 Found 35 hits with Last Name = 'pope' and Initial = 'aj'
Found 35 hits with Last Name = 'pope' and Initial = 'aj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium-transporting ATPase alpha chain 1
(Sus scrofa (Pig)) | BDBM50286575
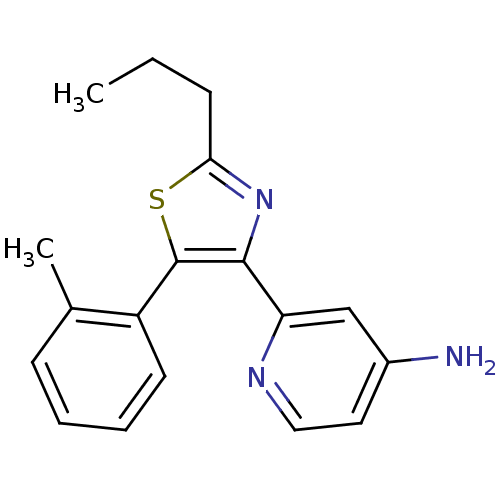
(2-(2-Propyl-5-o-tolyl-thiazol-4-yl)-pyridin-4-ylam...)Show InChI InChI=1S/C18H19N3S/c1-3-6-16-21-17(15-11-13(19)9-10-20-15)18(22-16)14-8-5-4-7-12(14)2/h4-5,7-11H,3,6H2,1-2H3,(H2,19,20) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 838 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to inhibit K+-stimulated ATPase activity |
Bioorg Med Chem Lett 5: 543-546 (1995)
Article DOI: 10.1016/0960-894X(95)00069-6
BindingDB Entry DOI: 10.7270/Q2R211CV |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM18128

((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...)Show SMILES [H][C@]1([C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(O)=O)[C@H](O)[C@]2(O)CO[C@@H]([C@@H]2O)N1O |r| Show InChI InChI=1S/C17H23N3O9/c18-9(5-7-1-3-8(21)4-2-7)14(24)19-10(16(25)26)11-12(22)17(27)6-29-15(13(17)23)20(11)28/h1-4,9-13,15,21-23,27-28H,5-6,18H2,(H,19,24)(H,25,26)/t9-,10-,11-,12-,13-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase |
Bioorg Med Chem Lett 11: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89BPK |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50097749
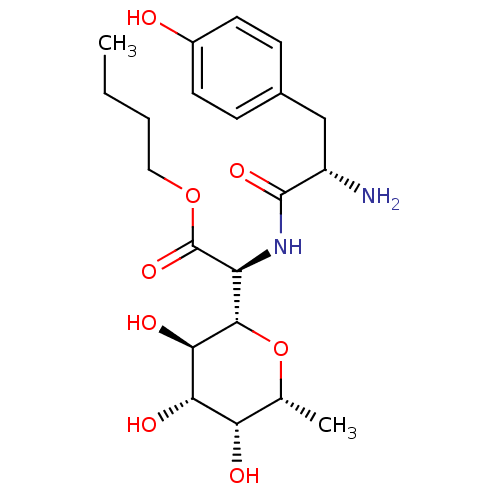
(CHEMBL163375 | [2-Amino-3-((S)-4-hydroxy-phenyl)-p...)Show SMILES CCCCOC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H]1O[C@H](C)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H32N2O8/c1-3-4-9-30-21(29)15(19-18(27)17(26)16(25)11(2)31-19)23-20(28)14(22)10-12-5-7-13(24)8-6-12/h5-8,11,14-19,24-27H,3-4,9-10,22H2,1-2H3,(H,23,28)/t11-,14+,15+,16+,17+,18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay |
Bioorg Med Chem Lett 11: 715-8 (2001)
BindingDB Entry DOI: 10.7270/Q20C4V1Q |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50112575
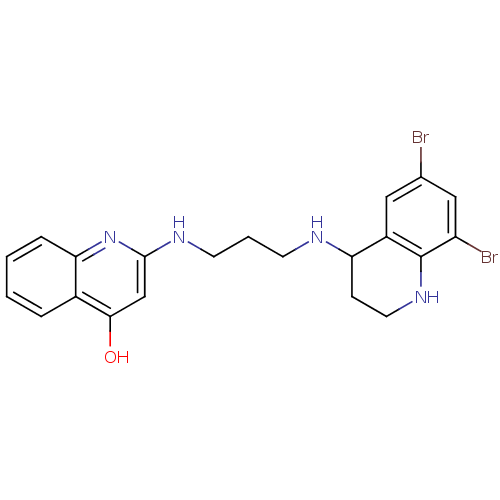
(2-[3-(6,8-Dibromo-1,2,3,4-tetrahydro-quinolin-4-yl...)Show SMILES Oc1cc(NCCCNC2CCNc3c(Br)cc(Br)cc23)nc2ccccc12 Show InChI InChI=1S/C21H22Br2N4O/c22-13-10-15-17(6-9-26-21(15)16(23)11-13)24-7-3-8-25-20-12-19(28)14-4-1-2-5-18(14)27-20/h1-2,4-5,10-12,17,24,26H,3,6-9H2,(H2,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Methionyl-tRNA synthetase in a pyrophosphate exchange assay |
J Med Chem 45: 1959-62 (2002)
BindingDB Entry DOI: 10.7270/Q2HQ3Z6X |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50091497
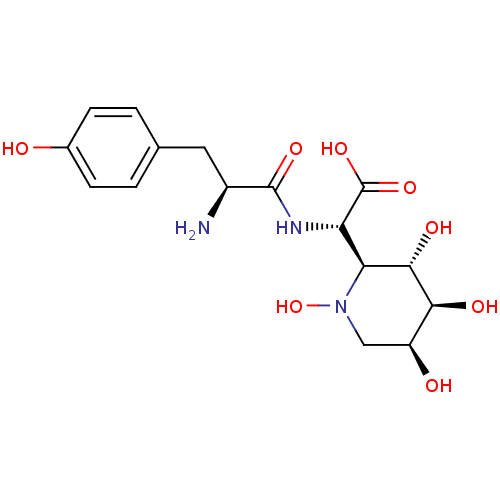
((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@H]1[C@H](O)[C@@H](O)[C@@H](O)CN1O)C(O)=O Show InChI InChI=1S/C16H23N3O8/c17-9(5-7-1-3-8(20)4-2-7)15(24)18-11(16(25)26)12-14(23)13(22)10(21)6-19(12)27/h1-4,9-14,20-23,27H,5-6,17H2,(H,18,24)(H,25,26)/t9-,10-,11-,12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay |
Bioorg Med Chem Lett 11: 711-4 (2001)
BindingDB Entry DOI: 10.7270/Q2445KR1 |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50091497

