

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Homo sapiens (Human)) | BDBM153128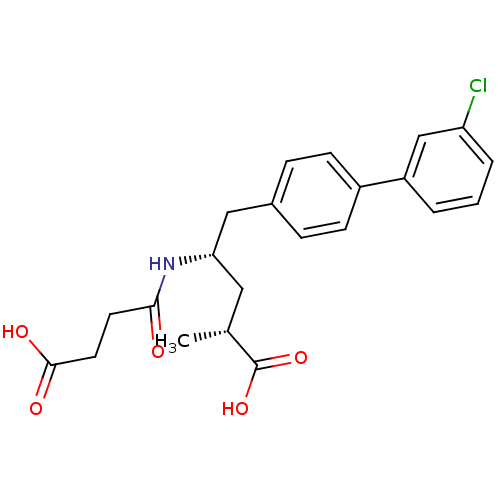 (US8993631, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130359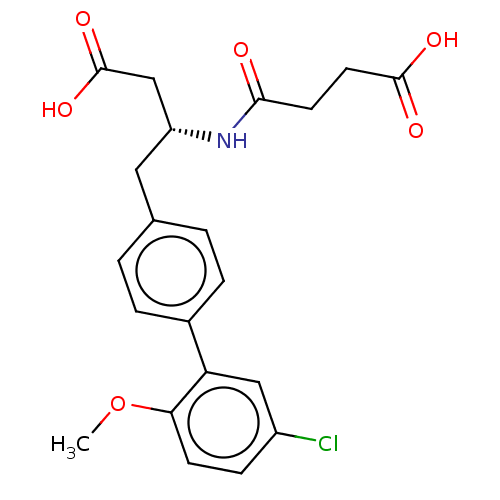 (US8822534, Example 5-39 | US8993631, 5-8) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542738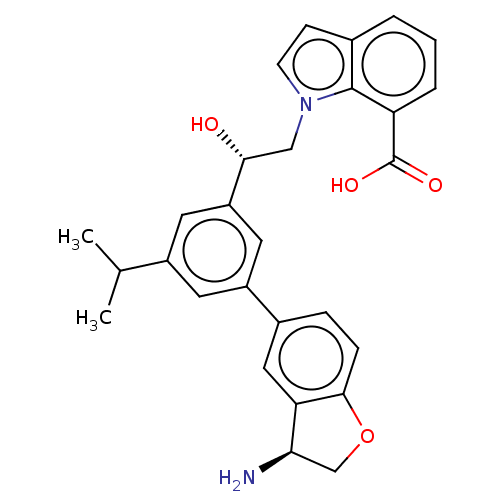 (CHEMBL4637027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16047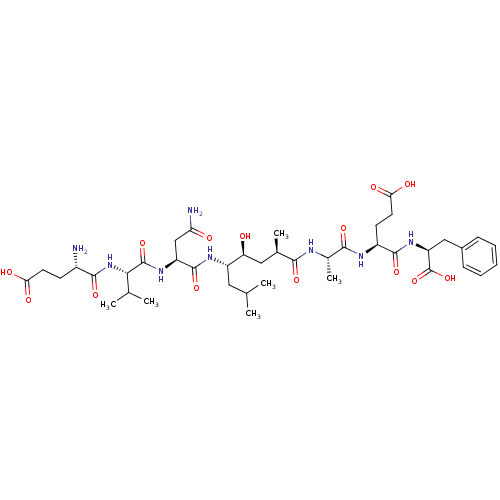 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542741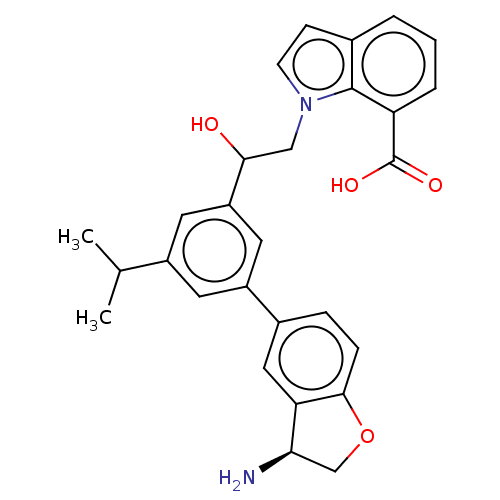 (CHEMBL4647950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542731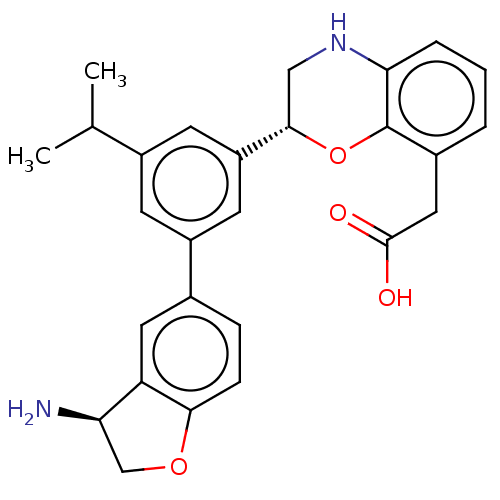 (CHEMBL4642845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509659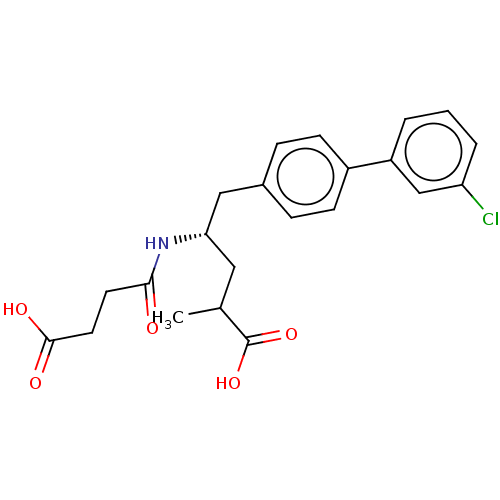 (CHEMBL4443138) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542731 (CHEMBL4642845) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542738 (CHEMBL4637027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130365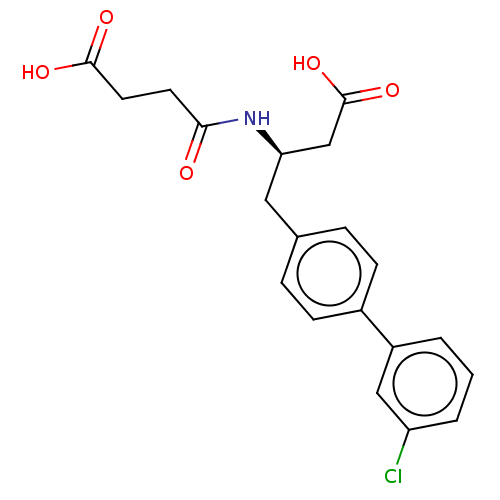 (US8822534, Example 11-1 | US8822534, Example 12-1 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509652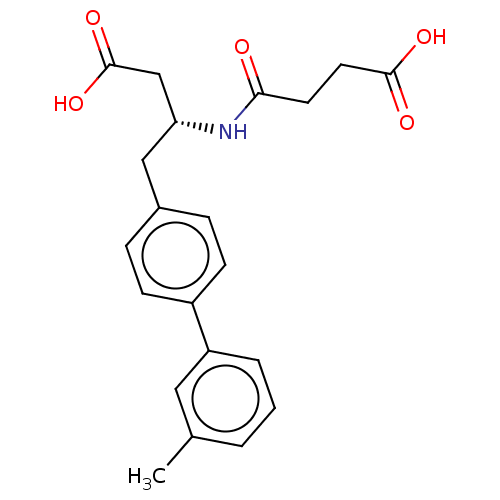 (CHEMBL4557208) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542724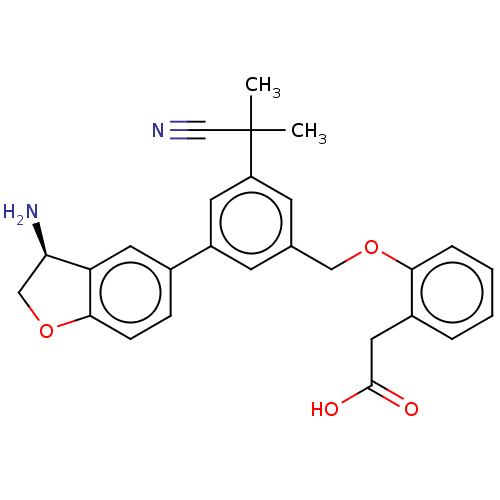 (CHEMBL4636415) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509666 (CHEMBL4442804) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542740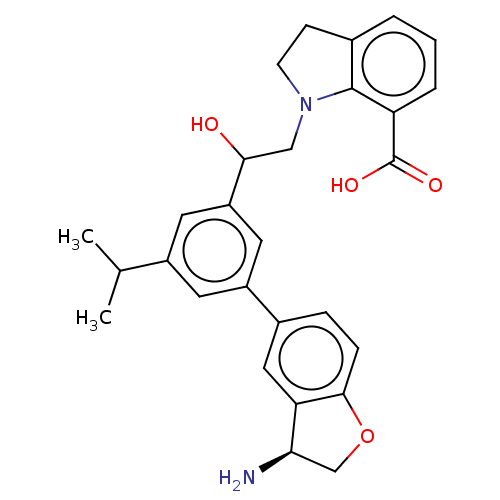 (CHEMBL4646398) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542740 (CHEMBL4646398) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542728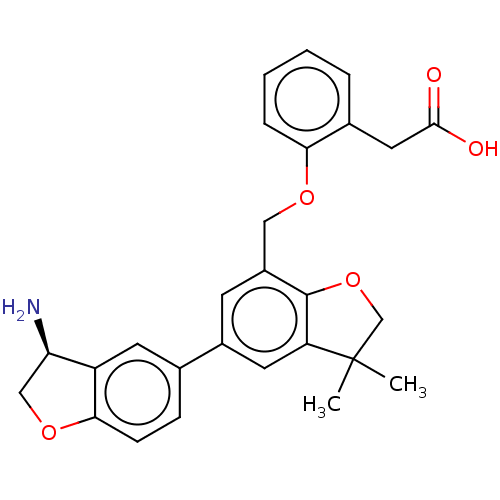 (CHEMBL4635912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542723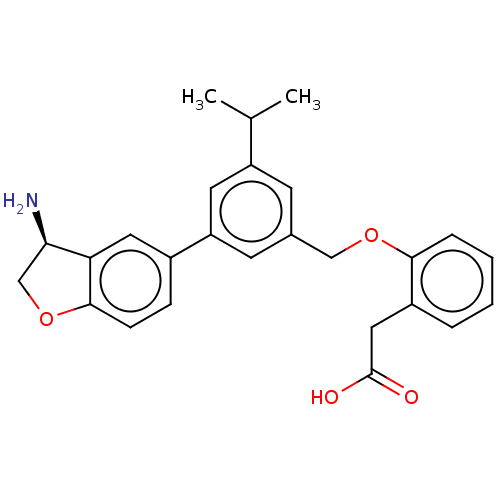 (CHEMBL4643449) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542723 (CHEMBL4643449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542733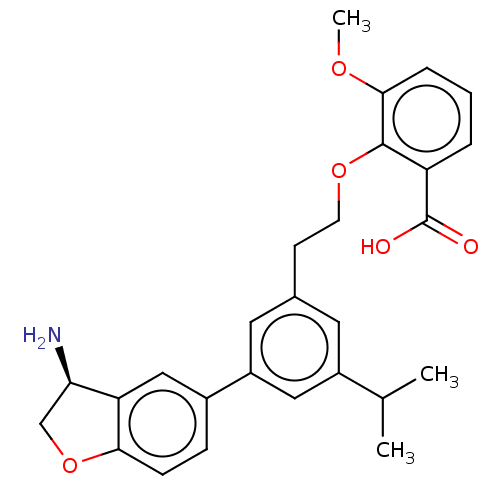 (CHEMBL4646141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542725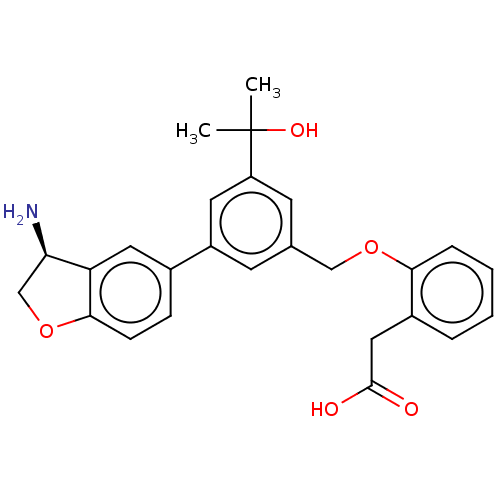 (CHEMBL4637683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542730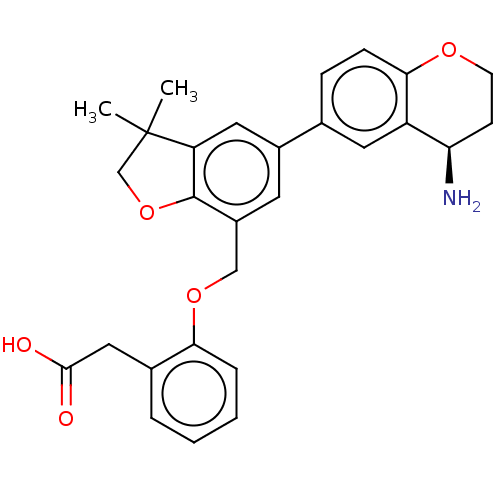 (CHEMBL4647909) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542724 (CHEMBL4636415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20606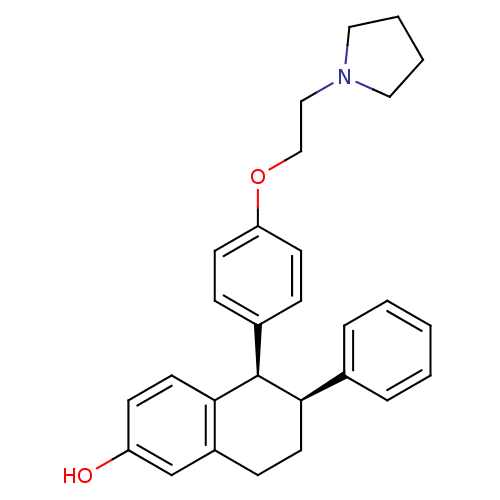 ((5R,6S)-6-phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | 3.10 | n/a | n/a | 7.4 | 22 |
Novartis Pharmaceuticals | Assay Description Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPA... | J Med Chem 48: 364-79 (2005) Article DOI: 10.1021/jm040858p BindingDB Entry DOI: 10.7270/Q2CC0XZJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130355 (US8822534, Example 5-7 | US8993631, 5-3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509647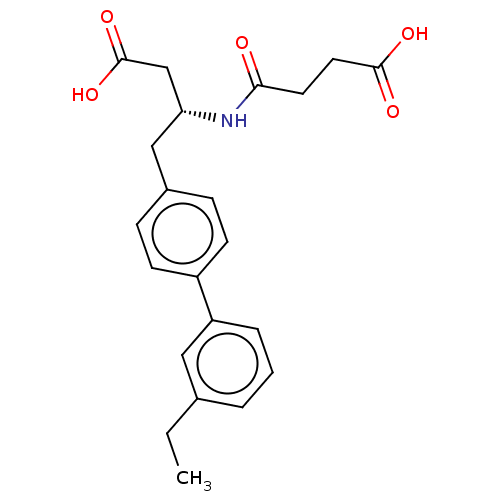 (CHEMBL4518426) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542725 (CHEMBL4637683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542730 (CHEMBL4647909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542735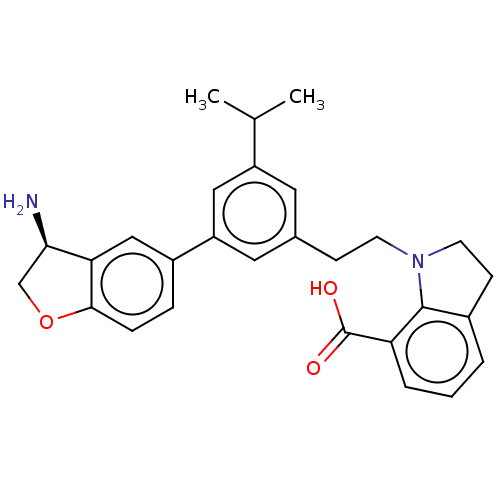 (CHEMBL4635286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor B (Homo sapiens (Human)) | BDBM50540314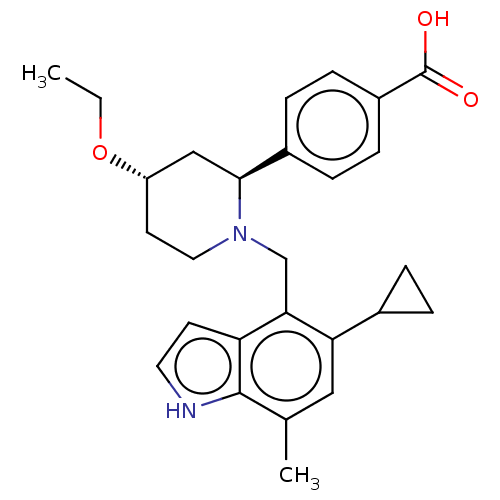 (CHEMBL4639592) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human serine protease factor B by TR-FRET based competition binding assay | J Med Chem 63: 5697-5722 (2020) Article DOI: 10.1021/acs.jmedchem.9b01870 BindingDB Entry DOI: 10.7270/Q21N84P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50034842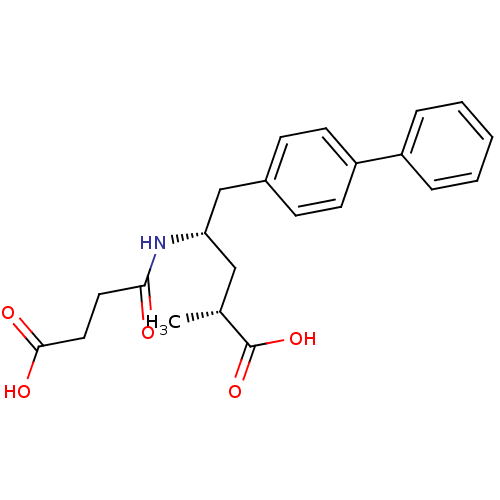 ((2R,4S)-5-Biphenyl-4-yl-4-(3-carboxy-propionylamin...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130354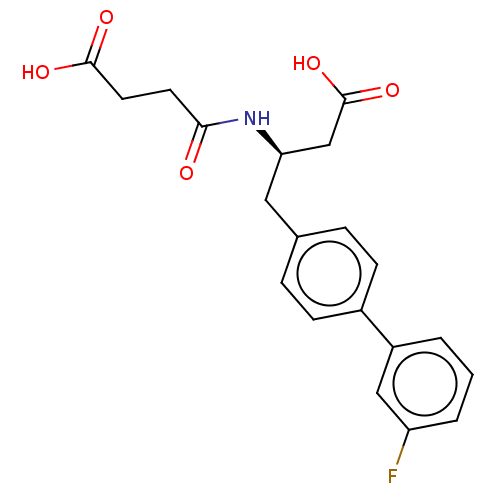 (US8822534, Example 5-4 | US8993631, 5-2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509655 (CHEMBL4466321) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542728 (CHEMBL4635912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50524338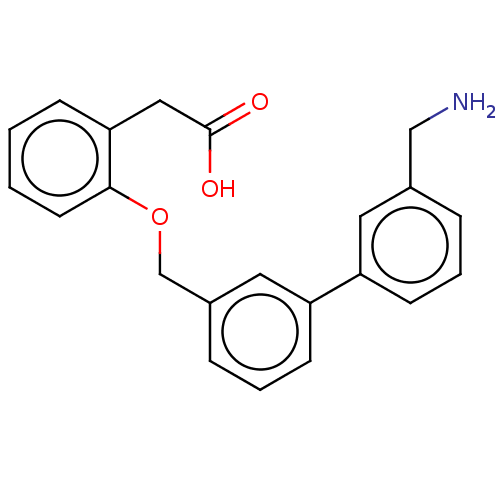 (CHEMBL4468000) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human complement FD by TR-FRET assay | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509649 (CHEMBL4474653) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542733 (CHEMBL4646141) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542726 (CHEMBL4642766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542731 (CHEMBL4642845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of N-terminal human plasma F11a catalytic domain expressed in Escherichia coli strain BL21(DE3) using D-Leu-Pro-Arg*Rh110-D-Pro as substra... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542738 (CHEMBL4637027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of N-terminal human plasma F11a catalytic domain expressed in Escherichia coli strain BL21(DE3) using D-Leu-Pro-Arg*Rh110-D-Pro as substra... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16057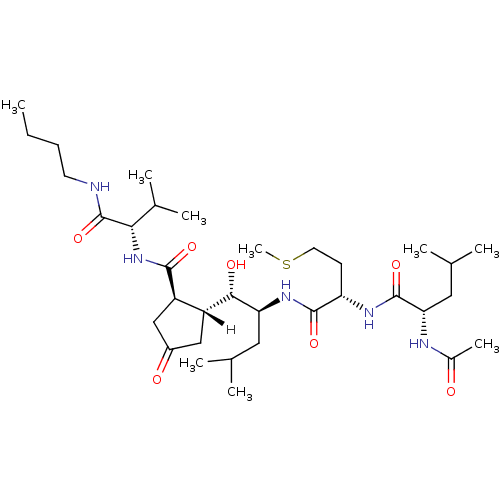 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16055 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16049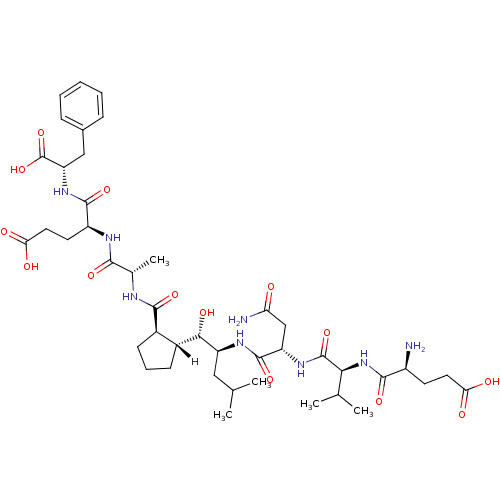 ((4S)-4-[(2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16054 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16060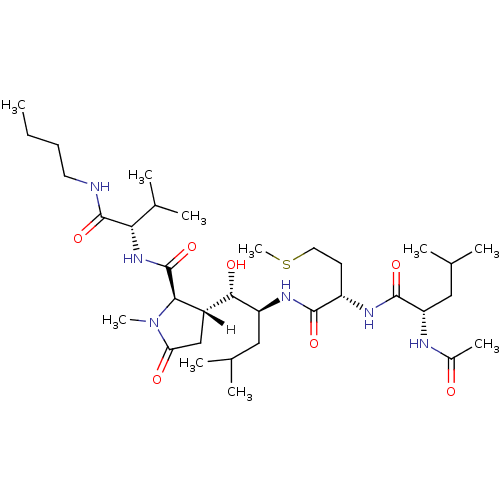 ((2R,3R)-3-[(1S,2S)-2-[[(2S)-2-[[(2S)-2-acetamido-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542726 (CHEMBL4642766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542732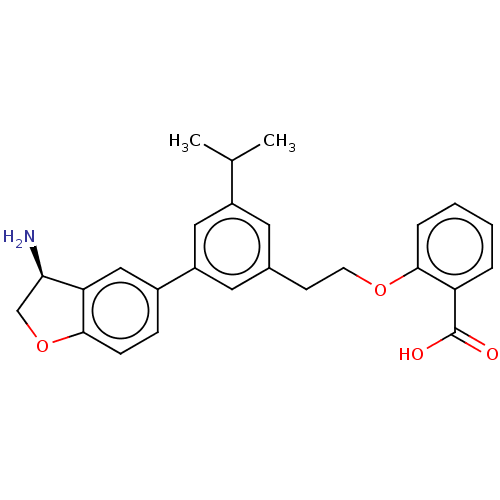 (CHEMBL4647925) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor B (Homo sapiens (Human)) | BDBM160475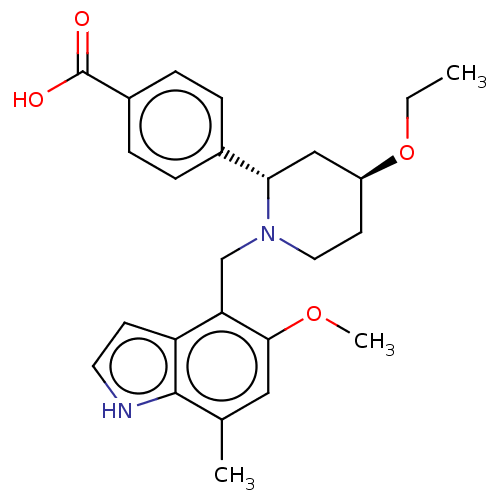 (US10093663, Example 26b | US9682968, Example-26a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human serine protease factor B by TR-FRET based competition binding assay | J Med Chem 63: 5697-5722 (2020) Article DOI: 10.1021/acs.jmedchem.9b01870 BindingDB Entry DOI: 10.7270/Q21N84P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16048 ((4S)-4-amino-4-{[(1S)-1-{[(1S)-2-carbamoyl-1-{[(1R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20606 ((5R,6S)-6-phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | 24 | n/a | n/a | n/a | 25 |
Novartis Pharmaceuticals | Assay Description Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPA... | J Med Chem 48: 364-79 (2005) Article DOI: 10.1021/jm040858p BindingDB Entry DOI: 10.7270/Q2CC0XZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 406 total ) | Next | Last >> |