 Found 172 hits with Last Name = 'pulido-rios' and Initial = 't'
Found 172 hits with Last Name = 'pulido-rios' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084436
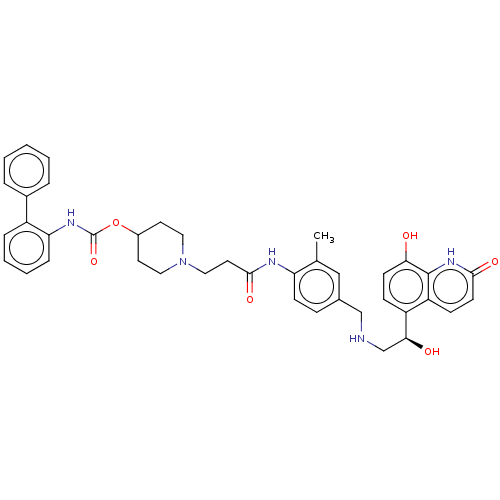
(CHEMBL3426693)Show SMILES Cc1cc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)ccc1NC(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C40H43N5O6/c1-26-23-27(24-41-25-36(47)31-12-15-35(46)39-32(31)13-16-37(48)44-39)11-14-33(26)42-38(49)19-22-45-20-17-29(18-21-45)51-40(50)43-34-10-6-5-9-30(34)28-7-3-2-4-8-28/h2-16,23,29,36,41,46-47H,17-22,24-25H2,1H3,(H,42,49)(H,43,50)(H,44,48)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084443
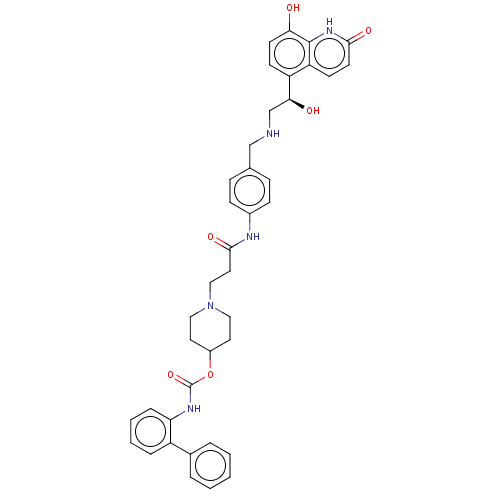
(CHEMBL3426687)Show SMILES O[C@@H](CNCc1ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C39H41N5O6/c45-34-16-14-31(32-15-17-36(47)43-38(32)34)35(46)25-40-24-26-10-12-28(13-11-26)41-37(48)20-23-44-21-18-29(19-22-44)50-39(49)42-33-9-5-4-8-30(33)27-6-2-1-3-7-27/h1-17,29,35,40,45-46H,18-25H2,(H,41,48)(H,42,49)(H,43,47)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084432
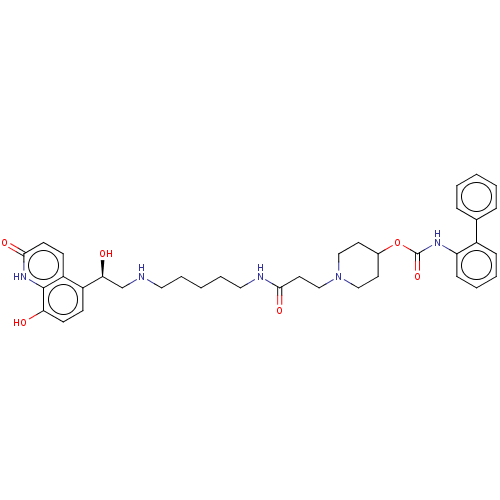
(CHEMBL3426697)Show SMILES O[C@@H](CNCCCCCNC(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C37H45N5O6/c43-32-15-13-29(30-14-16-35(46)41-36(30)32)33(44)25-38-20-7-2-8-21-39-34(45)19-24-42-22-17-27(18-23-42)48-37(47)40-31-12-6-5-11-28(31)26-9-3-1-4-10-26/h1,3-6,9-16,27,33,38,43-44H,2,7-8,17-25H2,(H,39,45)(H,40,47)(H,41,46)/t33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50337878
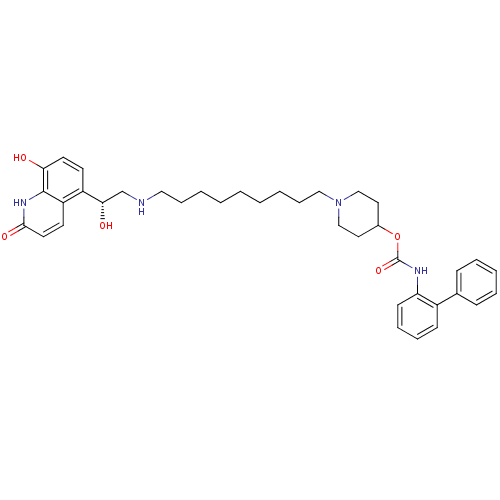
((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C38H48N4O5/c43-34-19-17-31(32-18-20-36(45)41-37(32)34)35(44)27-39-23-11-4-2-1-3-5-12-24-42-25-21-29(22-26-42)47-38(46)40-33-16-10-9-15-30(33)28-13-7-6-8-14-28/h6-10,13-20,29,35,39,43-44H,1-5,11-12,21-27H2,(H,40,46)(H,41,45)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084439
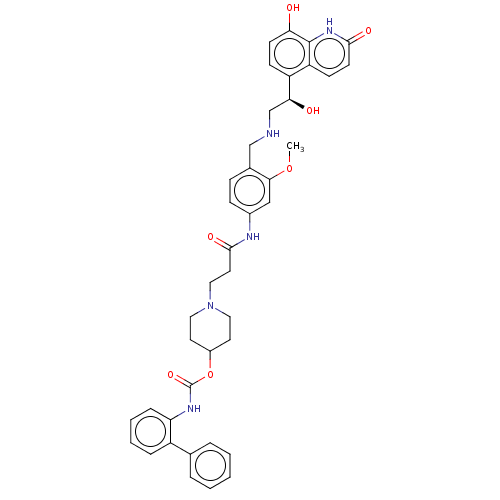
(CHEMBL3426691)Show SMILES COc1cc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)ccc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C40H43N5O7/c1-51-36-23-28(12-11-27(36)24-41-25-35(47)31-13-15-34(46)39-32(31)14-16-37(48)44-39)42-38(49)19-22-45-20-17-29(18-21-45)52-40(50)43-33-10-6-5-9-30(33)26-7-3-2-4-8-26/h2-16,23,29,35,41,46-47H,17-22,24-25H2,1H3,(H,42,49)(H,43,50)(H,44,48)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084440
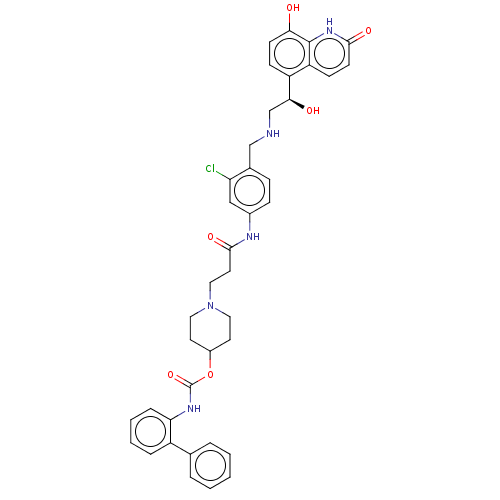