((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@H]1[C@H](O)[C@@H](O)[C@@H](O)CN1O)C(O)=O Show InChI InChI=1S/C16H23N3O8/c17-9(5-7-1-3-8(20)4-2-7)15(24)18-11(16(25)26)12-14(23)13(22)10(21)6-19(12)27/h1-4,9-14,20-23,27H,5-6,17H2,(H,18,24)(H,25,26)/t9-,10-,11-,12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against tyrosyl tRNA synthetase from Staphylococcus aureus was determined |
Bioorg Med Chem Lett 10: 1811-4 (2000)
BindingDB Entry DOI: 10.7270/Q23F4Q5G |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM18128

((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...)Show SMILES [H][C@]1([C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(O)=O)[C@H](O)[C@]2(O)CO[C@@H]([C@@H]2O)N1O |r| Show InChI InChI=1S/C17H23N3O9/c18-9(5-7-1-3-8(21)4-2-7)14(24)19-10(16(25)26)11-12(22)17(27)6-29-15(13(17)23)20(11)28/h1-4,9-13,15,21-23,27-28H,5-6,18H2,(H,19,24)(H,25,26)/t9-,10-,11-,12-,13-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against tyrosyl tRNA synthetase from Staphylococcus aureus was determined |
Bioorg Med Chem Lett 10: 1811-4 (2000)
BindingDB Entry DOI: 10.7270/Q23F4Q5G |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50097746
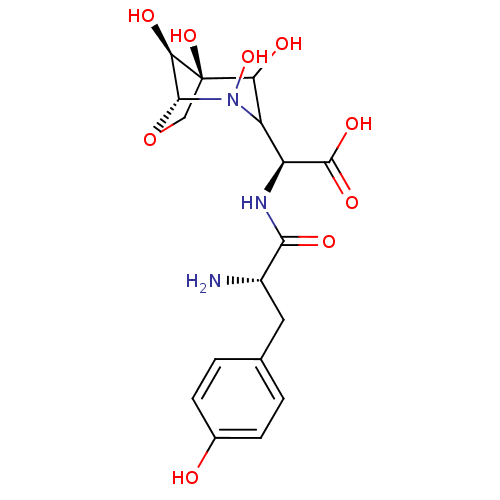
((S)-[2-Amino-3-((S)-4-hydroxy-phenyl)-propionylami...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C1C(O)[C@]2(O)CO[C@H]([C@H]2O)N1O)C(O)=O Show InChI InChI=1S/C17H23N3O9/c18-9(5-7-1-3-8(21)4-2-7)14(24)19-10(16(25)26)11-12(22)17(27)6-29-15(13(17)23)20(11)28/h1-4,9-13,15,21-23,27-28H,5-6,18H2,(H,19,24)(H,25,26)/t9-,10-,11?,12?,13+,15+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay |
Bioorg Med Chem Lett 11: 711-4 (2001)
BindingDB Entry DOI: 10.7270/Q2445KR1 |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50104311

