 Found 535 hits with Last Name = 'radi' and Initial = 'm'
Found 535 hits with Last Name = 'radi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Tested for inhibitor binding of wild-type HIV PR |
J Med Chem 47: 2030-6 (2004)
Article DOI: 10.1021/jm031105q
BindingDB Entry DOI: 10.7270/Q2CN74PV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50581791

(CHEMBL5073221)Show SMILES NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type HIV-1 protease assessed as initial inhibition constant by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02177
BindingDB Entry DOI: 10.7270/Q208695M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50143837
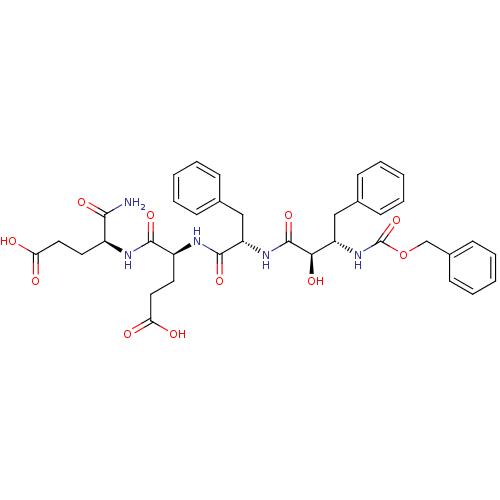
((S)-4-[(S)-2-((2R,3S)-3-Benzyloxycarbonylamino-2-h...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C37H43N5O11/c38-33(48)26(16-18-30(43)44)39-34(49)27(17-19-31(45)46)40-35(50)29(21-24-12-6-2-7-13-24)41-36(51)32(47)28(20-23-10-4-1-5-11-23)42-37(52)53-22-25-14-8-3-9-15-25/h1-15,26-29,32,47H,16-22H2,(H2,38,48)(H,39,49)(H,40,50)(H,41,51)(H,42,52)(H,43,44)(H,45,46)/t26-,27-,28-,29-,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Tested for inhibitor binding of Val82Ala mutant of HIV PR |
J Med Chem 47: 2030-6 (2004)
Article DOI: 10.1021/jm031105q
BindingDB Entry DOI: 10.7270/Q2CN74PV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Tested for inhibitor binding of D25N/V82A mutant of HIV PR |
J Med Chem 47: 2030-6 (2004)
Article DOI: 10.1021/jm031105q
BindingDB Entry DOI: 10.7270/Q2CN74PV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nischarin
(RAT) | BDBM50387827
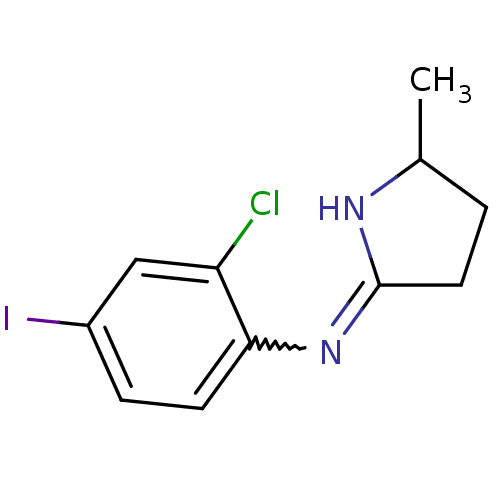
(CHEMBL2058635)Show InChI InChI=1S/C11H12ClIN2/c1-7-2-5-11(14-7)15-10-4-3-8(13)6-9(10)12/h3-4,6-7H,2,5H2,1H3,(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [125I]PIC from Imidazoline-1 receptor in rat PC12 cell membrane incubated for 30 mins by gamma counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50143837

