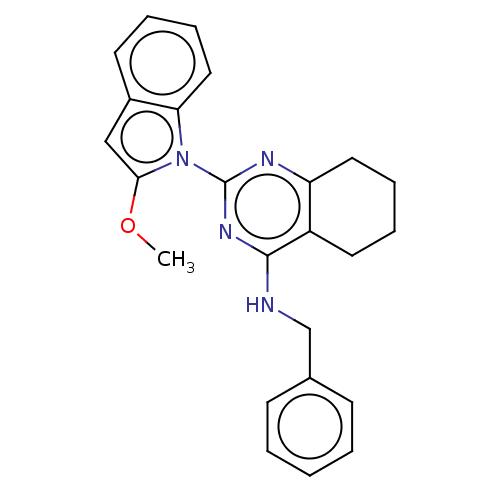 (US9062026, Table III, Compound 71) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM163134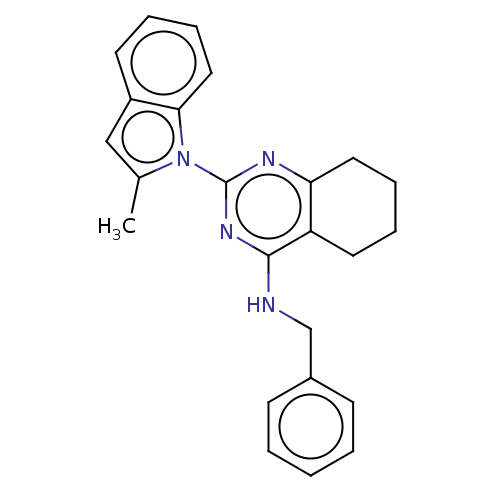 (US9062026, Table III, Compound 62) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383197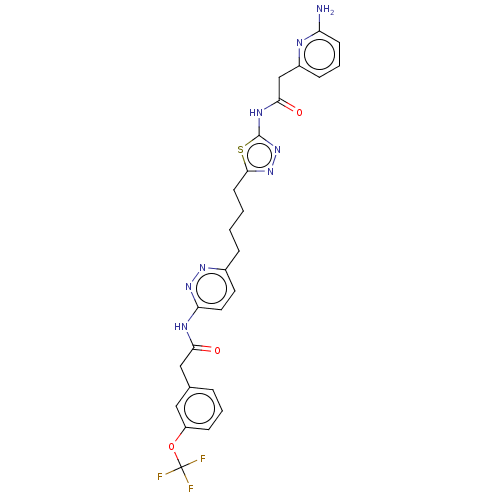 (US10278968, Compound 725 | US10793535, Cmpd ID 725) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM163144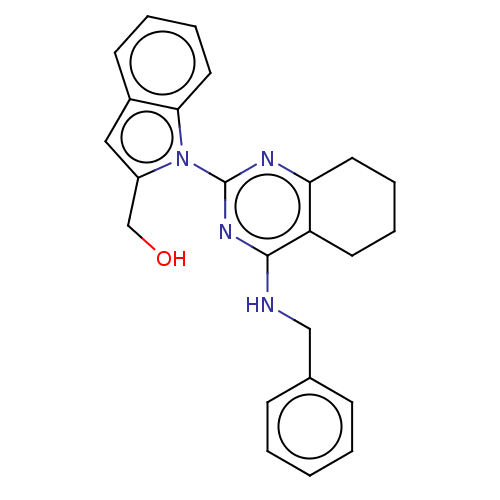 (US9062026, Table III, Compound 72) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500321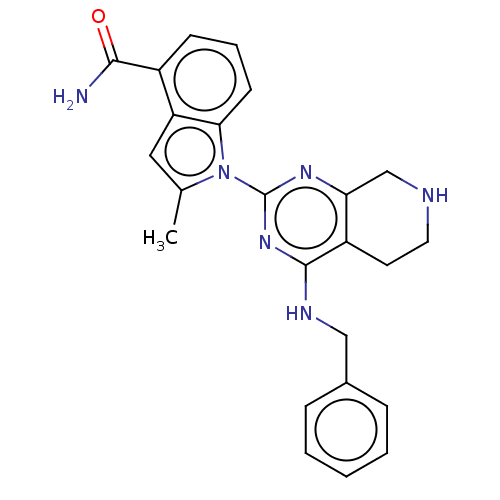 (CHEMBL3745888) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500323 (CHEMBL3746353) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277818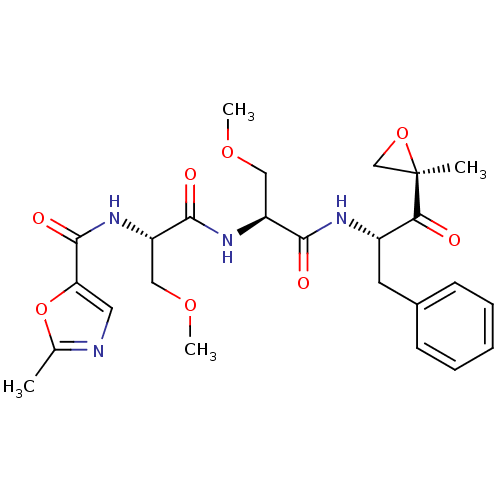 (2-Me-5-thiazole-Ser(OMe)-Ser(OMe)-Phe-ketoepoxide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277778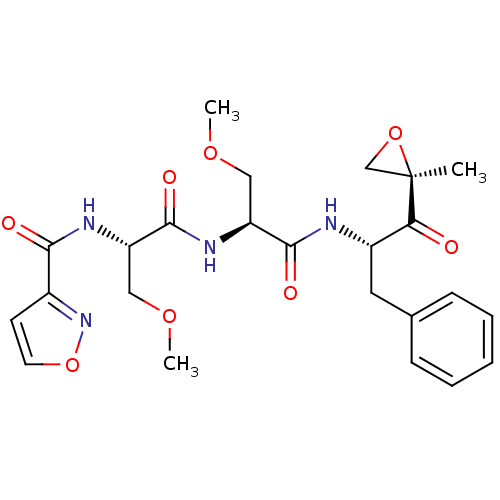 (CHEMBL484002 | N-((S)-3-methoxy-1-((S)-3-methoxy-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500318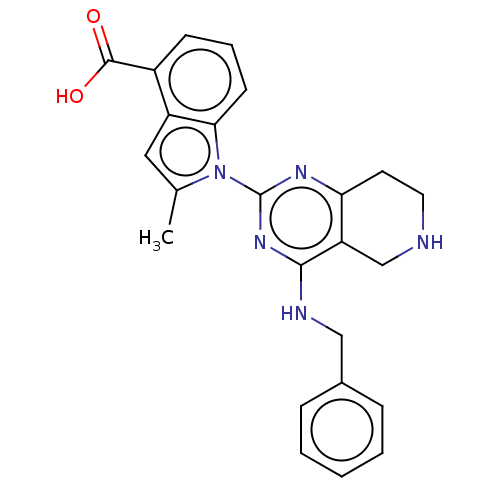 (CHEMBL3746650) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500339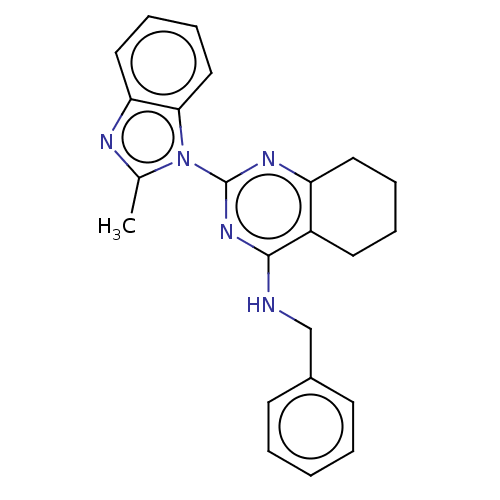 (CHEMBL3747498) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383196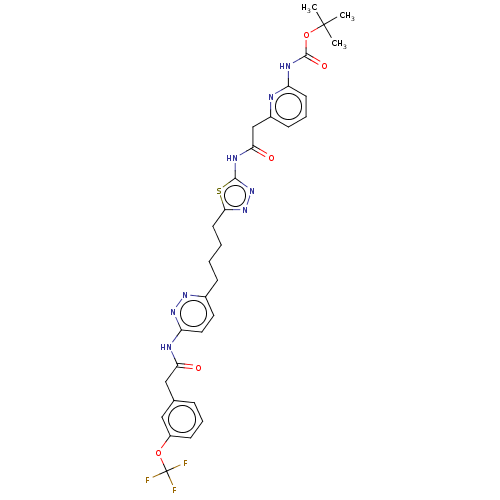 (US10278968, Compound 724 | US10793535, Cmpd ID 724) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500316 (CHEMBL3746000) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM163082 (US9062026, Table III, Compound 10) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500337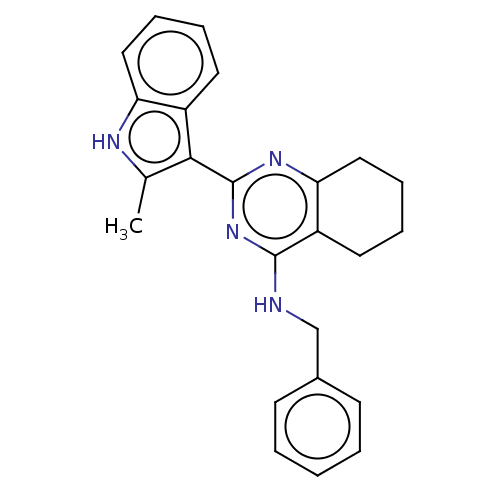 (CHEMBL3747075) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM163080 (US9062026, Table III, Compound 8) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM163136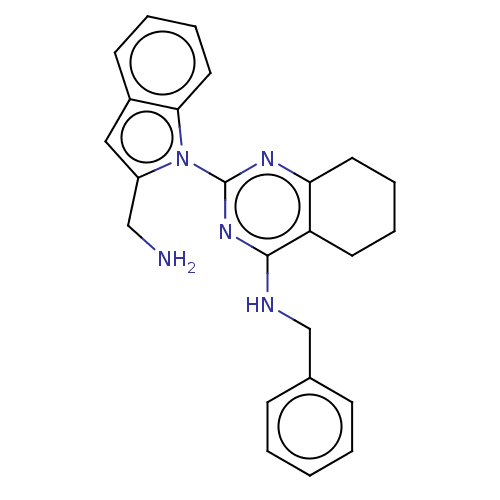 (US9062026, Table III, Compound 64 | US9062026, Tab...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM163092 (US9062026, Table III, Compound 20) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM383188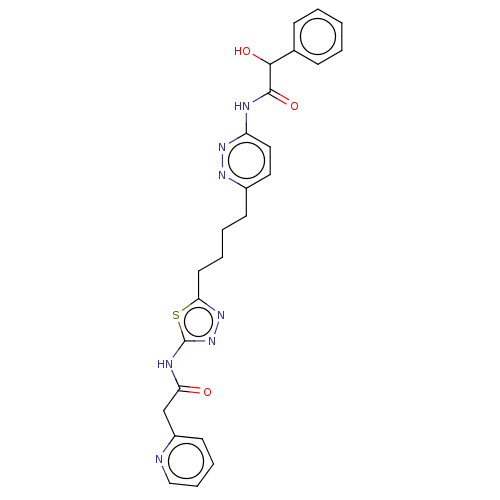 (US10278968, Compound 715 | US10793535, Cmpd ID 715) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th... | J Med Chem 51: 5594-607 (2008) BindingDB Entry DOI: 10.7270/Q27P91QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM163115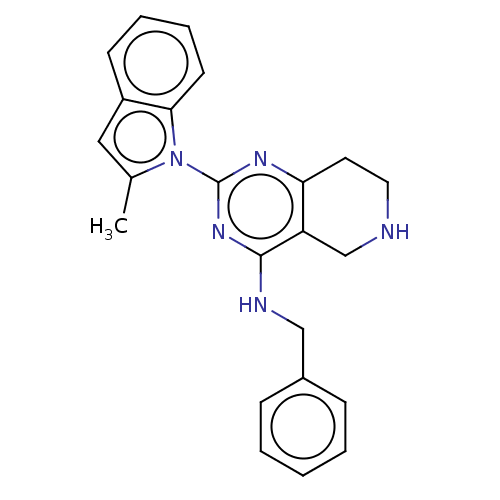 (US9062026, Table III, Compound 43) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 