((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...)Show SMILES CCOC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H]1C=C[C@H](O)[C@H](O)[C@H]1O |c:21| Show InChI InChI=1S/C19H26N2O7/c1-2-28-19(27)15(12-7-8-14(23)17(25)16(12)24)21-18(26)13(20)9-10-3-5-11(22)6-4-10/h3-8,12-17,22-25H,2,9,20H2,1H3,(H,21,26)/t12-,13-,14-,15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase |
Bioorg Med Chem Lett 11: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89BPK |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50097748

(CHEMBL351127 | [2-Amino-3-((S)-4-hydroxy-phenyl)-p...)Show SMILES CCCCOC(=O)C(NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@H]1C[C@@H](O)[C@@H](O)[C@@H](C)O1 Show InChI InChI=1S/C21H32N2O7/c1-3-4-9-29-21(28)18(17-11-16(25)19(26)12(2)30-17)23-20(27)15(22)10-13-5-7-14(24)8-6-13/h5-8,12,15-19,24-26H,3-4,9-11,22H2,1-2H3,(H,23,27)/t12-,15+,16-,17-,18?,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay |
Bioorg Med Chem Lett 11: 715-8 (2001)
BindingDB Entry DOI: 10.7270/Q20C4V1Q |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM18132
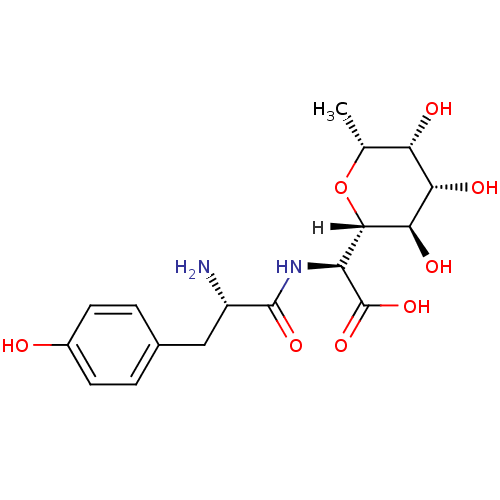
((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...)Show SMILES [H][C@]1(O[C@H](C)[C@H](O)[C@H](O)[C@H]1O)[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C17H24N2O8/c1-7-12(21)13(22)14(23)15(27-7)11(17(25)26)19-16(24)10(18)6-8-2-4-9(20)5-3-8/h2-5,7,10-15,20-23H,6,18H2,1H3,(H,19,24)(H,25,26)/t7-,10+,11+,12+,13+,14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay |
Bioorg Med Chem Lett 11: 715-8 (2001)
BindingDB Entry DOI: 10.7270/Q20C4V1Q |
More data for this
Ligand-Target Pair | |
Arginine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50091515

(Aminoalkyl adenylate and aminoacyl sulfamate analo...)Show SMILES N[C@@H](CCCNC(N)=[NH2+])C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C16H26N10O7S/c17-7(2-1-3-21-16(19)20)14(29)25-34(30,31)32-4-8-10(27)11(28)15(33-8)26-6-24-9-12(18)22-5-23-13(9)26/h5-8,10-11,15,27-28H,1-4,17H2,(H,25,29)(H2,18,22,23)(H4,19,20,21)/p+1/t7-,8+,10+,11+,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cognate Staphylococcus aureus Arginyl-tRNA synthetase |
Bioorg Med Chem Lett 10: 1871-4 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3FWJ |
More data for this
Ligand-Target Pair | |
Arginine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50091518