((S)-4-[(S)-2-((2R,3S)-3-Benzyloxycarbonylamino-2-h...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C37H43N5O11/c38-33(48)26(16-18-30(43)44)39-34(49)27(17-19-31(45)46)40-35(50)29(21-24-12-6-2-7-13-24)41-36(51)32(47)28(20-23-10-4-1-5-11-23)42-37(52)53-22-25-14-8-3-9-15-25/h1-15,26-29,32,47H,16-22H2,(H2,38,48)(H,39,49)(H,40,50)(H,41,51)(H,42,52)(H,43,44)(H,45,46)/t26-,27-,28-,29-,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Tested for inhibitor binding of wild-type HIV PR |
J Med Chem 47: 2030-6 (2004)
Article DOI: 10.1021/jm031105q
BindingDB Entry DOI: 10.7270/Q2CN74PV |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50021809
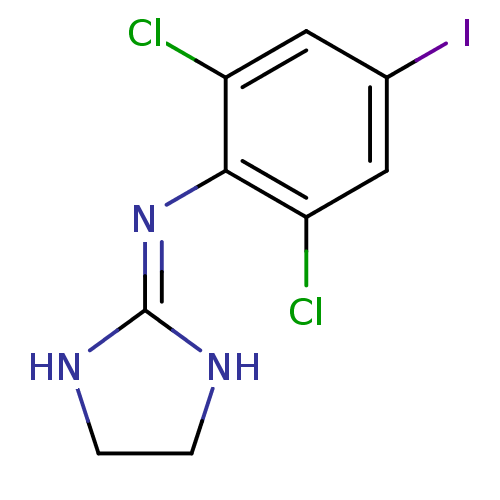
((2,6-Dichloro-4-iodo-phenyl)-(4,5-dihydro-1H-imida...)Show SMILES Clc1cc(I)cc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H8Cl2IN3/c10-6-3-5(12)4-7(11)8(6)15-9-13-1-2-14-9/h3-4H,1-2H2,(H2,13,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [125I]PIC from Imidazoline-1 receptor in rat PC12 cell membrane incubated for 30 mins by gamma counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50091345
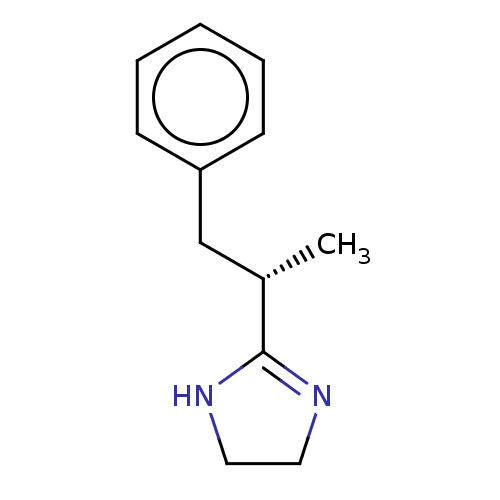
(CHEMBL2092861)Show InChI InChI=1S/C12H16N2/c1-10(12-13-7-8-14-12)9-11-5-3-2-4-6-11/h2-6,10H,7-9H2,1H3,(H,13,14)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [125I]PIC from Imidazoline-1 receptor in rat PC12 cell membrane by gamma counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81807
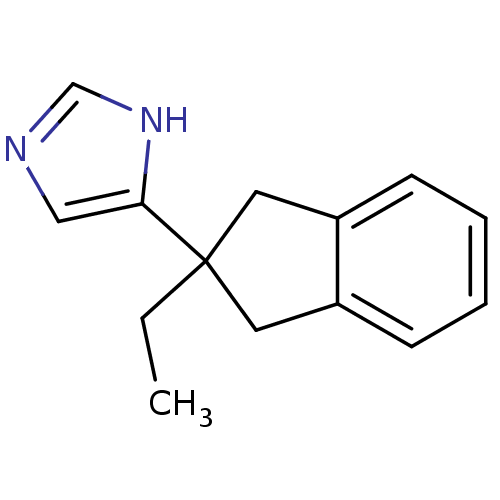
(ATIPAMEZOLE | CAS_104054-27-5 | NSC_71310)Show InChI InChI=1S/C14H16N2/c1-2-14(13-9-15-10-16-13)7-11-5-3-4-6-12(11)8-14/h3-6,9-10H,2,7-8H2,1H3,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [3H]RS-79948-197 from recombinant human alpha2A adrenoreceptor expressed in CHOK1 cell membrane by scintillation counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50240366
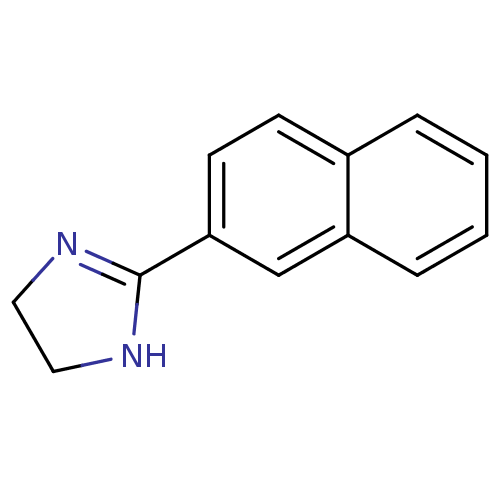
(2-(naphthalen-2-yl)-4,5-dihydro-1H-imidazole | 2-N...)Show InChI InChI=1S/C13H12N2/c1-2-4-11-9-12(6-5-10(11)3-1)13-14-7-8-15-13/h1-6,9H,7-8H2,(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [125I]PIC from Imidazoline-1 receptor in rat PC12 cell membrane incubated for 30 mins by gamma counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50085683
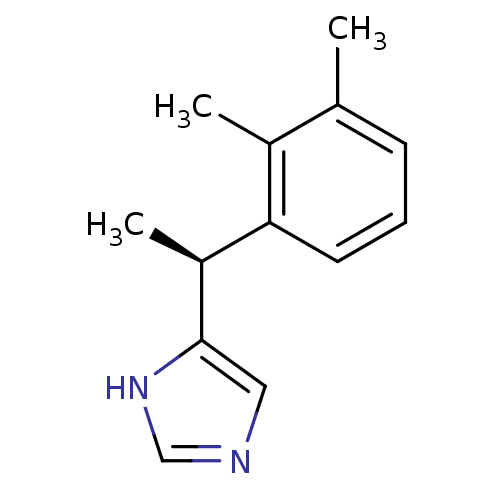
((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...)Show InChI InChI=1S/C13H16N2/c1-9-5-4-6-12(10(9)2)11(3)13-7-14-8-15-13/h4-8,11H,1-3H3,(H,14,15)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [3H]RS-79948-197 from recombinant human alpha2A adrenoreceptor expressed in CHOK1 cell membrane by scintillation counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50179393

