 Found 54 hits with Last Name = 'pethe' and Initial = 'k'
Found 54 hits with Last Name = 'pethe' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50022085
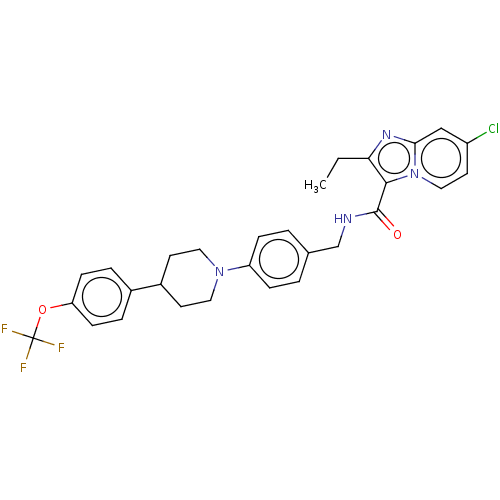
(CHEMBL3298909)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H28ClF3N4O2/c1-2-25-27(37-16-13-22(30)17-26(37)35-25)28(38)34-18-19-3-7-23(8-4-19)36-14-11-21(12-15-36)20-5-9-24(10-6-20)39-29(31,32)33/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C9 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50022080
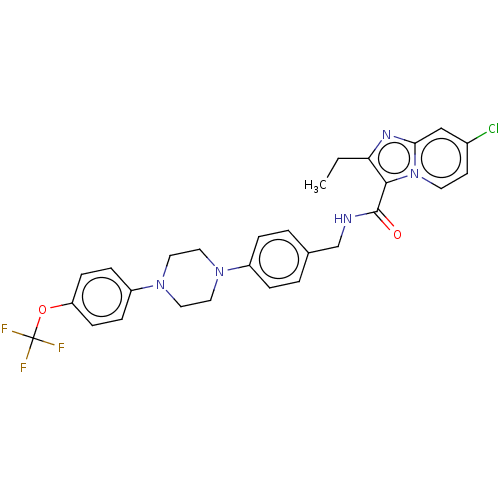
(CHEMBL3298837)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H27ClF3N5O2/c1-2-24-26(37-12-11-20(29)17-25(37)34-24)27(38)33-18-19-3-5-21(6-4-19)35-13-15-36(16-14-35)22-7-9-23(10-8-22)39-28(30,31)32/h3-12,17H,2,13-16,18H2,1H3,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C9 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50022083
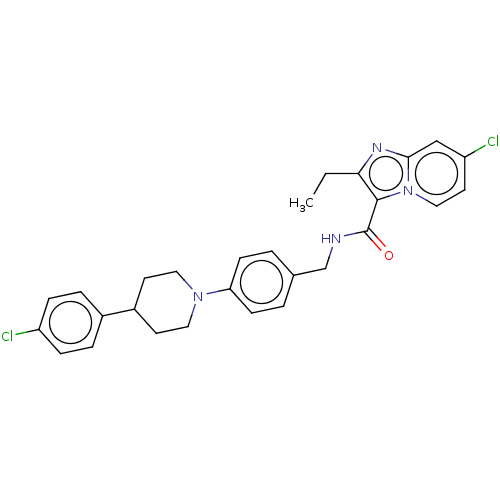
(CHEMBL3298907)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N4O/c1-2-25-27(34-16-13-23(30)17-26(34)32-25)28(35)31-18-19-3-9-24(10-4-19)33-14-11-21(12-15-33)20-5-7-22(29)8-6-20/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C9 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50022084
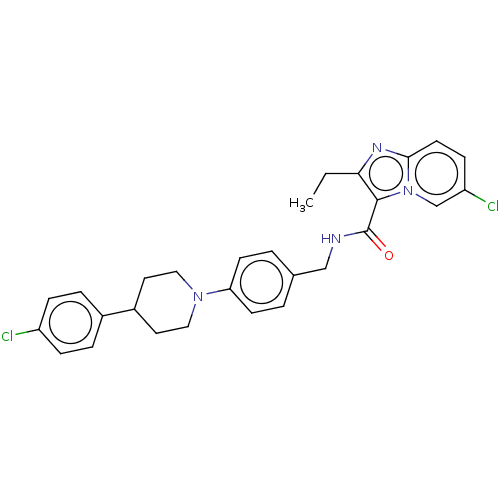
(CHEMBL3298908)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N4O/c1-2-25-27(34-18-23(30)9-12-26(34)32-25)28(35)31-17-19-3-10-24(11-4-19)33-15-13-21(14-16-33)20-5-7-22(29)8-6-20/h3-12,18,21H,2,13-17H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C9 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50022085