(CHEMBL434782 | Phosphoric acid (S)-2-amino-5-guani...)Show SMILES N[C@@H](CCCNC(N)=[NH2+])COP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C16H28N9O7P/c17-8(2-1-3-21-16(19)20)4-30-33(28,29)31-5-9-11(26)12(27)15(32-9)25-7-24-10-13(18)22-6-23-14(10)25/h6-9,11-12,15,26-27H,1-5,17H2,(H,28,29)(H2,18,22,23)(H4,19,20,21)/t8-,9+,11+,12+,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cognate Staphylococcus aureus Arginyl-tRNA synthetase |
Bioorg Med Chem Lett 10: 1871-4 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3FWJ |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50112577
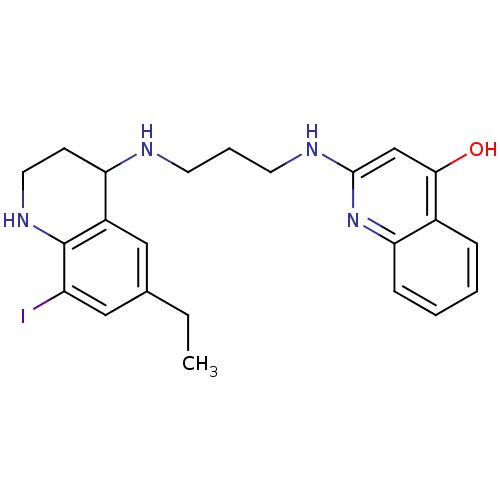
(2-[3-(6-Ethyl-8-iodo-1,2,3,4-tetrahydro-quinolin-4...)Show SMILES CCc1cc(I)c2NCCC(NCCCNc3cc(O)c4ccccc4n3)c2c1 Show InChI InChI=1S/C23H27IN4O/c1-2-15-12-17-19(8-11-27-23(17)18(24)13-15)25-9-5-10-26-22-14-21(29)16-6-3-4-7-20(16)28-22/h3-4,6-7,12-14,19,25,27H,2,5,8-11H2,1H3,(H2,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Staphylococcus aureus Methionyl-tRNA synthetase |
J Med Chem 45: 1959-62 (2002)
BindingDB Entry DOI: 10.7270/Q2HQ3Z6X |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase 1, cytoplasmic
(Homo sapiens (Human)) | BDBM50366646
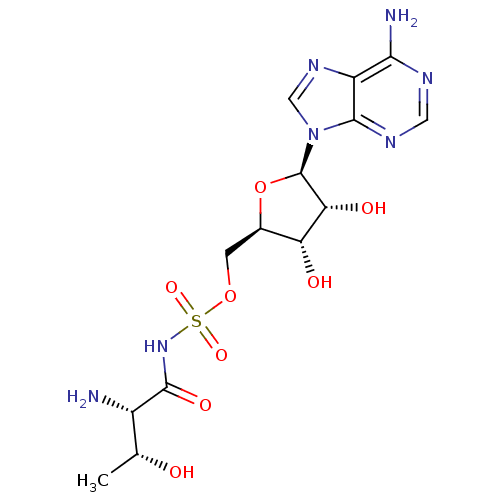
(CHEMBL1163068)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H21N7O8S/c1-5(22)7(15)13(25)20-30(26,27)28-2-6-9(23)10(24)14(29-6)21-4-19-8-11(16)17-3-18-12(8)21/h3-7,9-10,14,22-24H,2,15H2,1H3,(H,20,25)(H2,16,17,18)/t5-,6-,7+,9-,10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cognate Staphylococcus aureus threonyl tRNA synthetase |
Bioorg Med Chem Lett 10: 1871-4 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3FWJ |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50091496
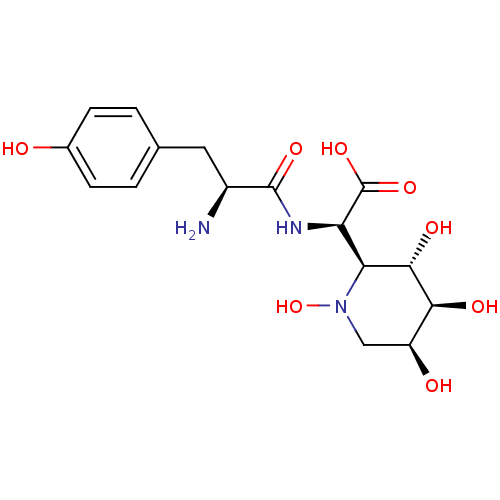
((R)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]([C@H]1[C@H](O)[C@@H](O)[C@@H](O)CN1O)C(O)=O Show InChI InChI=1S/C16H23N3O8/c17-9(5-7-1-3-8(20)4-2-7)15(24)18-11(16(25)26)12-14(23)13(22)10(21)6-19(12)27/h1-4,9-14,20-23,27H,5-6,17H2,(H,18,24)(H,25,26)/t9-,10-,11+,12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against tyrosyl tRNA synthetase from Staphylococcus aureus was determined |
Bioorg Med Chem Lett 10: 1811-4 (2000)
BindingDB Entry DOI: 10.7270/Q23F4Q5G |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50097744