(Efaroxan)Show InChI InChI=1S/C13H16N2O/c1-2-13(12-14-7-8-15-12)9-10-5-3-4-6-11(10)16-13/h3-6H,2,7-9H2,1H3,(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [3H]RS-79948-197 from recombinant human alpha2A adrenoreceptor expressed in CHOK1 cell membrane by scintillation counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50436581
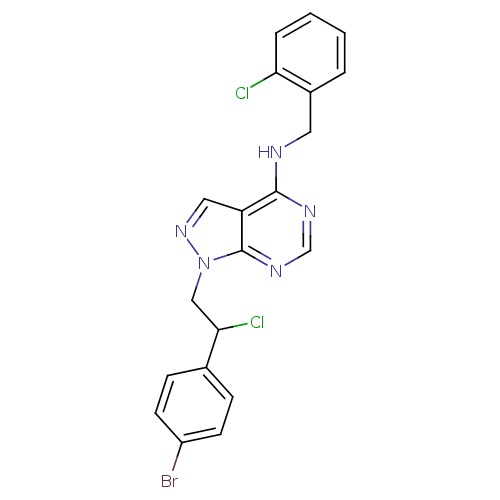
(CHEMBL2397805)Show SMILES ClC(Cn1ncc2c(NCc3ccccc3Cl)ncnc12)c1ccc(Br)cc1 Show InChI InChI=1S/C20H16BrCl2N5/c21-15-7-5-13(6-8-15)18(23)11-28-20-16(10-27-28)19(25-12-26-20)24-9-14-3-1-2-4-17(14)22/h1-8,10,12,18H,9,11H2,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant wild type human Abl using abitide as substrate |
J Med Chem 56: 5382-94 (2014)
Article DOI: 10.1021/jm400233w
BindingDB Entry DOI: 10.7270/Q29G5P63 |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50019646

(2-(alpha-(2,6-Dichlorophenoxy)ethyl)2-imidazoline ...)Show InChI InChI=1S/C11H12Cl2N2O/c1-7(11-14-5-6-15-11)16-10-8(12)3-2-4-9(10)13/h2-4,7H,5-6H2,1H3,(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [125I]PIC from Imidazoline-1 receptor in rat PC12 cell membrane incubated for 30 mins by gamma counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50102904
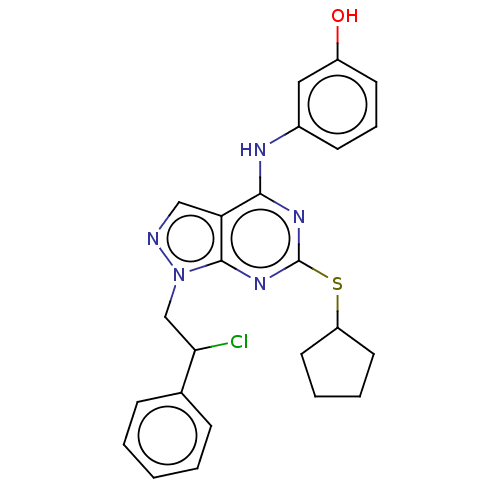
(CHEMBL3394091)Show SMILES Oc1cccc(Nc2nc(SC3CCCC3)nc3n(CC(Cl)c4ccccc4)ncc23)c1 Show InChI InChI=1S/C24H24ClN5OS/c25-21(16-7-2-1-3-8-16)15-30-23-20(14-26-30)22(27-17-9-6-10-18(31)13-17)28-24(29-23)32-19-11-4-5-12-19/h1-3,6-10,13-14,19,21,31H,4-5,11-12,15H2,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP |
J Med Chem 58: 347-61 (2015)
Article DOI: 10.1021/jm5013159
BindingDB Entry DOI: 10.7270/Q2416ZTT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479681

(CHEMBL478258)Show SMILES [H][C@@]1(CSc2nc([C@H](C)c3c(F)cccc3F)c(C)c(=O)[nH]2)C[C@@]1([H])c1ccc(OC)cc1 |r| Show InChI InChI=1S/C24H24F2N2O2S/c1-13(21-19(25)5-4-6-20(21)26)22-14(2)23(29)28-24(27-22)31-12-16-11-18(16)15-7-9-17(30-3)10-8-15/h4-10,13,16,18H,11-12H2,1-3H3,(H,27,28,29)/t13-,16+,18+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479681

(CHEMBL478258)Show SMILES [H][C@@]1(CSc2nc([C@H](C)c3c(F)cccc3F)c(C)c(=O)[nH]2)C[C@@]1([H])c1ccc(OC)cc1 |r| Show InChI InChI=1S/C24H24F2N2O2S/c1-13(21-19(25)5-4-6-20(21)26)22-14(2)23(29)28-24(27-22)31-12-16-11-18(16)15-7-9-17(30-3)10-8-15/h4-10,13,16,18H,11-12H2,1-3H3,(H,27,28,29)/t13-,16+,18+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50102908
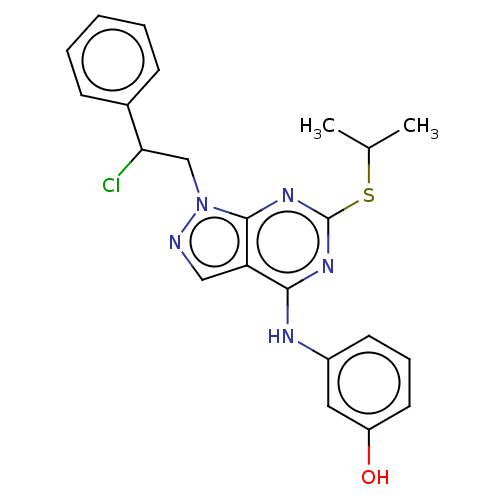
(CHEMBL3393071)Show SMILES CC(C)Sc1nc(Nc2cccc(O)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 Show InChI InChI=1S/C22H22ClN5OS/c1-14(2)30-22-26-20(25-16-9-6-10-17(29)11-16)18-12-24-28(21(18)27-22)13-19(23)15-7-4-3-5-8-15/h3-12,14,19,29H,13H2,1-2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP |
J Med Chem 58: 347-61 (2015)
Article DOI: 10.1021/jm5013159
BindingDB Entry DOI: 10.7270/Q2416ZTT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479681