(CHEMBL3298909)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H28ClF3N4O2/c1-2-25-27(37-16-13-22(30)17-26(37)35-25)28(38)34-18-19-3-7-23(8-4-19)36-14-11-21(12-15-36)20-5-9-24(10-6-20)39-29(31,32)33/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50022083

(CHEMBL3298907)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N4O/c1-2-25-27(34-16-13-23(30)17-26(34)32-25)28(35)31-18-19-3-9-24(10-4-19)33-14-11-21(12-15-33)20-5-7-22(29)8-6-20/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50022079
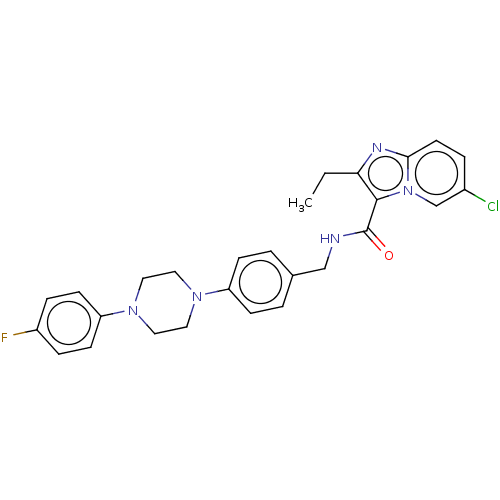
(CHEMBL3298836)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C27H27ClFN5O/c1-2-24-26(34-18-20(28)5-12-25(34)31-24)27(35)30-17-19-3-8-22(9-4-19)32-13-15-33(16-14-32)23-10-6-21(29)7-11-23/h3-12,18H,2,13-17H2,1H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP3A4 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50022078
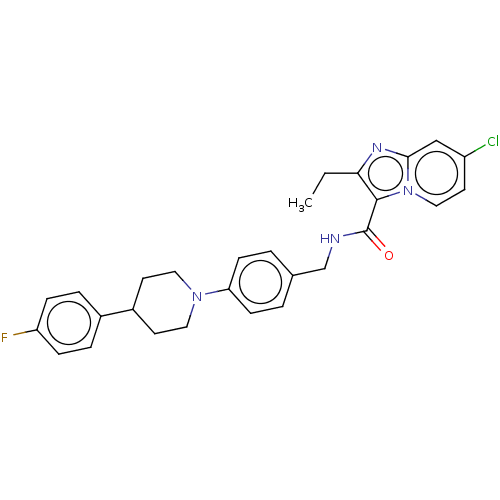
(CHEMBL3298835)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H28ClFN4O/c1-2-25-27(34-16-13-22(29)17-26(34)32-25)28(35)31-18-19-3-9-24(10-4-19)33-14-11-21(12-15-33)20-5-7-23(30)8-6-20/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C9 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50022081
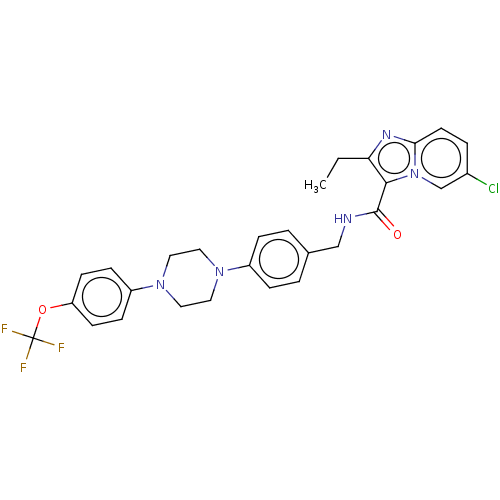
(CHEMBL3298905)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H27ClF3N5O2/c1-2-24-26(37-18-20(29)5-12-25(37)34-24)27(38)33-17-19-3-6-21(7-4-19)35-13-15-36(16-14-35)22-8-10-23(11-9-22)39-28(30,31)32/h3-12,18H,2,13-17H2,1H3,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C9 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50022078