(CHEMBL3426690)Show SMILES O[C@@H](CNCc1ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1Cl)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C39H40ClN5O6/c40-32-22-27(11-10-26(32)23-41-24-35(47)30-12-14-34(46)38-31(30)13-15-36(48)44-38)42-37(49)18-21-45-19-16-28(17-20-45)51-39(50)43-33-9-5-4-8-29(33)25-6-2-1-3-7-25/h1-15,22,28,35,41,46-47H,16-21,23-24H2,(H,42,49)(H,43,50)(H,44,48)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084442
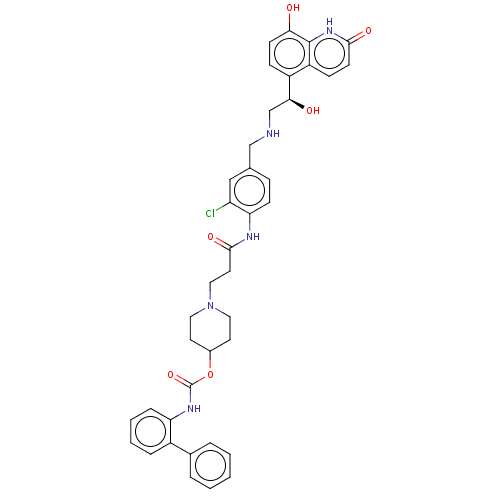
(CHEMBL3426688)Show SMILES O[C@@H](CNCc1ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(Cl)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C39H40ClN5O6/c40-31-22-25(23-41-24-35(47)29-11-14-34(46)38-30(29)12-15-36(48)44-38)10-13-33(31)42-37(49)18-21-45-19-16-27(17-20-45)51-39(50)43-32-9-5-4-8-28(32)26-6-2-1-3-7-26/h1-15,22,27,35,41,46-47H,16-21,23-24H2,(H,42,49)(H,43,50)(H,44,48)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084424
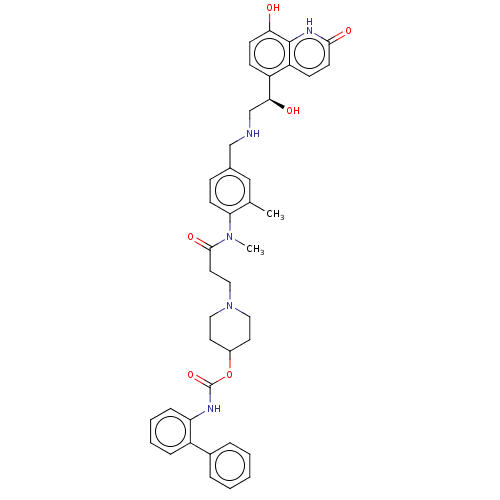
(CHEMBL3426705)Show SMILES CN(C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1C |r| Show InChI InChI=1S/C41H45N5O6/c1-27-24-28(25-42-26-37(48)32-13-16-36(47)40-33(32)14-17-38(49)44-40)12-15-35(27)45(2)39(50)20-23-46-21-18-30(19-22-46)52-41(51)43-34-11-7-6-10-31(34)29-8-4-3-5-9-29/h3-17,24,30,37,42,47-48H,18-23,25-26H2,1-2H3,(H,43,51)(H,44,49)/t37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084431
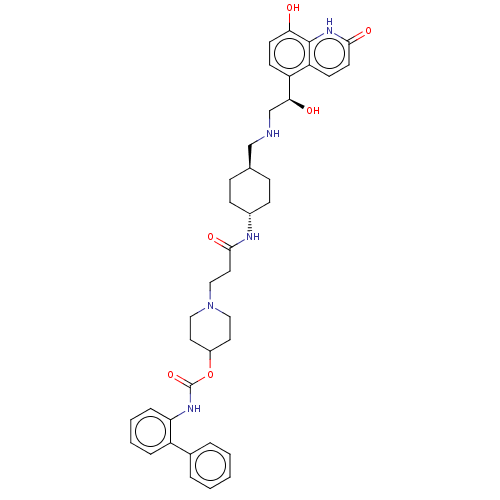
(CHEMBL3426698)Show SMILES O[C@@H](CNC[C@H]1CC[C@@H](CC1)NC(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:5.4,wD:1.0,8.11,(.27,-3.7,;1.33,-3.08,;2.67,-3.85,;2.67,-5.39,;4.01,-6.16,;4.01,-7.7,;5.35,-8.47,;5.35,-10.01,;4.01,-10.78,;2.68,-10.01,;2.68,-8.47,;4.01,-12.32,;5.35,-13.09,;6.42,-12.47,;5.35,-14.63,;6.69,-15.4,;6.69,-16.94,;8.03,-17.71,;8.03,-19.25,;6.69,-20.02,;5.36,-19.25,;5.36,-17.71,;6.69,-21.56,;5.35,-22.33,;4.29,-21.71,;5.35,-23.87,;4.02,-24.64,;2.68,-23.86,;1.35,-24.63,;1.34,-26.17,;2.67,-26.94,;4.01,-26.18,;5.34,-26.95,;6.68,-26.19,;8.01,-26.96,;8,-28.5,;6.66,-29.27,;5.33,-28.49,;1.33,-1.54,;2.66,-.77,;2.66,.77,;1.33,1.54,;1.33,2.77,;,.77,;-1.33,1.54,;-2.68,.77,;-3.75,1.39,;-2.68,-.77,;-1.33,-1.54,;,-.77,)| Show InChI InChI=1S/C39H47N5O6/c45-34-16-14-31(32-15-17-36(47)43-38(32)34)35(46)25-40-24-26-10-12-28(13-11-26)41-37(48)20-23-44-21-18-29(19-22-44)50-39(49)42-33-9-5-4-8-30(33)27-6-2-1-3-7-27/h1-9,14-17,26,28-29,35,40,45-46H,10-13,18-25H2,(H,41,48)(H,42,49)(H,43,47)/t26-,28-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084433
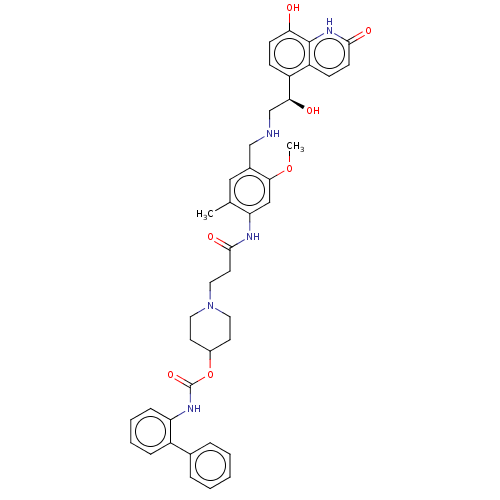