(CHEMBL478258)Show SMILES [H][C@@]1(CSc2nc([C@H](C)c3c(F)cccc3F)c(C)c(=O)[nH]2)C[C@@]1([H])c1ccc(OC)cc1 |r| Show InChI InChI=1S/C24H24F2N2O2S/c1-13(21-19(25)5-4-6-20(21)26)22-14(2)23(29)28-24(27-22)31-12-16-11-18(16)15-7-9-17(30-3)10-8-15/h4-10,13,16,18H,11-12H2,1-3H3,(H,27,28,29)/t13-,16+,18+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 free reverse transcriptase |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Abl by filter-binding assay |
J Med Chem 54: 2610-26 (2011)
Article DOI: 10.1021/jm1012819
BindingDB Entry DOI: 10.7270/Q28K79F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nischarin
(RAT) | BDBM50070328

(CHEBI:8862 | Rilmenidine)Show InChI InChI=1S/C10H16N2O/c1-2-7(1)9(8-3-4-8)12-10-11-5-6-13-10/h7-9H,1-6H2,(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [125I]PIC from Imidazoline-1 receptor in rat PC12 cell membrane incubated for 30 mins by gamma counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50354489
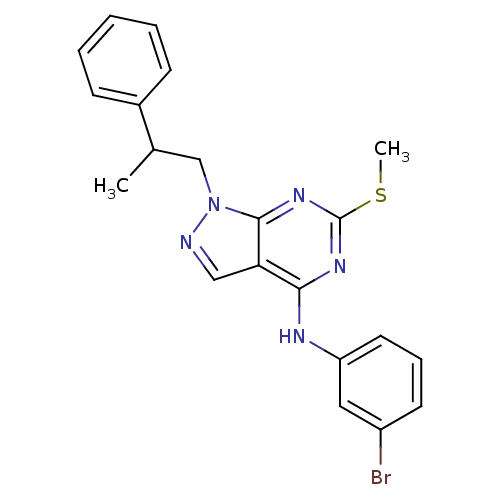
(CHEMBL1836680)Show SMILES CSc1nc(Nc2cccc(Br)c2)c2cnn(CC(C)c3ccccc3)c2n1 Show InChI InChI=1S/C21H20BrN5S/c1-14(15-7-4-3-5-8-15)13-27-20-18(12-23-27)19(25-21(26-20)28-2)24-17-10-6-9-16(22)11-17/h3-12,14H,13H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition human recombinant cSRC using KVEKIGEGTYGVVYK peptide substrate in presence of [gamma-32P]-ATP |
Bioorg Med Chem Lett 21: 5928-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.079
BindingDB Entry DOI: 10.7270/Q29G5N6P |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50315603
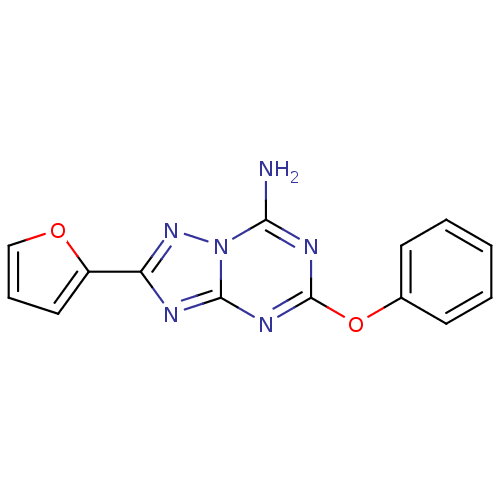
(2-(furan-2-yl)-5-phenoxy-[1,2,4]triazolo[1,5-a][1,...)Show InChI InChI=1S/C14H10N6O2/c15-12-17-14(22-9-5-2-1-3-6-9)18-13-16-11(19-20(12)13)10-7-4-8-21-10/h1-8H,(H2,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from adenosine A2A receptor in rat striatal membrane |
Bioorg Med Chem 18: 2524-36 (2010)
Article DOI: 10.1016/j.bmc.2010.02.039
BindingDB Entry DOI: 10.7270/Q2P84C13 |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50138500