(CHEMBL3298835)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H28ClFN4O/c1-2-25-27(34-16-13-22(29)17-26(34)32-25)28(35)31-18-19-3-9-24(10-4-19)33-14-11-21(12-15-33)20-5-7-23(30)8-6-20/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50022079

(CHEMBL3298836)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C27H27ClFN5O/c1-2-24-26(34-18-20(28)5-12-25(34)31-24)27(35)30-17-19-3-8-22(9-4-19)32-13-15-33(16-14-32)23-10-6-21(29)7-11-23/h3-12,18H,2,13-17H2,1H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50022079

(CHEMBL3298836)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C27H27ClFN5O/c1-2-24-26(34-18-20(28)5-12-25(34)31-24)27(35)30-17-19-3-8-22(9-4-19)32-13-15-33(16-14-32)23-10-6-21(29)7-11-23/h3-12,18H,2,13-17H2,1H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C9 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50022078

(CHEMBL3298835)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H28ClFN4O/c1-2-25-27(34-16-13-22(29)17-26(34)32-25)28(35)31-18-19-3-9-24(10-4-19)33-14-11-21(12-15-33)20-5-7-23(30)8-6-20/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP3A4 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50022084

(CHEMBL3298908)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N4O/c1-2-25-27(34-18-23(30)9-12-26(34)32-25)28(35)31-17-19-3-10-24(11-4-19)33-15-13-21(14-16-33)20-5-7-22(29)8-6-20/h3-12,18,21H,2,13-17H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50022081