(CHEMBL3426696)Show SMILES COc1cc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(C)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H45N5O7/c1-26-22-28(24-42-25-36(48)31-12-14-35(47)40-32(31)13-15-38(49)45-40)37(52-2)23-34(26)43-39(50)18-21-46-19-16-29(17-20-46)53-41(51)44-33-11-7-6-10-30(33)27-8-4-3-5-9-27/h3-15,22-23,29,36,42,47-48H,16-21,24-25H2,1-2H3,(H,43,50)(H,44,51)(H,45,49)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084426
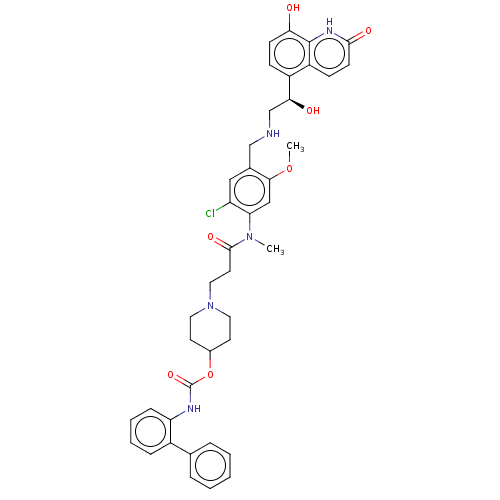
(CHEMBL3426703)Show SMILES COc1cc(N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(Cl)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H44ClN5O7/c1-46(34-23-37(53-2)27(22-32(34)42)24-43-25-36(49)30-12-14-35(48)40-31(30)13-15-38(50)45-40)39(51)18-21-47-19-16-28(17-20-47)54-41(52)44-33-11-7-6-10-29(33)26-8-4-3-5-9-26/h3-15,22-23,28,36,43,48-49H,16-21,24-25H2,1-2H3,(H,44,52)(H,45,50)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50249134
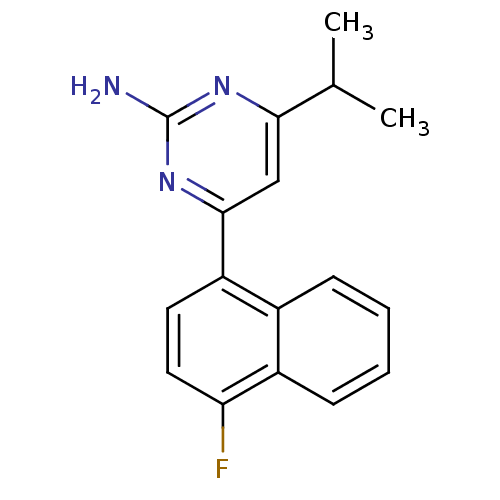
(4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...)Show InChI InChI=1S/C17H16FN3/c1-10(2)15-9-16(21-17(19)20-15)13-7-8-14(18)12-6-4-3-5-11(12)13/h3-10H,1-2H3,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Br J Pharmacol 127: 1075-82 (1999)
Article DOI: 10.1038/sj.bjp.0702632
BindingDB Entry DOI: 10.7270/Q24748DG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084434
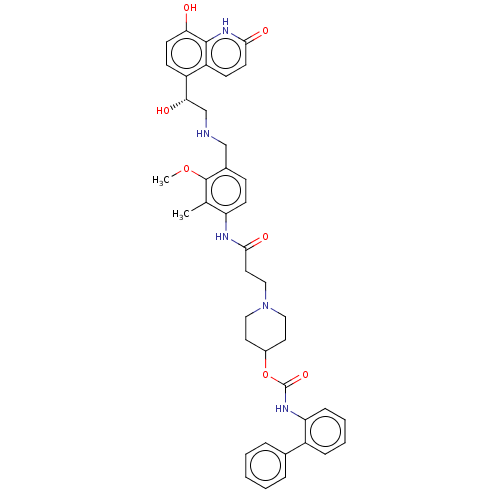
(CHEMBL3426695)Show SMILES COc1c(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1C |r| Show InChI InChI=1S/C41H45N5O7/c1-26-33(15-12-28(40(26)52-2)24-42-25-36(48)31-13-16-35(47)39-32(31)14-17-37(49)45-39)43-38(50)20-23-46-21-18-29(19-22-46)53-41(51)44-34-11-7-6-10-30(34)27-8-4-3-5-9-27/h3-17,29,36,42,47-48H,18-25H2,1-2H3,(H,43,50)(H,44,51)(H,45,49)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084425
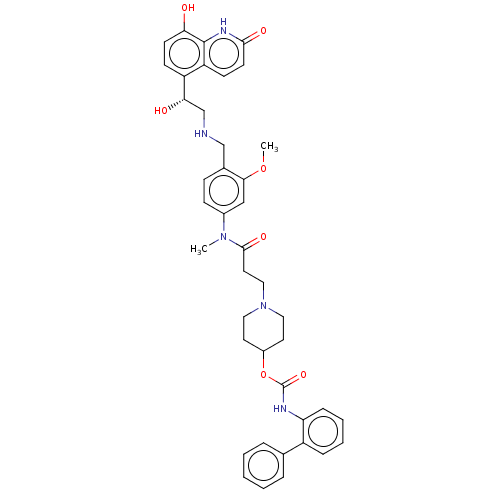
(CHEMBL3426704 | US9394275, I-25)Show SMILES COc1cc(ccc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12)N(C)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C41H45N5O7/c1-45(29-13-12-28(37(24-29)52-2)25-42-26-36(48)32-14-16-35(47)40-33(32)15-17-38(49)44-40)39(50)20-23-46-21-18-30(19-22-46)53-41(51)43-34-11-7-6-10-31(34)27-8-4-3-5-9-27/h3-17,24,30,36,42,47-48H,18-23,25-26H2,1-2H3,(H,43,51)(H,44,49)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084430

(CHEMBL3426699)Show SMILES O[C@@H](CNCc1ccc(cc1)C(=O)NCCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C39H41N5O6/c45-34-16-14-31(32-15-17-36(47)43-37(32)34)35(46)25-40-24-26-10-12-28(13-11-26)38(48)41-20-23-44-21-18-29(19-22-44)50-39(49)42-33-9-5-4-8-30(33)27-6-2-1-3-7-27/h1-17,29,35,40,45-46H,18-25H2,(H,41,48)(H,42,49)(H,43,47)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084438