(2-((E)-Styryl)-4,5-dihydro-1H-imidazole | 2-styryl...)Show InChI InChI=1S/C11H12N2/c1-2-4-10(5-3-1)6-7-11-12-8-9-13-11/h1-7H,8-9H2,(H,12,13)/b7-6+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [125I]PIC from Imidazoline-1 receptor in rat PC12 cell membrane by gamma counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50436582
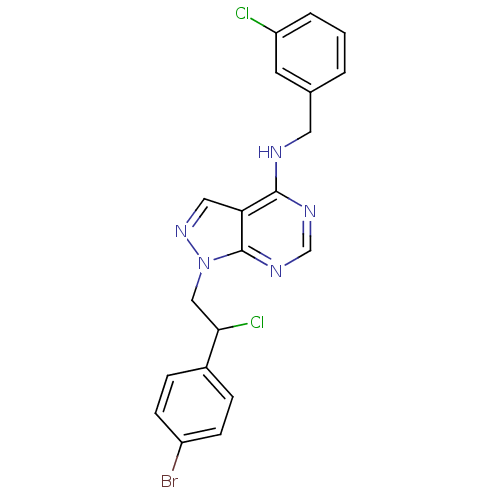
(CHEMBL2397804)Show SMILES ClC(Cn1ncc2c(NCc3cccc(Cl)c3)ncnc12)c1ccc(Br)cc1 Show InChI InChI=1S/C20H16BrCl2N5/c21-15-6-4-14(5-7-15)18(23)11-28-20-17(10-27-28)19(25-12-26-20)24-9-13-2-1-3-16(22)8-13/h1-8,10,12,18H,9,11H2,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant wild type human Abl using abitide as substrate |
J Med Chem 56: 5382-94 (2014)
Article DOI: 10.1021/jm400233w
BindingDB Entry DOI: 10.7270/Q29G5P63 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50354481
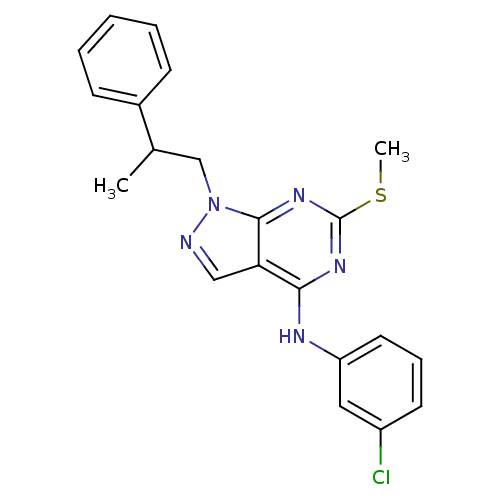
(CHEMBL1836679)Show SMILES CSc1nc(Nc2cccc(Cl)c2)c2cnn(CC(C)c3ccccc3)c2n1 Show InChI InChI=1S/C21H20ClN5S/c1-14(15-7-4-3-5-8-15)13-27-20-18(12-23-27)19(25-21(26-20)28-2)24-17-10-6-9-16(22)11-17/h3-12,14H,13H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition human recombinant cSRC using KVEKIGEGTYGVVYK peptide substrate in presence of [gamma-32P]-ATP |
Bioorg Med Chem Lett 21: 5928-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.079
BindingDB Entry DOI: 10.7270/Q29G5N6P |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50354480
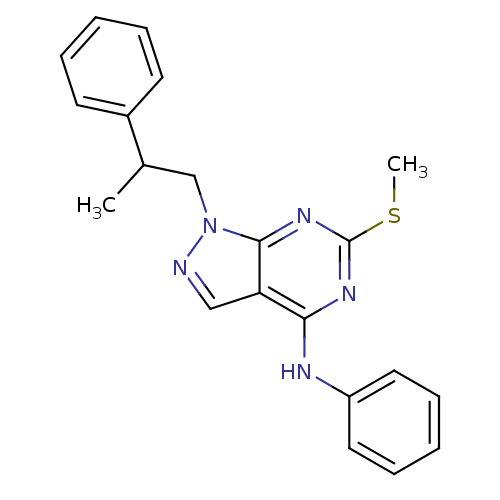
(CHEMBL1836678)Show InChI InChI=1S/C21H21N5S/c1-15(16-9-5-3-6-10-16)14-26-20-18(13-22-26)19(24-21(25-20)27-2)23-17-11-7-4-8-12-17/h3-13,15H,14H2,1-2H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition human recombinant cSRC using KVEKIGEGTYGVVYK peptide substrate in presence of [gamma-32P]-ATP |
Bioorg Med Chem Lett 21: 5928-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.079
BindingDB Entry DOI: 10.7270/Q29G5N6P |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50179397