((S)-[2-Amino-3-((S)-4-hydroxy-phenyl)-propionylami...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H]1OC[C@H](O)[C@H](O)[C@H]1O)C(O)=O Show InChI InChI=1S/C16H22N2O8/c17-9(5-7-1-3-8(19)4-2-7)15(23)18-11(16(24)25)14-13(22)12(21)10(20)6-26-14/h1-4,9-14,19-22H,5-6,17H2,(H,18,23)(H,24,25)/t9-,10-,11-,12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay |
Bioorg Med Chem Lett 11: 715-8 (2001)
BindingDB Entry DOI: 10.7270/Q20C4V1Q |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50097744

((S)-[2-Amino-3-((S)-4-hydroxy-phenyl)-propionylami...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H]1OC[C@H](O)[C@H](O)[C@H]1O)C(O)=O Show InChI InChI=1S/C16H22N2O8/c17-9(5-7-1-3-8(19)4-2-7)15(23)18-11(16(24)25)14-13(22)12(21)10(20)6-26-14/h1-4,9-14,19-22H,5-6,17H2,(H,18,23)(H,24,25)/t9-,10-,11-,12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay |
Bioorg Med Chem Lett 11: 711-4 (2001)
BindingDB Entry DOI: 10.7270/Q2445KR1 |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50104310