(CHEMBL3298905)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H27ClF3N5O2/c1-2-24-26(37-18-20(29)5-12-25(37)34-24)27(38)33-17-19-3-6-21(7-4-19)35-13-15-36(16-14-35)22-8-10-23(11-9-22)39-28(30,31)32/h3-12,18H,2,13-17H2,1H3,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP3A4 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Glutamine-dependent NAD(+) synthetase
(Homo sapiens (Human)) | BDBM92599
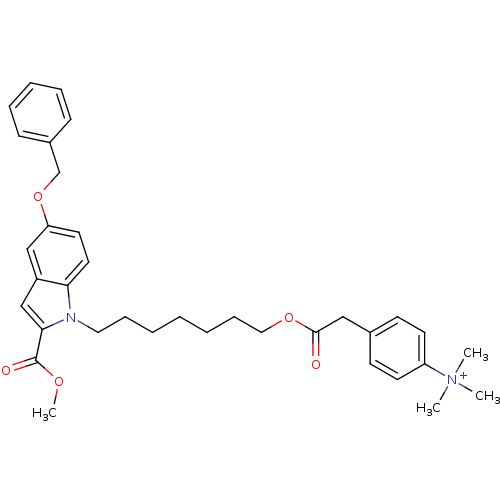
(CHEMBL263057 | NAD Synthetase Inhibitor, 4)Show SMILES COC(=O)c1cc2cc(OCc3ccccc3)ccc2n1CCCCCCCOC(=O)Cc1ccc(cc1)[N+](C)(C)C Show InChI InChI=1S/C35H43N2O5/c1-37(2,3)30-17-15-27(16-18-30)23-34(38)41-22-12-7-5-6-11-21-36-32-20-19-31(42-26-28-13-9-8-10-14-28)24-29(32)25-33(36)35(39)40-4/h8-10,13-20,24-25H,5-7,11-12,21-23,26H2,1-4H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | 8.5 | 37 |
National Institutes of Health
| Assay Description
Inhibition assay using NAD synthetase. |
J Biol Chem 283: 19329-41 (2008)
Article DOI: 10.1074/jbc.M800694200
BindingDB Entry DOI: 10.7270/Q27P8X0W |
More data for this
Ligand-Target Pair | |
Glutamine-dependent NAD(+) synthetase
(Homo sapiens (Human)) | BDBM92597
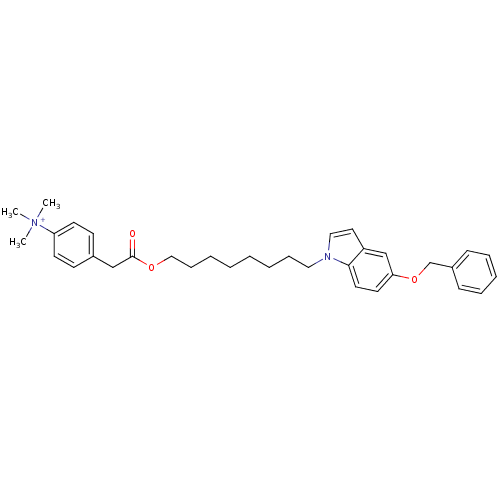
(CHEMBL324501 | NAD Synthetase Inhibitor, 2)Show SMILES C[N+](C)(C)c1ccc(CC(=O)OCCCCCCCCn2ccc3cc(OCc4ccccc4)ccc23)cc1 Show InChI InChI=1S/C34H43N2O3/c1-36(2,3)31-17-15-28(16-18-31)25-34(37)38-24-12-7-5-4-6-11-22-35-23-21-30-26-32(19-20-33(30)35)39-27-29-13-9-8-10-14-29/h8-10,13-21,23,26H,4-7,11-12,22,24-25,27H2,1-3H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | 8.5 | 37 |
National Institutes of Health
| Assay Description
Inhibition assay using NAD synthetase. |
J Biol Chem 283: 19329-41 (2008)
Article DOI: 10.1074/jbc.M800694200
BindingDB Entry DOI: 10.7270/Q27P8X0W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50022082
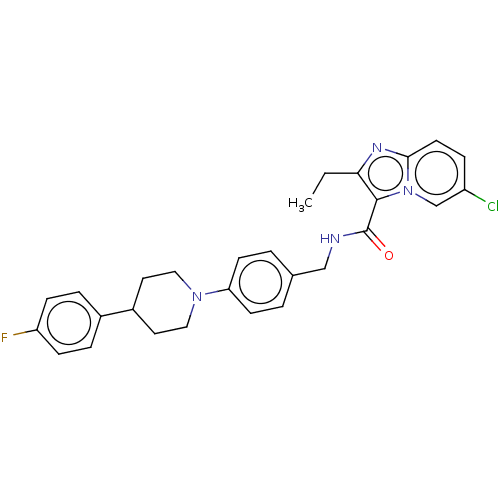
(CHEMBL3298906)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H28ClFN4O/c1-2-25-27(34-18-22(29)7-12-26(34)32-25)28(35)31-17-19-3-10-24(11-4-19)33-15-13-21(14-16-33)20-5-8-23(30)9-6-20/h3-12,18,21H,2,13-17H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP1A2 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50022083

(CHEMBL3298907)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N4O/c1-2-25-27(34-16-13-23(30)17-26(34)32-25)28(35)31-18-19-3-9-24(10-4-19)33-14-11-21(12-15-33)20-5-7-22(29)8-6-20/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP1A2 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50022084

(CHEMBL3298908)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N4O/c1-2-25-27(34-18-23(30)9-12-26(34)32-25)28(35)31-17-19-3-10-24(11-4-19)33-15-13-21(14-16-33)20-5-7-22(29)8-6-20/h3-12,18,21H,2,13-17H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP1A2 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50022077
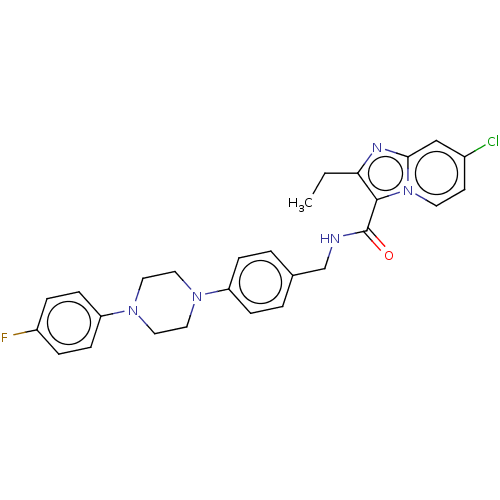
(CHEMBL3109797)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C27H27ClFN5O/c1-2-24-26(34-12-11-20(28)17-25(34)31-24)27(35)30-18-19-3-7-22(8-4-19)32-13-15-33(16-14-32)23-9-5-21(29)6-10-23/h3-12,17H,2,13-16,18H2,1H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C9 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50022081