(CHEMBL1162356)Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [125I]PIC from Imidazoline-1 receptor in rat PC12 cell membrane incubated for 30 mins by gamma counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50016897
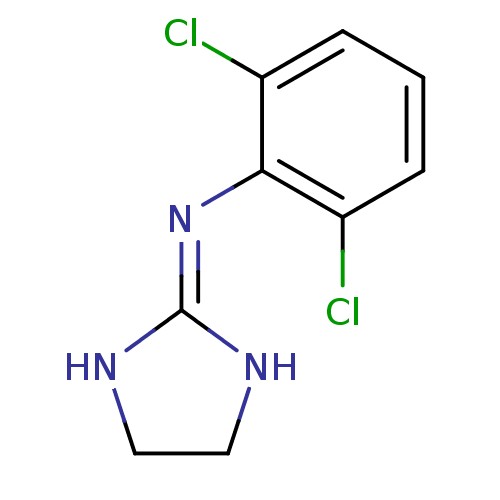
(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Displacement of [3H]RS-79948-197 from recombinant human alpha2A adrenoreceptor expressed in CHOK1 cell membrane by scintillation counting method |
Bioorg Med Chem 24: 3174-83 (2016)
Article DOI: 10.1016/j.bmc.2016.05.043
BindingDB Entry DOI: 10.7270/Q2NZ89J3 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 free reverse transcriptase |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50102872
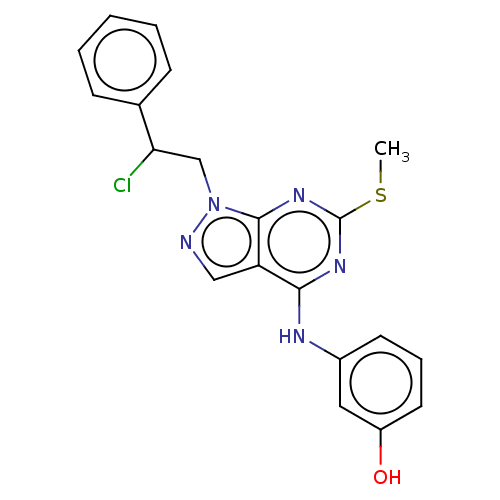
(CHEMBL3394083)Show SMILES CSc1nc(Nc2cccc(O)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 Show InChI InChI=1S/C20H18ClN5OS/c1-28-20-24-18(23-14-8-5-9-15(27)10-14)16-11-22-26(19(16)25-20)12-17(21)13-6-3-2-4-7-13/h2-11,17,27H,12H2,1H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP |
J Med Chem 58: 347-61 (2015)
Article DOI: 10.1021/jm5013159
BindingDB Entry DOI: 10.7270/Q2416ZTT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479680
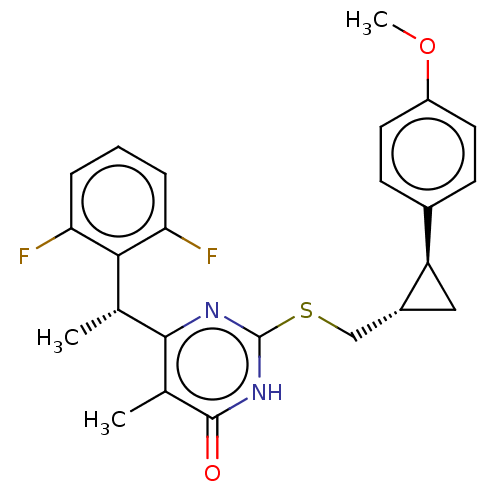
(CHEMBL459082)Show SMILES [H][C@]1(CSc2nc([C@H](C)c3c(F)cccc3F)c(C)c(=O)[nH]2)C[C@]1([H])c1ccc(OC)cc1 |r| Show InChI InChI=1S/C24H24F2N2O2S/c1-13(21-19(25)5-4-6-20(21)26)22-14(2)23(29)28-24(27-22)31-12-16-11-18(16)15-7-9-17(30-3)10-8-15/h4-10,13,16,18H,11-12H2,1-3H3,(H,27,28,29)/t13-,16-,18-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50479681

(CHEMBL478258)Show SMILES [H][C@@]1(CSc2nc([C@H](C)c3c(F)cccc3F)c(C)c(=O)[nH]2)C[C@@]1([H])c1ccc(OC)cc1 |r| Show InChI InChI=1S/C24H24F2N2O2S/c1-13(21-19(25)5-4-6-20(21)26)22-14(2)23(29)28-24(27-22)31-12-16-11-18(16)15-7-9-17(30-3)10-8-15/h4-10,13,16,18H,11-12H2,1-3H3,(H,27,28,29)/t13-,16+,18+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA-dNTP ternary complex expressed in Escherichia coli BL21 |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50315595
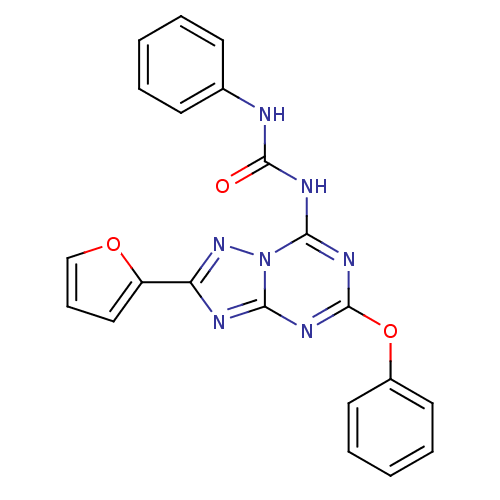
(CHEMBL1089440 | N-(2-(furan-2-yl)-5-phenoxy-[1,2,4...)Show SMILES O=C(Nc1ccccc1)Nc1nc(Oc2ccccc2)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C21H15N7O3/c29-20(22-14-8-3-1-4-9-14)24-19-26-21(31-15-10-5-2-6-11-15)25-18-23-17(27-28(18)19)16-12-7-13-30-16/h1-13H,(H2,22,23,24,25,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from adenosine A2A receptor in rat striatal membrane |
Bioorg Med Chem 18: 2524-36 (2010)
Article DOI: 10.1016/j.bmc.2010.02.039
BindingDB Entry DOI: 10.7270/Q2P84C13 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50479681

(CHEMBL478258)Show SMILES [H][C@@]1(CSc2nc([C@H](C)c3c(F)cccc3F)c(C)c(=O)[nH]2)C[C@@]1([H])c1ccc(OC)cc1 |r| Show InChI InChI=1S/C24H24F2N2O2S/c1-13(21-19(25)5-4-6-20(21)26)22-14(2)23(29)28-24(27-22)31-12-16-11-18(16)15-7-9-17(30-3)10-8-15/h4-10,13,16,18H,11-12H2,1-3H3,(H,27,28,29)/t13-,16+,18+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA binary complex expressed in Escherichia coli BL21 |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479680