26S proteasome non-ATPase regulatory subunit 14 (Homo sapiens (Human)) | BDBM50264457 (CHEMBL4080490) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Diego Curated by ChEMBL | Assay Description Inhibition of 26S proteasome regulatory subunit RPN11 deubiquitinating activity in human erythrocytes using Ub4-pepOG protein substrate preincubated ... | J Med Chem 60: 1343-1361 (2017) Article DOI: 10.1021/acs.jmedchem.6b01379 BindingDB Entry DOI: 10.7270/Q22F7QX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 26S proteasome non-ATPase regulatory subunit 14 (Homo sapiens (Human)) | BDBM50264417 (CHEMBL4104719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Diego Curated by ChEMBL | Assay Description Inhibition of 26S proteasome regulatory subunit RPN11 deubiquitinating activity in human erythrocytes using Ub4-pepOG protein substrate preincubated ... | J Med Chem 60: 1343-1361 (2017) Article DOI: 10.1021/acs.jmedchem.6b01379 BindingDB Entry DOI: 10.7270/Q22F7QX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 26S proteasome non-ATPase regulatory subunit 14 (Homo sapiens (Human)) | BDBM50264415 (CHEMBL4096289) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Diego Curated by ChEMBL | Assay Description Inhibition of 26S proteasome regulatory subunit RPN11 deubiquitinating activity in human erythrocytes using Ub4-pepOG protein substrate preincubated ... | J Med Chem 60: 1343-1361 (2017) Article DOI: 10.1021/acs.jmedchem.6b01379 BindingDB Entry DOI: 10.7270/Q22F7QX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| STAM-binding protein (Homo sapiens (Human)) | BDBM50264455 (CHEMBL4060337) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Diego Curated by ChEMBL | Assay Description Inhibition of AMSH (unknown origin) using DiUbK63TAMRA as substrate by fluorescence assay | J Med Chem 60: 1343-1361 (2017) Article DOI: 10.1021/acs.jmedchem.6b01379 BindingDB Entry DOI: 10.7270/Q22F7QX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500320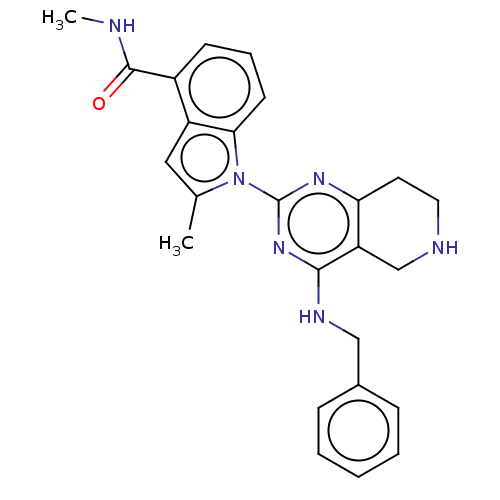 (CHEMBL3747639) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM163098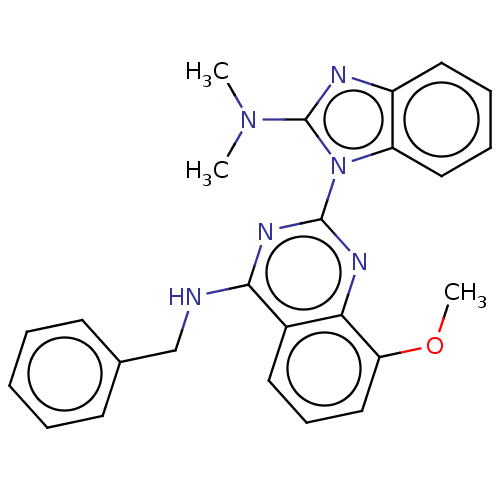 (US9062026, Table III, Compound 26) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM163097 (US9062026, Table III, Compound 25) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277733 ((2S)-3-methoxy-2-[(2S)-3-methoxy-2-[(5-methyl-1,2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 372 total ) | Next | Last >> |