(CHEMBL3298905)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H27ClF3N5O2/c1-2-24-26(37-18-20(29)5-12-25(37)34-24)27(38)33-17-19-3-6-21(7-4-19)35-13-15-36(16-14-35)22-8-10-23(11-9-22)39-28(30,31)32/h3-12,18H,2,13-17H2,1H3,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP1A2 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50022080

(CHEMBL3298837)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H27ClF3N5O2/c1-2-24-26(37-12-11-20(29)17-25(37)34-24)27(38)33-18-19-3-5-21(6-4-19)35-13-15-36(16-14-35)22-7-9-23(10-8-22)39-28(30,31)32/h3-12,17H,2,13-16,18H2,1H3,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP1A2 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50022078

(CHEMBL3298835)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H28ClFN4O/c1-2-25-27(34-16-13-22(29)17-26(34)32-25)28(35)31-18-19-3-9-24(10-4-19)33-14-11-21(12-15-33)20-5-7-23(30)8-6-20/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP1A2 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50022077

(CHEMBL3109797)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C27H27ClFN5O/c1-2-24-26(34-12-11-20(28)17-25(34)31-24)27(35)30-18-19-3-7-22(8-4-19)32-13-15-33(16-14-32)23-9-5-21(29)6-10-23/h3-12,17H,2,13-16,18H2,1H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP1A2 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50022084

(CHEMBL3298908)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N4O/c1-2-25-27(34-18-23(30)9-12-26(34)32-25)28(35)31-17-19-3-10-24(11-4-19)33-15-13-21(14-16-33)20-5-7-22(29)8-6-20/h3-12,18,21H,2,13-17H2,1H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2D6 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50022083

(CHEMBL3298907)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N4O/c1-2-25-27(34-16-13-23(30)17-26(34)32-25)28(35)31-18-19-3-9-24(10-4-19)33-14-11-21(12-15-33)20-5-7-22(29)8-6-20/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2D6 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50022082

(CHEMBL3298906)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H28ClFN4O/c1-2-25-27(34-18-22(29)7-12-26(34)32-25)28(35)31-17-19-3-10-24(11-4-19)33-15-13-21(14-16-33)20-5-8-23(30)9-6-20/h3-12,18,21H,2,13-17H2,1H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2D6 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50022081

(CHEMBL3298905)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H27ClF3N5O2/c1-2-24-26(37-18-20(29)5-12-25(37)34-24)27(38)33-17-19-3-6-21(7-4-19)35-13-15-36(16-14-35)22-8-10-23(11-9-22)39-28(30,31)32/h3-12,18H,2,13-17H2,1H3,(H,33,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2D6 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50022080

(CHEMBL3298837)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H27ClF3N5O2/c1-2-24-26(37-12-11-20(29)17-25(37)34-24)27(38)33-18-19-3-5-21(6-4-19)35-13-15-36(16-14-35)22-7-9-23(10-8-22)39-28(30,31)32/h3-12,17H,2,13-16,18H2,1H3,(H,33,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2D6 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50022079

(CHEMBL3298836)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C27H27ClFN5O/c1-2-24-26(34-18-20(28)5-12-25(34)31-24)27(35)30-17-19-3-8-22(9-4-19)32-13-15-33(16-14-32)23-10-6-21(29)7-11-23/h3-12,18H,2,13-17H2,1H3,(H,30,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2D6 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50022078

(CHEMBL3298835)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H28ClFN4O/c1-2-25-27(34-16-13-22(29)17-26(34)32-25)28(35)31-18-19-3-9-24(10-4-19)33-14-11-21(12-15-33)20-5-7-23(30)8-6-20/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2D6 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50022077

(CHEMBL3109797)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C27H27ClFN5O/c1-2-24-26(34-12-11-20(28)17-25(34)31-24)27(35)30-18-19-3-7-22(8-4-19)32-13-15-33(16-14-32)23-9-5-21(29)6-10-23/h3-12,17H,2,13-16,18H2,1H3,(H,30,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2D6 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50022084