((R)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...)Show SMILES CCOC(=O)[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H]1C=C[C@H](O)[C@H](O)[C@H]1O |c:21| Show InChI InChI=1S/C19H26N2O7/c1-2-28-19(27)15(12-7-8-14(23)17(25)16(12)24)21-18(26)13(20)9-10-3-5-11(22)6-4-10/h3-8,12-17,22-25H,2,9,20H2,1H3,(H,21,26)/t12-,13-,14-,15+,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase |
Bioorg Med Chem Lett 11: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89BPK |
More data for this
Ligand-Target Pair | |
Histidine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50091516
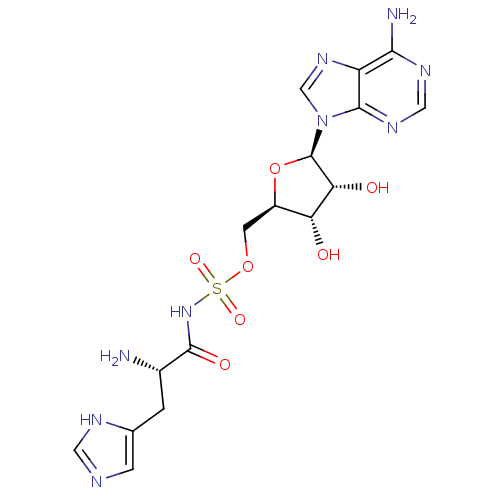
(5'-O-[(L-HISTIDYLAMINO)SULFONYL]ADENOSINE | CHEMBL...)Show SMILES N[C@@H](Cc1cnc[nH]1)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C16H21N9O7S/c17-8(1-7-2-19-4-20-7)15(28)24-33(29,30)31-3-9-11(26)12(27)16(32-9)25-6-23-10-13(18)21-5-22-14(10)25/h2,4-6,8-9,11-12,16,26-27H,1,3,17H2,(H,19,20)(H,24,28)(H2,18,21,22)/t8-,9+,11+,12+,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cognate Staphylococcus aureus Histidyl-tRNA synthetase |
Bioorg Med Chem Lett 10: 1871-4 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3FWJ |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50097747
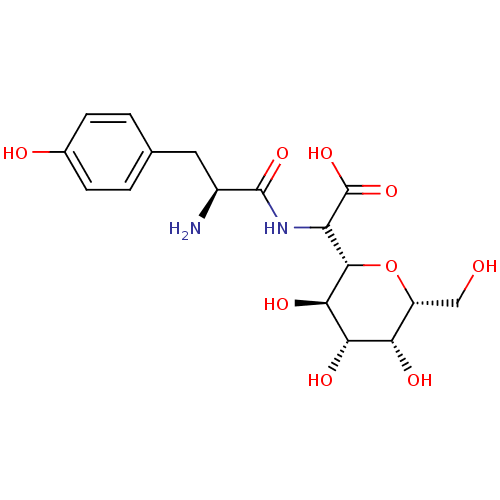
(CHEMBL162552 | [2-Amino-3-((S)-4-hydroxy-phenyl)-p...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)NC([C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O)C(O)=O Show InChI InChI=1S/C17H24N2O9/c18-9(5-7-1-3-8(21)4-2-7)16(25)19-11(17(26)27)15-14(24)13(23)12(22)10(6-20)28-15/h1-4,9-15,20-24H,5-6,18H2,(H,19,25)(H,26,27)/t9-,10+,11?,12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay |
Bioorg Med Chem Lett 11: 715-8 (2001)
BindingDB Entry DOI: 10.7270/Q20C4V1Q |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50104313
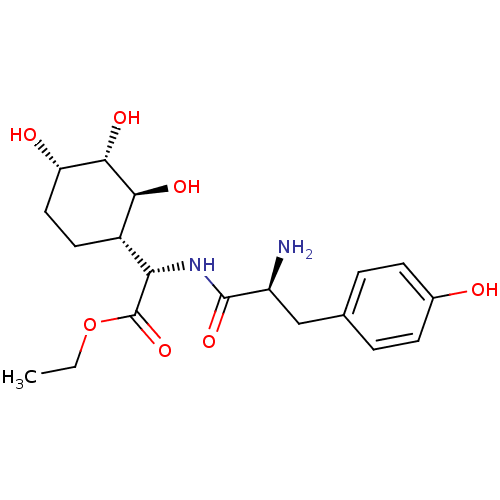
((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...)Show SMILES CCOC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H]1CC[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C19H28N2O7/c1-2-28-19(27)15(12-7-8-14(23)17(25)16(12)24)21-18(26)13(20)9-10-3-5-11(22)6-4-10/h3-6,12-17,22-25H,2,7-9,20H2,1H3,(H,21,26)/t12-,13-,14-,15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase |
Bioorg Med Chem Lett 11: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89BPK |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50097745
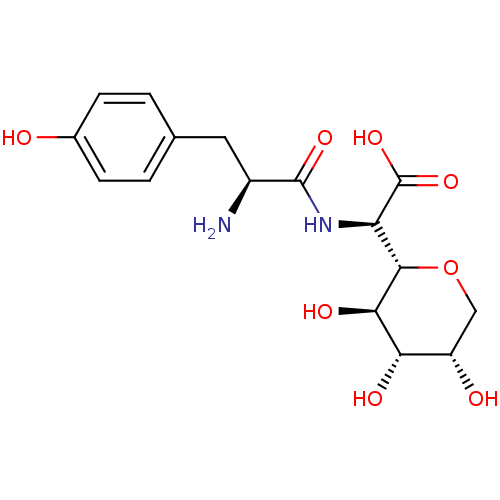
(CHEMBL159436 | [2-Amino-3-((S)-4-hydroxy-phenyl)-p...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]([C@@H]1OC[C@H](O)[C@H](O)[C@H]1O)C(O)=O Show InChI InChI=1S/C16H22N2O8/c17-9(5-7-1-3-8(19)4-2-7)15(23)18-11(16(24)25)14-13(22)12(21)10(20)6-26-14/h1-4,9-14,19-22H,5-6,17H2,(H,18,23)(H,24,25)/t9-,10-,11+,12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay |
Bioorg Med Chem Lett 11: 711-4 (2001)
BindingDB Entry DOI: 10.7270/Q2445KR1 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50112576
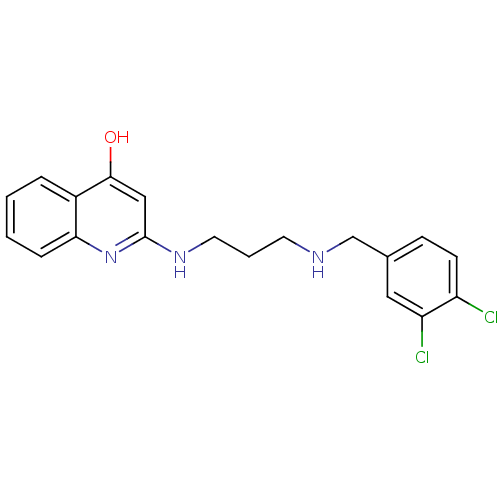
(2-(3-(3,4-dichlorobenzylamino)propylamino)quinolin...)Show InChI InChI=1S/C19H19Cl2N3O/c20-15-7-6-13(10-16(15)21)12-22-8-3-9-23-19-11-18(25)14-4-1-2-5-17(14)24-19/h1-2,4-7,10-11,22H,3,8-9,12H2,(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Methionyl-tRNA synthetase in a pyrophosphate exchange assay |
J Med Chem 45: 1959-62 (2002)
BindingDB Entry DOI: 10.7270/Q2HQ3Z6X |
More data for this
Ligand-Target Pair | |
Potassium-transporting ATPase alpha chain 1
(Sus scrofa (Pig)) | BDBM50000685