(CHEMBL459082)Show SMILES [H][C@]1(CSc2nc([C@H](C)c3c(F)cccc3F)c(C)c(=O)[nH]2)C[C@]1([H])c1ccc(OC)cc1 |r| Show InChI InChI=1S/C24H24F2N2O2S/c1-13(21-19(25)5-4-6-20(21)26)22-14(2)23(29)28-24(27-22)31-12-16-11-18(16)15-7-9-17(30-3)10-8-15/h4-10,13,16,18H,11-12H2,1-3H3,(H,27,28,29)/t13-,16-,18-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479680

(CHEMBL459082)Show SMILES [H][C@]1(CSc2nc([C@H](C)c3c(F)cccc3F)c(C)c(=O)[nH]2)C[C@]1([H])c1ccc(OC)cc1 |r| Show InChI InChI=1S/C24H24F2N2O2S/c1-13(21-19(25)5-4-6-20(21)26)22-14(2)23(29)28-24(27-22)31-12-16-11-18(16)15-7-9-17(30-3)10-8-15/h4-10,13,16,18H,11-12H2,1-3H3,(H,27,28,29)/t13-,16-,18-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 free reverse transcriptase |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50354485
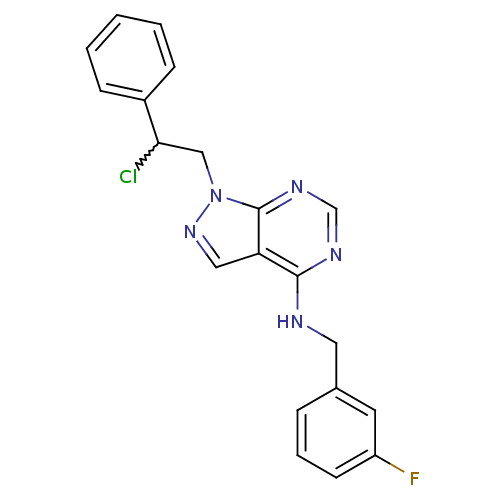
(CHEMBL412298)Show SMILES Fc1cccc(CNc2ncnc3n(CC(Cl)c4ccccc4)ncc23)c1 |w:15.15| Show InChI InChI=1S/C20H17ClFN5/c21-18(15-6-2-1-3-7-15)12-27-20-17(11-26-27)19(24-13-25-20)23-10-14-5-4-8-16(22)9-14/h1-9,11,13,18H,10,12H2,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition human recombinant Abl using Abtide peptide substrate in presence of [gamma-32P]-ATP |
Bioorg Med Chem Lett 21: 5928-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.079
BindingDB Entry DOI: 10.7270/Q29G5N6P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50354485

(CHEMBL412298)Show SMILES Fc1cccc(CNc2ncnc3n(CC(Cl)c4ccccc4)ncc23)c1 |w:15.15| Show InChI InChI=1S/C20H17ClFN5/c21-18(15-6-2-1-3-7-15)12-27-20-17(11-26-27)19(24-13-25-20)23-10-14-5-4-8-16(22)9-14/h1-9,11,13,18H,10,12H2,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant wild type human Abl using abitide as substrate |
J Med Chem 56: 5382-94 (2014)
Article DOI: 10.1021/jm400233w
BindingDB Entry DOI: 10.7270/Q29G5P63 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50101585
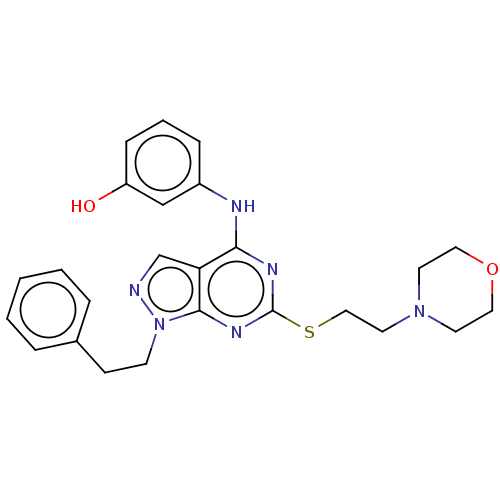
(CHEMBL3393986)Show SMILES Cl.Oc1cccc(Nc2nc(SCCN3CCOCC3)nc3n(CCc4ccccc4)ncc23)c1 Show InChI InChI=1S/C25H28N6O2S.ClH/c32-21-8-4-7-20(17-21)27-23-22-18-26-31(10-9-19-5-2-1-3-6-19)24(22)29-25(28-23)34-16-13-30-11-14-33-15-12-30;/h1-8,17-18,32H,9-16H2,(H,27,28,29);1H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP |
J Med Chem 58: 347-61 (2015)
Article DOI: 10.1021/jm5013159
BindingDB Entry DOI: 10.7270/Q2416ZTT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50230100

(4-fluoro-N-(5-(4-fluorobenzylthio)-1,3,4-thiadiazo...)Show InChI InChI=1S/C16H11F2N3OS2/c17-12-5-1-10(2-6-12)9-23-16-21-20-15(24-16)19-14(22)11-3-7-13(18)8-4-11/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86859