(CHEMBL3298908)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N4O/c1-2-25-27(34-18-23(30)9-12-26(34)32-25)28(35)31-17-19-3-10-24(11-4-19)33-15-13-21(14-16-33)20-5-7-22(29)8-6-20/h3-12,18,21H,2,13-17H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP3A4 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50022083

(CHEMBL3298907)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N4O/c1-2-25-27(34-16-13-23(30)17-26(34)32-25)28(35)31-18-19-3-9-24(10-4-19)33-14-11-21(12-15-33)20-5-7-22(29)8-6-20/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP3A4 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50022082

(CHEMBL3298906)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H28ClFN4O/c1-2-25-27(34-18-22(29)7-12-26(34)32-25)28(35)31-17-19-3-10-24(11-4-19)33-15-13-21(14-16-33)20-5-8-23(30)9-6-20/h3-12,18,21H,2,13-17H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP3A4 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50022080

(CHEMBL3298837)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H27ClF3N5O2/c1-2-24-26(37-12-11-20(29)17-25(37)34-24)27(38)33-18-19-3-5-21(6-4-19)35-13-15-36(16-14-35)22-7-9-23(10-8-22)39-28(30,31)32/h3-12,17H,2,13-16,18H2,1H3,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP3A4 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50022077

(CHEMBL3109797)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C27H27ClFN5O/c1-2-24-26(34-12-11-20(28)17-25(34)31-24)27(35)30-18-19-3-7-22(8-4-19)32-13-15-33(16-14-32)23-9-5-21(29)6-10-23/h3-12,17H,2,13-16,18H2,1H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP3A4 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50022079

(CHEMBL3298836)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C27H27ClFN5O/c1-2-24-26(34-18-20(28)5-12-25(34)31-24)27(35)30-17-19-3-8-22(9-4-19)32-13-15-33(16-14-32)23-10-6-21(29)7-11-23/h3-12,18H,2,13-17H2,1H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP1A2 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50022082

(CHEMBL3298906)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H28ClFN4O/c1-2-25-27(34-18-22(29)7-12-26(34)32-25)28(35)31-17-19-3-10-24(11-4-19)33-15-13-21(14-16-33)20-5-8-23(30)9-6-20/h3-12,18,21H,2,13-17H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50022081

(CHEMBL3298905)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H27ClF3N5O2/c1-2-24-26(37-18-20(29)5-12-25(37)34-24)27(38)33-17-19-3-6-21(7-4-19)35-13-15-36(16-14-35)22-8-10-23(11-9-22)39-28(30,31)32/h3-12,18H,2,13-17H2,1H3,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50022080

(CHEMBL3298837)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H27ClF3N5O2/c1-2-24-26(37-12-11-20(29)17-25(37)34-24)27(38)33-18-19-3-5-21(6-4-19)35-13-15-36(16-14-35)22-7-9-23(10-8-22)39-28(30,31)32/h3-12,17H,2,13-16,18H2,1H3,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50022077

(CHEMBL3109797)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCN(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C27H27ClFN5O/c1-2-24-26(34-12-11-20(28)17-25(34)31-24)27(35)30-18-19-3-7-22(8-4-19)32-13-15-33(16-14-32)23-9-5-21(29)6-10-23/h3-12,17H,2,13-16,18H2,1H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50022082