(6-Methyl-1-o-tolyl-2,3-dihydro-1H-pyrrolo[3,2-c]qu...)Show InChI InChI=1S/C19H18N2/c1-13-6-3-4-9-17(13)21-11-10-15-12-20-18-14(2)7-5-8-16(18)19(15)21/h3-9,12H,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit H+/K+ stimulated ATPase activity in lympholised gastric vesicles |
Bioorg Med Chem Lett 5: 543-546 (1995)
Article DOI: 10.1016/0960-894X(95)00069-6
BindingDB Entry DOI: 10.7270/Q2R211CV |
More data for this
Ligand-Target Pair | |
Potassium-transporting ATPase alpha chain 1
(Sus scrofa (Pig)) | BDBM50286575

(2-(2-Propyl-5-o-tolyl-thiazol-4-yl)-pyridin-4-ylam...)Show InChI InChI=1S/C18H19N3S/c1-3-6-16-21-17(15-11-13(19)9-10-20-15)18(22-16)14-8-5-4-7-12(14)2/h4-5,7-11H,3,6H2,1-2H3,(H2,19,20) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit H+/K+ stimulated ATPase activity in lympholised gastric vesicles |
Bioorg Med Chem Lett 5: 543-546 (1995)
Article DOI: 10.1016/0960-894X(95)00069-6
BindingDB Entry DOI: 10.7270/Q2R211CV |
More data for this
Ligand-Target Pair | |
Potassium-transporting ATPase alpha chain 1
(Sus scrofa (Pig)) | BDBM50286576
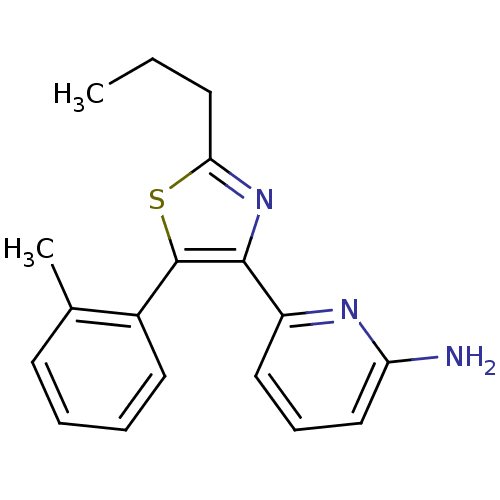
(6-(2-Propyl-5-o-tolyl-thiazol-4-yl)-pyridin-2-ylam...)Show InChI InChI=1S/C18H19N3S/c1-3-7-16-21-17(14-10-6-11-15(19)20-14)18(22-16)13-9-5-4-8-12(13)2/h4-6,8-11H,3,7H2,1-2H3,(H2,19,20) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit H+/K+ stimulated ATPase activity in lympholised gastric vesicles |
Bioorg Med Chem Lett 5: 543-546 (1995)
Article DOI: 10.1016/0960-894X(95)00069-6
BindingDB Entry DOI: 10.7270/Q2R211CV |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50104314
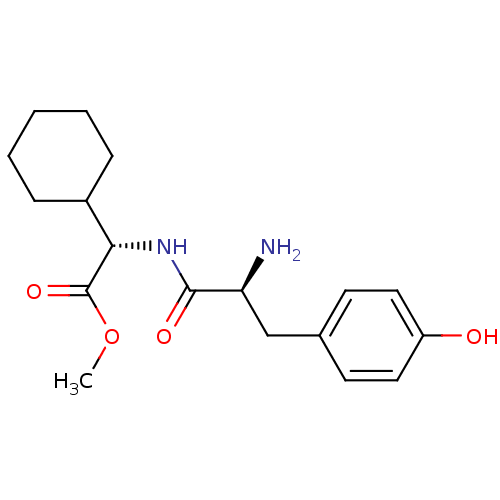
((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C1CCCCC1 Show InChI InChI=1S/C18H26N2O4/c1-24-18(23)16(13-5-3-2-4-6-13)20-17(22)15(19)11-12-7-9-14(21)10-8-12/h7-10,13,15-16,21H,2-6,11,19H2,1H3,(H,20,22)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase |
Bioorg Med Chem Lett 11: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89BPK |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50104312

((R)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...)Show SMILES CCOC(=O)[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H]1CC[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C19H28N2O7/c1-2-28-19(27)15(12-7-8-14(23)17(25)16(12)24)21-18(26)13(20)9-10-3-5-11(22)6-4-10/h3-6,12-17,22-25H,2,7-9,20H2,1H3,(H,21,26)/t12-,13-,14-,15+,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase |
Bioorg Med Chem Lett 11: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89BPK |
More data for this
Ligand-Target Pair | |
Potassium-transporting ATPase alpha chain 1
(Sus scrofa (Pig)) | BDBM50286571