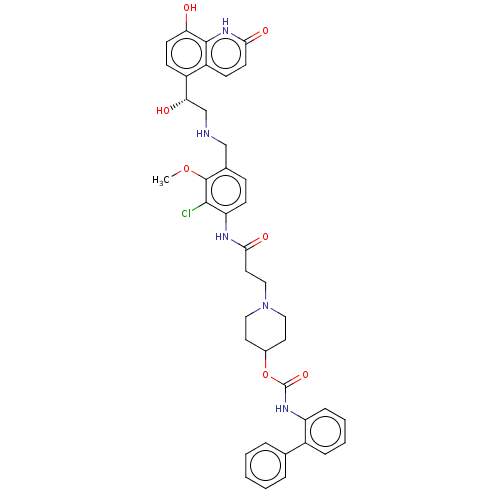
(CHEMBL3426692)Show SMILES COc1c(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1Cl |r| Show InChI InChI=1S/C40H42ClN5O7/c1-52-39-26(23-42-24-34(48)29-12-15-33(47)38-30(29)13-16-35(49)45-38)11-14-32(37(39)41)43-36(50)19-22-46-20-17-27(18-21-46)53-40(51)44-31-10-6-5-9-28(31)25-7-3-2-4-8-25/h2-16,27,34,42,47-48H,17-24H2,1H3,(H,43,50)(H,44,51)(H,45,49)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084427
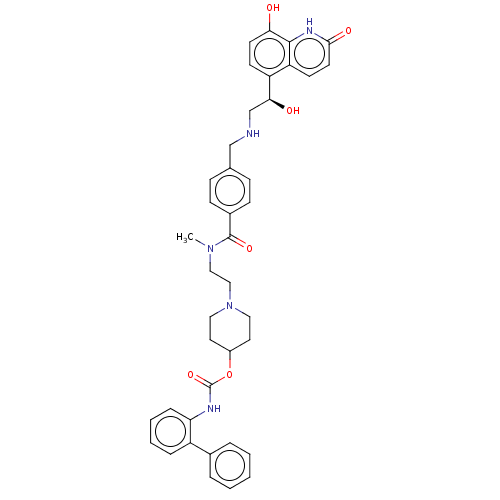
(CHEMBL3426702)Show SMILES CN(CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)C(=O)c1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1 |r| Show InChI InChI=1S/C40H43N5O6/c1-44(23-24-45-21-19-30(20-22-45)51-40(50)42-34-10-6-5-9-31(34)28-7-3-2-4-8-28)39(49)29-13-11-27(12-14-29)25-41-26-36(47)32-15-17-35(46)38-33(32)16-18-37(48)43-38/h2-18,30,36,41,46-47H,19-26H2,1H3,(H,42,50)(H,43,48)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85522
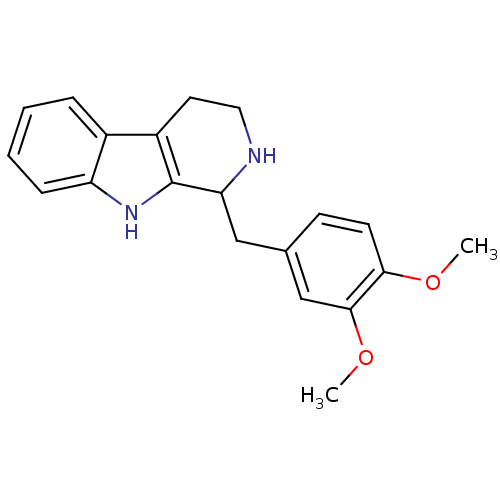
(LY-23728 | LY23728)Show InChI InChI=1S/C20H22N2O2/c1-23-18-8-7-13(12-19(18)24-2)11-17-20-15(9-10-21-17)14-5-3-4-6-16(14)22-20/h3-8,12,17,21-22H,9-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Br J Pharmacol 127: 1075-82 (1999)
Article DOI: 10.1038/sj.bjp.0702632
BindingDB Entry DOI: 10.7270/Q24748DG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084423
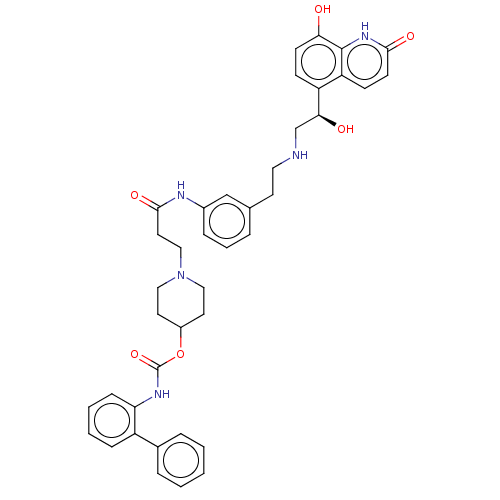
(CHEMBL3426706)Show SMILES O[C@@H](CNCCc1cccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C40H43N5O6/c46-35-15-13-32(33-14-16-37(48)44-39(33)35)36(47)26-41-21-17-27-7-6-10-29(25-27)42-38(49)20-24-45-22-18-30(19-23-45)51-40(50)43-34-12-5-4-11-31(34)28-8-2-1-3-9-28/h1-16,25,30,36,41,46-47H,17-24,26H2,(H,42,49)(H,43,50)(H,44,48)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Br J Pharmacol 127: 1075-82 (1999)
Article DOI: 10.1038/sj.bjp.0702632
BindingDB Entry DOI: 10.7270/Q24748DG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084435
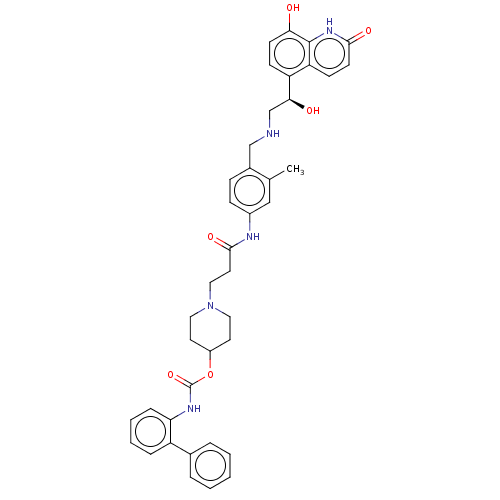
(CHEMBL3426694)Show SMILES Cc1cc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)ccc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C40H43N5O6/c1-26-23-29(12-11-28(26)24-41-25-36(47)32-13-15-35(46)39-33(32)14-16-37(48)44-39)42-38(49)19-22-45-20-17-30(18-21-45)51-40(50)43-34-10-6-5-9-31(34)27-7-3-2-4-8-27/h2-16,23,30,36,41,46-47H,17-22,24-25H2,1H3,(H,42,49)(H,43,50)(H,44,48)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM35256
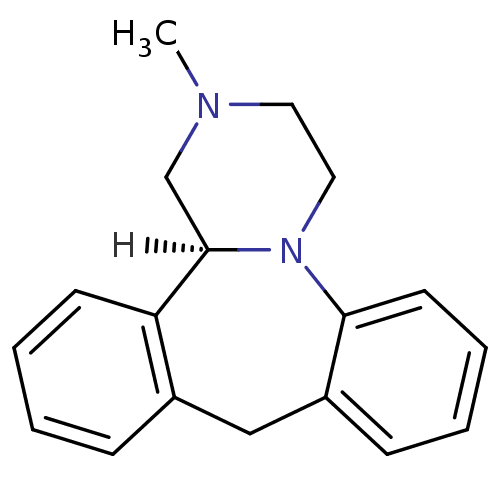