(CHEMBL3298906)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H28ClFN4O/c1-2-25-27(34-18-22(29)7-12-26(34)32-25)28(35)31-17-19-3-10-24(11-4-19)33-15-13-21(14-16-33)20-5-8-23(30)9-6-20/h3-12,18,21H,2,13-17H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C9 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Glutamine-dependent NAD(+) synthetase
(Homo sapiens (Human)) | BDBM92600
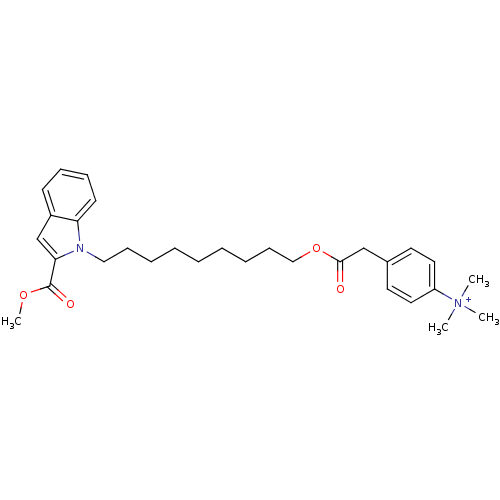
(CHEMBL419944 | NAD Synthetase Inhibitor, 5)Show SMILES COC(=O)c1cc2ccccc2n1CCCCCCCCCOC(=O)Cc1ccc(cc1)[N+](C)(C)C Show InChI InChI=1S/C30H41N2O4/c1-32(2,3)26-18-16-24(17-19-26)22-29(33)36-21-13-9-7-5-6-8-12-20-31-27-15-11-10-14-25(27)23-28(31)30(34)35-4/h10-11,14-19,23H,5-9,12-13,20-22H2,1-4H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.78E+4 | n/a | n/a | n/a | n/a | 8.5 | 37 |
National Institutes of Health
| Assay Description
Inhibition assay using NAD synthetase. |
J Biol Chem 283: 19329-41 (2008)
Article DOI: 10.1074/jbc.M800694200
BindingDB Entry DOI: 10.7270/Q27P8X0W |
More data for this
Ligand-Target Pair | |
Glutamine-dependent NAD(+) synthetase
(Homo sapiens (Human)) | BDBM92598

(NAD Synthetase Inhibitor, 3)Show SMILES COC(=O)c1cc2cc(OCc3ccccc3)ccc2n1CCCCCCCOC(=O)Cc1ccc[n+](C)c1 Show InChI InChI=1S/C32H37N2O5/c1-33-17-11-14-26(23-33)20-31(35)38-19-10-5-3-4-9-18-34-29-16-15-28(39-24-25-12-7-6-8-13-25)21-27(29)22-30(34)32(36)37-2/h6-8,11-17,21-23H,3-5,9-10,18-20,24H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.33E+4 | n/a | n/a | n/a | n/a | 8.5 | 37 |
National Institutes of Health
| Assay Description
Inhibition assay using NAD synthetase. |
J Biol Chem 283: 19329-41 (2008)
Article DOI: 10.1074/jbc.M800694200
BindingDB Entry DOI: 10.7270/Q27P8X0W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50022085

(CHEMBL3298909)Show SMILES CCc1nc2cc(Cl)ccn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H28ClF3N4O2/c1-2-25-27(37-16-13-22(30)17-26(37)35-25)28(38)34-18-19-3-7-23(8-4-19)36-14-11-21(12-15-36)20-5-9-24(10-6-20)39-29(31,32)33/h3-10,13,16-17,21H,2,11-12,14-15,18H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP3A4 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50022086
(6-chloro-2-ethyl-N-(4-(4-(4-(trifluoromethoxy)phen...)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H28ClF3N4O2/c1-2-25-27(37-18-22(30)7-12-26(37)35-25)28(38)34-17-19-3-8-23(9-4-19)36-15-13-21(14-16-36)20-5-10-24(11-6-20)39-29(31,32)33/h3-12,18,21H,2,13-17H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP1A2 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50022086

(6-chloro-2-ethyl-N-(4-(4-(4-(trifluoromethoxy)phen...)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H28ClF3N4O2/c1-2-25-27(37-18-22(30)7-12-26(37)35-25)28(38)34-17-19-3-8-23(9-4-19)36-15-13-21(14-16-36)20-5-10-24(11-6-20)39-29(31,32)33/h3-12,18,21H,2,13-17H2,1H3,(H,34,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2D6 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50022086

(6-chloro-2-ethyl-N-(4-(4-(4-(trifluoromethoxy)phen...)Show SMILES CCc1nc2ccc(Cl)cn2c1C(=O)NCc1ccc(cc1)N1CCC(CC1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H28ClF3N4O2/c1-2-25-27(37-18-22(30)7-12-26(37)35-25)28(38)34-17-19-3-8-23(9-4-19)36-15-13-21(14-16-36)20-5-10-24(11-6-20)39-29(31,32)33/h3-12,18,21H,2,13-17H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) by LC/MS/MS analysis |
J Med Chem 57: 5293-305 (2014)
Article DOI: 10.1021/jm5003606
BindingDB Entry DOI: 10.7270/Q2WS8VTR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data