(CAS_1327207 | N-(5-(4-bromobenzylthio)-1,3,4-thiad...)Show InChI InChI=1S/C16H11BrClN3OS2/c17-12-5-1-10(2-6-12)9-23-16-21-20-15(24-16)19-14(22)11-3-7-13(18)8-4-11/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50436570
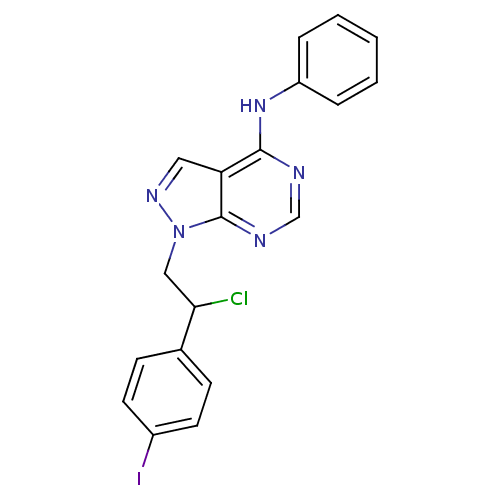
(CHEMBL2397818)Show InChI InChI=1S/C19H15ClIN5/c20-17(13-6-8-14(21)9-7-13)11-26-19-16(10-24-26)18(22-12-23-19)25-15-4-2-1-3-5-15/h1-10,12,17H,11H2,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant wild type human Abl using abitide as substrate |
J Med Chem 56: 5382-94 (2014)
Article DOI: 10.1021/jm400233w
BindingDB Entry DOI: 10.7270/Q29G5P63 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50436590

(CHEMBL2397796)Show SMILES Fc1cccc(CNc2ncnc3n(CC(Cl)c4ccc(Br)cc4)ncc23)c1 Show InChI InChI=1S/C20H16BrClFN5/c21-15-6-4-14(5-7-15)18(22)11-28-20-17(10-27-28)19(25-12-26-20)24-9-13-2-1-3-16(23)8-13/h1-8,10,12,18H,9,11H2,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant wild type human Abl using abitide as substrate |
J Med Chem 56: 5382-94 (2014)
Article DOI: 10.1021/jm400233w
BindingDB Entry DOI: 10.7270/Q29G5P63 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Citrobacter freundii) | BDBM50049708

(CHEMBL148 | IMIPENEM)Show SMILES C[C@@H](O)[C@@H]1[C@H]2CC(SCCN=CN)=C(N2C1=O)C(O)=O |r,w:11.11,c:12| Show InChI InChI=1S/C12H17N3O4S/c1-6(16)9-7-4-8(20-3-2-14-5-13)10(12(18)19)15(7)11(9)17/h5-7,9,16H,2-4H2,1H3,(H2,13,14)(H,18,19)/t6-,7-,9-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires
Curated by ChEMBL
| Assay Description
Inhibition of Citrobacter freundii PER2 beta lactamase |
Antimicrob Agents Chemother 51: 2359-65 (2007)
Article DOI: 10.1128/AAC.01395-06
BindingDB Entry DOI: 10.7270/Q24X57J4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50436571
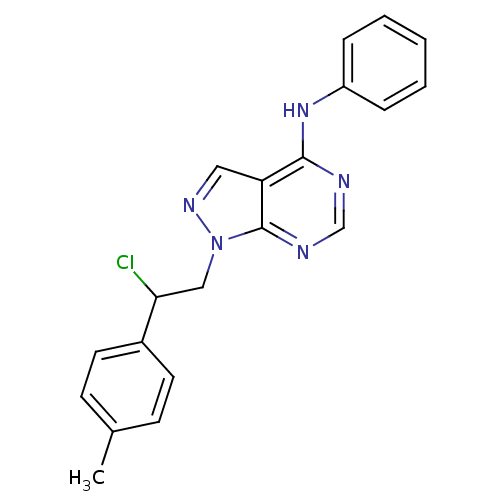
(CHEMBL2397817)Show InChI InChI=1S/C20H18ClN5/c1-14-7-9-15(10-8-14)18(21)12-26-20-17(11-24-26)19(22-13-23-20)25-16-5-3-2-4-6-16/h2-11,13,18H,12H2,1H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant wild type human Abl using abitide as substrate |
J Med Chem 56: 5382-94 (2014)
Article DOI: 10.1021/jm400233w
BindingDB Entry DOI: 10.7270/Q29G5P63 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50354484
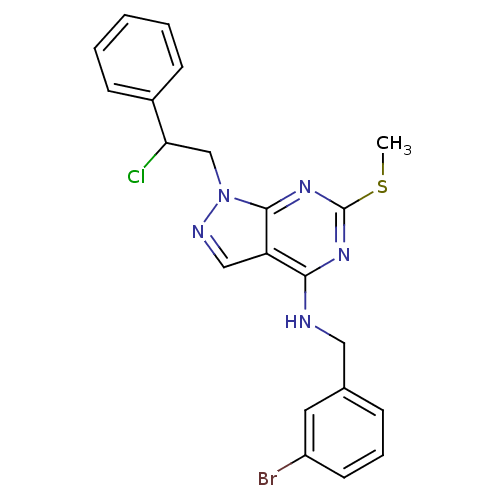
(CHEMBL1629808)Show SMILES CSc1nc(NCc2cccc(Br)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 Show InChI InChI=1S/C21H19BrClN5S/c1-29-21-26-19(24-11-14-6-5-9-16(22)10-14)17-12-25-28(20(17)27-21)13-18(23)15-7-3-2-4-8-15/h2-10,12,18H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition human recombinant Abl using Abtide peptide substrate in presence of [gamma-32P]-ATP |
Bioorg Med Chem Lett 21: 5928-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.079
BindingDB Entry DOI: 10.7270/Q29G5N6P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data