((S)-mianserin | Lerivon | MIANSERIN | MIANSERIN (+...)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Br J Pharmacol 127: 1075-82 (1999)
Article DOI: 10.1038/sj.bjp.0702632
BindingDB Entry DOI: 10.7270/Q24748DG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084441
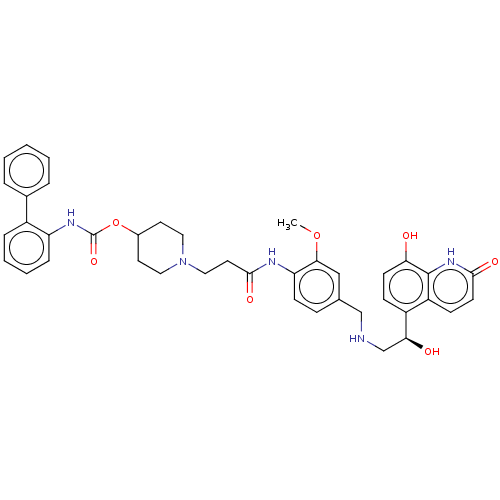
(CHEMBL3426689)Show SMILES COc1cc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)ccc1NC(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C40H43N5O7/c1-51-36-23-26(24-41-25-35(47)30-12-15-34(46)39-31(30)13-16-37(48)44-39)11-14-33(36)42-38(49)19-22-45-20-17-28(18-21-45)52-40(50)43-32-10-6-5-9-29(32)27-7-3-2-4-8-27/h2-16,23,28,35,41,46-47H,17-22,24-25H2,1H3,(H,42,49)(H,43,50)(H,44,48)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084437
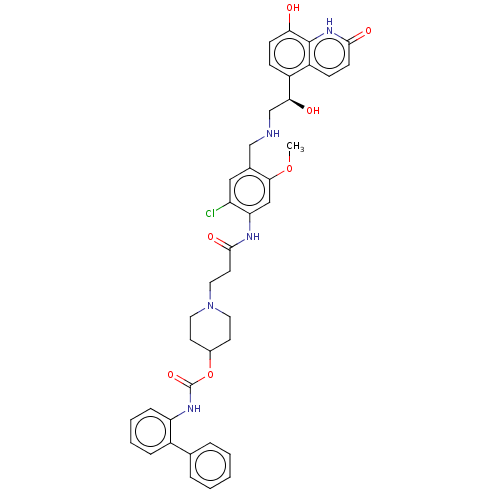
(Batefenterol | GSK961081 | GSK961081A | TD-5959)Show SMILES COc1cc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(Cl)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C40H42ClN5O7/c1-52-36-22-33(31(41)21-26(36)23-42-24-35(48)29-11-13-34(47)39-30(29)12-14-37(49)45-39)43-38(50)17-20-46-18-15-27(16-19-46)53-40(51)44-32-10-6-5-9-28(32)25-7-3-2-4-8-25/h2-14,21-22,27,35,42,47-48H,15-20,23-24H2,1H3,(H,43,50)(H,44,51)(H,45,49)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084421

(CHEMBL3426708)Show SMILES COc1ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1CCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H45N5O7/c1-52-37-15-11-29(25-28(37)17-21-42-26-36(48)32-12-14-35(47)40-33(32)13-16-38(49)45-40)43-39(50)20-24-46-22-18-30(19-23-46)53-41(51)44-34-10-6-5-9-31(34)27-7-3-2-4-8-27/h2-16,25,30,36,42,47-48H,17-24,26H2,1H3,(H,43,50)(H,44,51)(H,45,49)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771
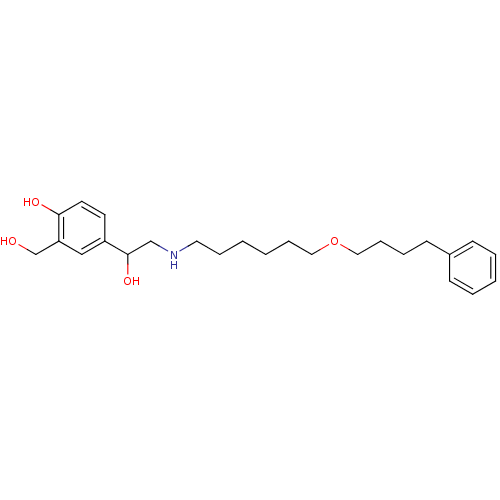
(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50419652

(MILVETEROL)Show SMILES O[C@@H](CNCCc1ccc(NC[C@H](O)c2ccccc2)cc1)c1ccc(O)c(NC=O)c1 |r| Show InChI InChI=1S/C25H29N3O4/c29-17-28-22-14-20(8-11-23(22)30)24(31)15-26-13-12-18-6-9-21(10-7-18)27-16-25(32)19-4-2-1-3-5-19/h1-11,14,17,24-27,30-32H,12-13,15-16H2,(H,28,29)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084428
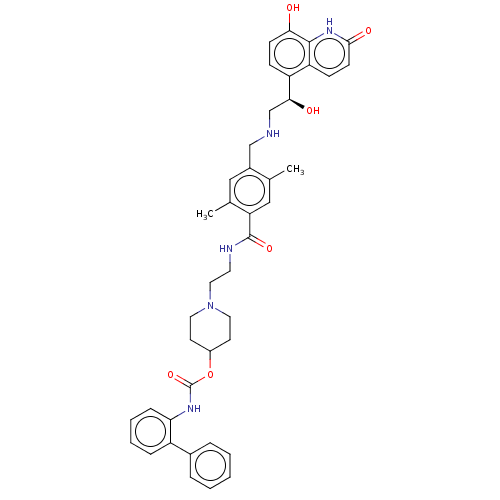
(CHEMBL3426701)Show SMILES Cc1cc(C(=O)NCCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(C)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H45N5O6/c1-26-23-34(27(2)22-29(26)24-42-25-37(48)32-12-14-36(47)39-33(32)13-15-38(49)45-39)40(50)43-18-21-46-19-16-30(17-20-46)52-41(51)44-35-11-7-6-10-31(35)28-8-4-3-5-9-28/h3-15,22-23,30,37,42,47-48H,16-21,24-25H2,1-2H3,(H,43,50)(H,44,51)(H,45,49)/t37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084429