(2-(2,5-Di-o-tolyl-thiazol-4-yl)-pyridine | CHEMBL3...)Show InChI InChI=1S/C22H18N2S/c1-15-9-3-5-11-17(15)21-20(19-13-7-8-14-23-19)24-22(25-21)18-12-6-4-10-16(18)2/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit H+/K+ stimulated ATPase activity in lympholised gastric vesicles |
Bioorg Med Chem Lett 5: 543-546 (1995)
Article DOI: 10.1016/0960-894X(95)00069-6
BindingDB Entry DOI: 10.7270/Q2R211CV |
More data for this
Ligand-Target Pair | |
Potassium-transporting ATPase alpha chain 1
(Sus scrofa (Pig)) | BDBM50286572

(2-(2-Propyl-5-o-tolyl-thiazol-4-yl)-pyridine | CHE...)Show InChI InChI=1S/C18H18N2S/c1-3-8-16-20-17(15-11-6-7-12-19-15)18(21-16)14-10-5-4-9-13(14)2/h4-7,9-12H,3,8H2,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit H+/K+ stimulated ATPase activity in lympholised gastric vesicles |
Bioorg Med Chem Lett 5: 543-546 (1995)
Article DOI: 10.1016/0960-894X(95)00069-6
BindingDB Entry DOI: 10.7270/Q2R211CV |
More data for this
Ligand-Target Pair | |
Potassium-transporting ATPase alpha chain 1
(Sus scrofa (Pig)) | BDBM50286570
(2-(2-Benzyl-5-o-tolyl-thiazol-4-yl)-pyridine | CHE...)Show InChI InChI=1S/C22H18N2S/c1-16-9-5-6-12-18(16)22-21(19-13-7-8-14-23-19)24-20(25-22)15-17-10-3-2-4-11-17/h2-14H,15H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit H+/K+ stimulated ATPase activity in lympholised gastric vesicles |
Bioorg Med Chem Lett 5: 543-546 (1995)
Article DOI: 10.1016/0960-894X(95)00069-6
BindingDB Entry DOI: 10.7270/Q2R211CV |
More data for this
Ligand-Target Pair | |
Potassium-transporting ATPase alpha chain 1
(Sus scrofa (Pig)) | BDBM50286573
(CHEMBL149912 | Methyl-[6-(2-propyl-5-o-tolyl-thiaz...)Show InChI InChI=1S/C19H21N3S/c1-4-8-17-22-18(15-11-7-12-16(20-3)21-15)19(23-17)14-10-6-5-9-13(14)2/h5-7,9-12H,4,8H2,1-3H3,(H,20,21) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit H+/K+ stimulated ATPase activity in lympholised gastric vesicles |
Bioorg Med Chem Lett 5: 543-546 (1995)
Article DOI: 10.1016/0960-894X(95)00069-6
BindingDB Entry DOI: 10.7270/Q2R211CV |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM18128

((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...)Show SMILES [H][C@]1([C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(O)=O)[C@H](O)[C@]2(O)CO[C@@H]([C@@H]2O)N1O |r| Show InChI InChI=1S/C17H23N3O9/c18-9(5-7-1-3-8(21)4-2-7)14(24)19-10(16(25)26)11-12(22)17(27)6-29-15(13(17)23)20(11)28/h1-4,9-13,15,21-23,27-28H,5-6,18H2,(H,19,24)(H,25,26)/t9-,10-,11-,12-,13-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against tyrosyl tRNA synthetase from mammalian cells was determined |
Bioorg Med Chem Lett 10: 1811-4 (2000)
BindingDB Entry DOI: 10.7270/Q23F4Q5G |
More data for this
Ligand-Target Pair | |
Potassium-transporting ATPase alpha chain 1
(Sus scrofa (Pig)) | BDBM50286574

(2-(5-Phenyl-2-propyl-thiazol-4-yl)-pyridine | CHEM...)Show InChI InChI=1S/C17H16N2S/c1-2-8-15-19-16(14-11-6-7-12-18-14)17(20-15)13-9-4-3-5-10-13/h3-7,9-12H,2,8H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit H+/K+ stimulated ATPase activity in lympholised gastric vesicles |
Bioorg Med Chem Lett 5: 543-546 (1995)
Article DOI: 10.1016/0960-894X(95)00069-6
BindingDB Entry DOI: 10.7270/Q2R211CV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data