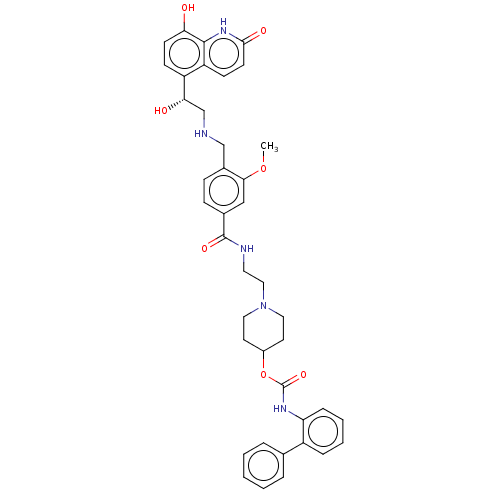
(CHEMBL3426700)Show SMILES COc1cc(ccc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12)C(=O)NCCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C40H43N5O7/c1-51-36-23-27(11-12-28(36)24-41-25-35(47)31-13-15-34(46)38-32(31)14-16-37(48)44-38)39(49)42-19-22-45-20-17-29(18-21-45)52-40(50)43-33-10-6-5-9-30(33)26-7-3-2-4-8-26/h2-16,23,29,35,41,46-47H,17-22,24-25H2,1H3,(H,42,49)(H,43,50)(H,44,48)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084422
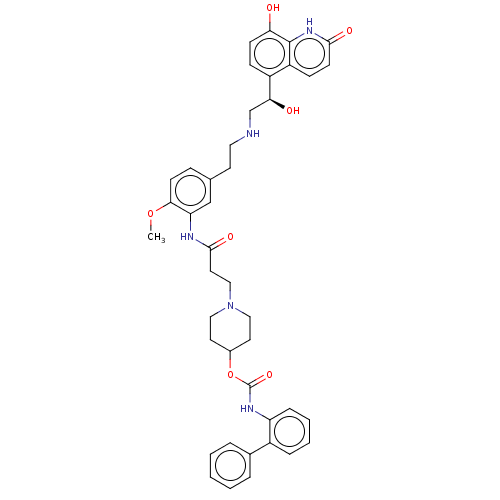
(CHEMBL3426707)Show SMILES COc1ccc(CCNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1NC(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C41H45N5O7/c1-52-37-15-11-27(17-21-42-26-36(48)31-12-14-35(47)40-32(31)13-16-38(49)45-40)25-34(37)43-39(50)20-24-46-22-18-29(19-23-46)53-41(51)44-33-10-6-5-9-30(33)28-7-3-2-4-8-28/h2-16,25,29,36,42,47-48H,17-24,26H2,1H3,(H,43,50)(H,44,51)(H,45,49)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Br J Pharmacol 127: 1075-82 (1999)
Article DOI: 10.1038/sj.bjp.0702632
BindingDB Entry DOI: 10.7270/Q24748DG |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM86453

(CAS_73573-87-2 | Formoterol | NSC_3083544)Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018608
(CHEMBL3290989)Show SMILES COc1ccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)cc1-c1ccccc1 Show InChI InChI=1S/C30H32N2O4/c1-36-30-14-12-26(18-27(30)22-5-3-2-4-6-22)32-25-10-7-21(8-11-25)15-16-31-19-29(35)23-9-13-28(34)24(17-23)20-33/h2-14,17-18,29,31-35H,15-16,19-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
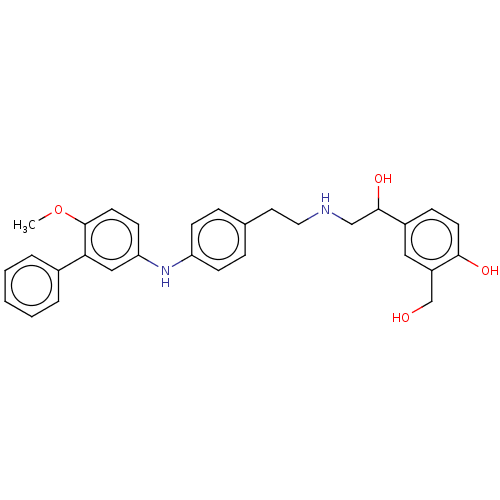
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018557
(CHEMBL3290992)Show SMILES CSc1ccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)cc1 Show InChI InChI=1S/C24H28N2O3S/c1-30-22-9-7-21(8-10-22)26-20-5-2-17(3-6-20)12-13-25-15-24(29)18-4-11-23(28)19(14-18)16-27/h2-11,14,24-29H,12-13,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
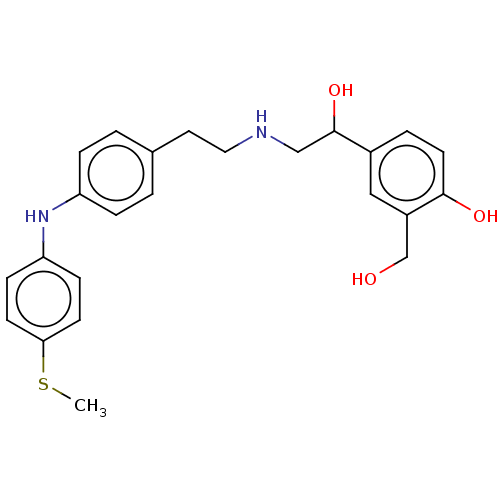
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018558
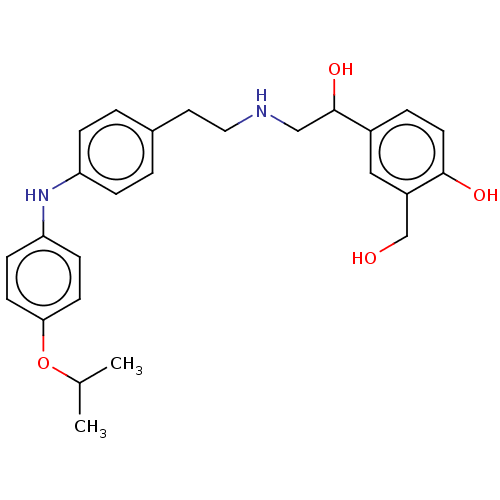
(CHEMBL3290993)Show SMILES CC(C)Oc1ccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)cc1 Show InChI InChI=1S/C26H32N2O4/c1-18(2)32-24-10-8-23(9-11-24)28-22-6-3-19(4-7-22)13-14-27-16-26(31)20-5-12-25(30)21(15-20)17-29/h3-12,15,18,26-31H,13-14,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018563
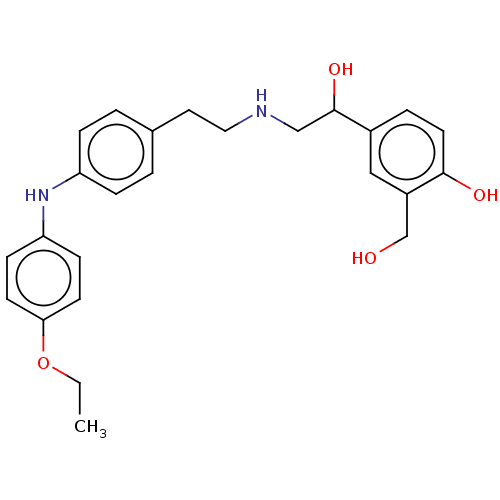
(CHEMBL3290991)Show SMILES CCOc1ccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)cc1 Show InChI InChI=1S/C25H30N2O4/c1-2-31-23-10-8-22(9-11-23)27-21-6-3-18(4-7-21)13-14-26-16-25(30)19-5-12-24(29)20(15-19)17-28/h3-12,15,25-30H,2,13-14,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018562
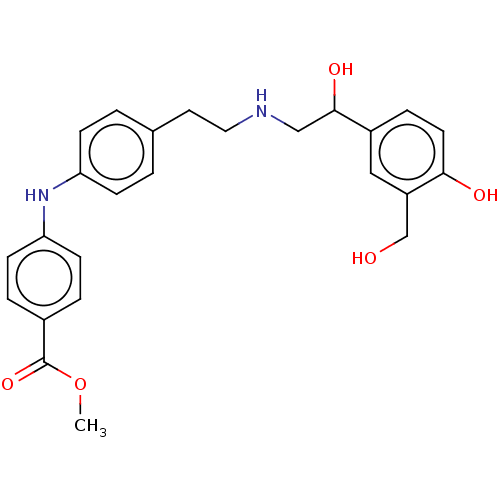
(CHEMBL3290996)Show SMILES COC(=O)c1ccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)cc1 Show InChI InChI=1S/C25H28N2O5/c1-32-25(31)18-4-9-22(10-5-18)27-21-7-2-17(3-8-21)12-13-26-15-24(30)19-6-11-23(29)20(14-19)16-28/h2-11,14,24,26-30H,12-13,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018601
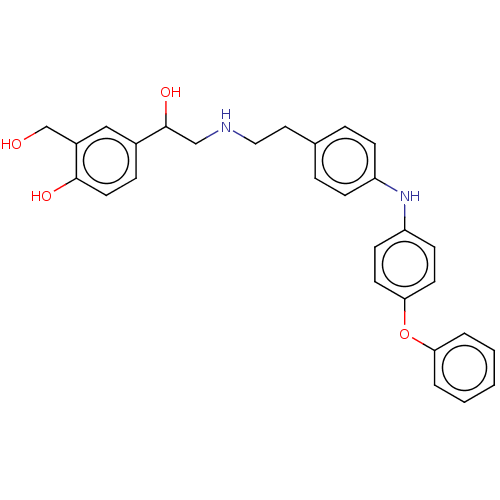
(CHEMBL3290982)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C29H30N2O4/c32-20-23-18-22(8-15-28(23)33)29(34)19-30-17-16-21-6-9-24(10-7-21)31-25-11-13-27(14-12-25)35-26-4-2-1-3-5-26/h1-15,18,29-34H,16-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018607

(CHEMBL3290988)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2cccc(c2)-c2ccccc2)cc1 Show InChI InChI=1S/C29H30N2O3/c32-20-25-17-24(11-14-28(25)33)29(34)19-30-16-15-21-9-12-26(13-10-21)31-27-8-4-7-23(18-27)22-5-2-1-3-6-22/h1-14,17-18,29-34H,15-16,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018606
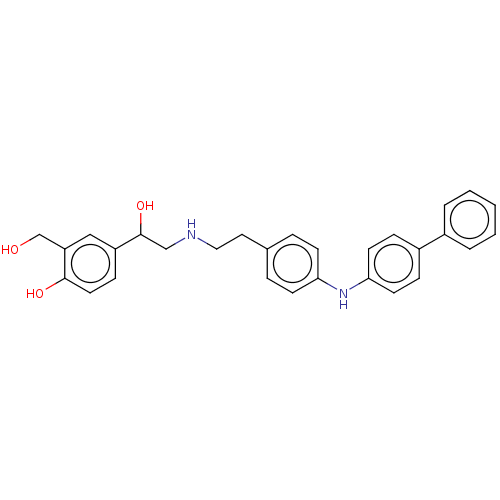
(CHEMBL3290987)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C29H30N2O3/c32-20-25-18-24(10-15-28(25)33)29(34)19-30-17-16-21-6-11-26(12-7-21)31-27-13-8-23(9-14-27)22-4-2-1-3-5-22/h1-15,18,29-34H,16-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
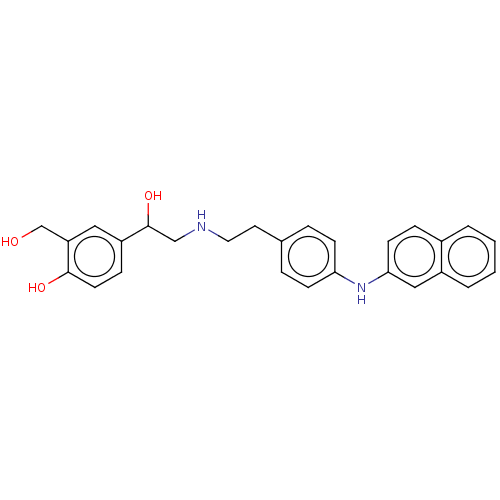
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018602
(CHEMBL3290983)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C27H28N2O3/c30-18-23-15-22(8-12-26(23)31)27(32)17-28-14-13-19-5-9-24(10-6-19)29-25-11-7-20-3-1-2-4-21(20)16-25/h1-12,15-16,27-32H,13-14,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50249134

(4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...)Show InChI InChI=1S/C17H16FN3/c1-10(2)15-9-16(21-17(19)20-15)13-7-8-14(18)12-6-4-3-5-11(12)13/h3-10H,1-2H3,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Br J Pharmacol 127: 1075-82 (1999)
Article DOI: 10.1038/sj.bjp.0702632
BindingDB Entry DOI: 10.7270/Q24748DG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50249134

(4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...)Show InChI InChI=1S/C17H16FN3/c1-10(2)15-9-16(21-17(19)20-15)13-7-8-14(18)12-6-4-3-5-11(12)13/h3-10H,1-2H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Br J Pharmacol 127: 1075-82 (1999)
Article DOI: 10.1038/sj.bjp.0702632
BindingDB Entry DOI: 10.7270/Q24748DG |
More data for this
Ligand-Target Pair | |
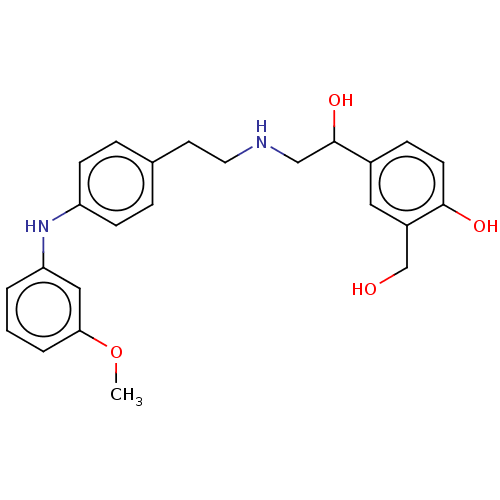
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018609
(CHEMBL3290990)Show SMILES COc1cccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)c1 Show InChI InChI=1S/C24H28N2O4/c1-30-22-4-2-3-21(14-22)26-20-8-5-17(6-9-20)11-12-25-15-24(29)18-7-10-23(28)19(13-18)16-27/h2-10,13-14,24-29H,11-12,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
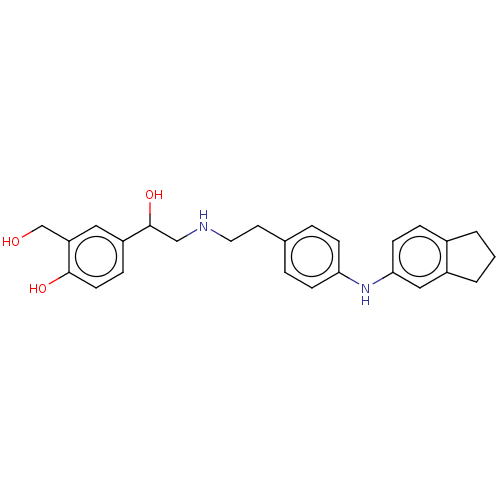
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018605
(CHEMBL3290986)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2ccc3CCCc3c2)cc1 Show InChI InChI=1S/C26H30N2O3/c29-17-22-14-21(7-11-25(22)30)26(31)16-27-13-12-18-4-8-23(9-5-18)28-24-10-6-19-2-1-3-20(19)15-24/h4-11,14-15,26-31H,1-3,12-13,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50249134

(4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...)Show InChI InChI=1S/C17H16FN3/c1-10(2)15-9-16(21-17(19)20-15)13-7-8-14(18)12-6-4-3-5-11(12)13/h3-10H,1-2H3,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Br J Pharmacol 127: 1075-82 (1999)
Article DOI: 10.1038/sj.bjp.0702632
BindingDB Entry DOI: 10.7270/Q24748DG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 5A
(Homo sapiens (Human)) | BDBM50249134

(4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...)Show InChI InChI=1S/C17H16FN3/c1-10(2)15-9-16(21-17(19)20-15)13-7-8-14(18)12-6-4-3-5-11(12)13/h3-10H,1-2H3,(H2,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Br J Pharmacol 127: 1075-82 (1999)
Article DOI: 10.1038/sj.bjp.0702632
BindingDB Entry DOI: 10.7270/Q24748DG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50249134

(4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...)Show InChI InChI=1S/C17H16FN3/c1-10(2)15-9-16(21-17(19)20-15)13-7-8-14(18)12-6-4-3-5-11(12)13/h3-10H,1-2H3,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Br J Pharmacol 127: 1075-82 (1999)
Article DOI: 10.1038/sj.bjp.0702632
BindingDB Entry DOI: 10.7270/Q24748DG |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018561
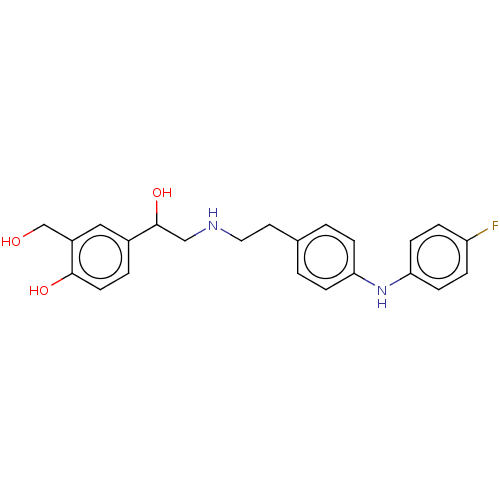
(CHEMBL3290995)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2ccc(F)cc2)cc1 Show InChI InChI=1S/C23H25FN2O3/c24-19-4-8-21(9-5-19)26-20-6-1-16(2-7-20)11-12-25-14-23(29)17-3-10-22(28)18(13-17)15-27/h1-10,13,23,25-29H,11-12,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data