

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50343621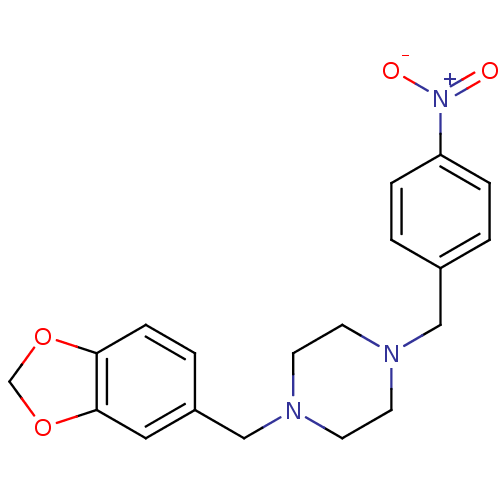 (1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-nitrobenzyl)pi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human AChE assessed as inhibition constant using acetylthiocholine iodide as substrate by Cornish-Bowden plot an... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50343619 (1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-bromobenzyl)pi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50343620 (1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-iodobenzyl)pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50343618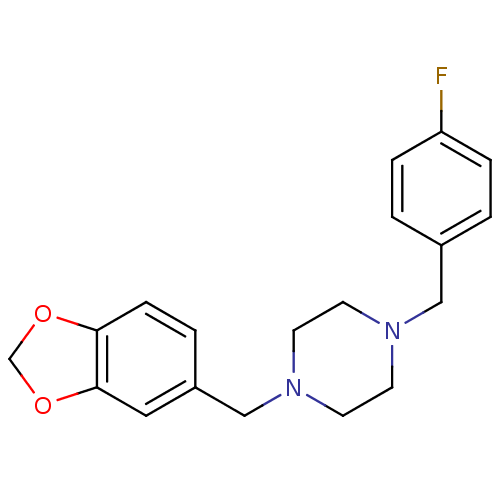 (1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-fluorobenzyl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50343622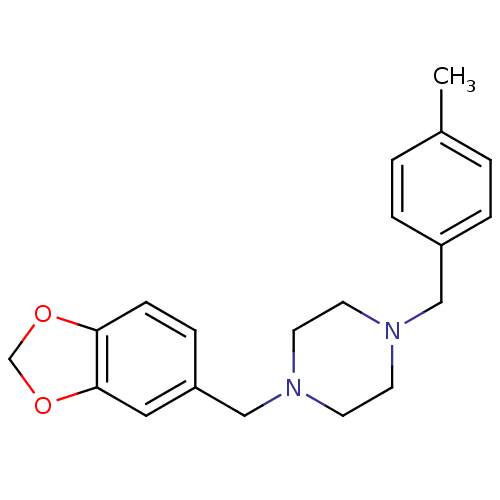 (1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-methylbenzyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human AChE assessed as dissociation constant for protein-substrate-compound complex using acetylthiocholine iodi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50338990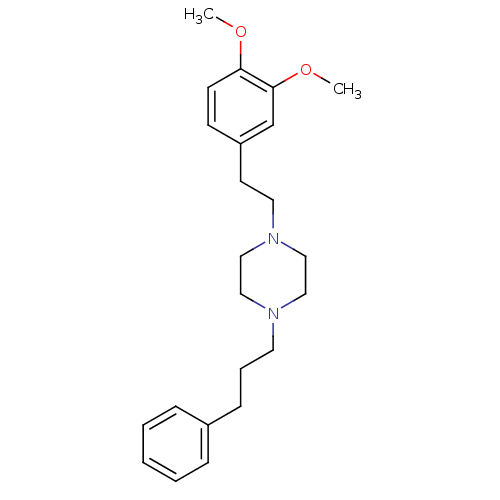 (1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Binding affinity to emopamil binding protein | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50540526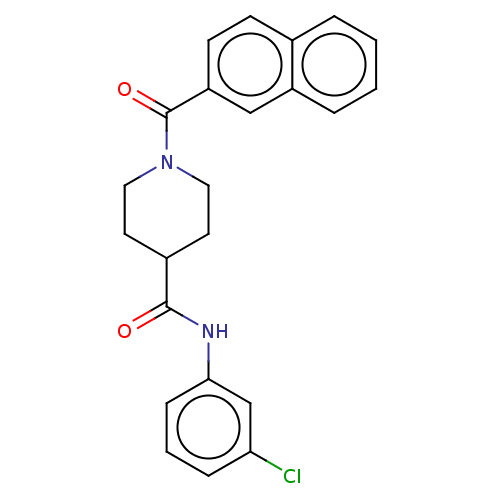 (CHEMBL4637658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAGL at 31.25 nM to 125 nM pre-incubated for 5 mins before MAGL substrate addition and further incubated ... | J Med Chem 63: 5783-5796 (2020) Article DOI: 10.1021/acs.jmedchem.9b02137 BindingDB Entry DOI: 10.7270/Q2GQ7288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM288689 (TC001262 | US10092529, Compound 38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.7 | -44.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
National Institute of Biological Sciences, Beijing US Patent | Assay Description Materials: Recombinant full-length RIPK1 protein with N-terminal GST-tag (Cat#R07-34G) was purchased from SignalChem. The ADP-Glo kinase assay kit (C... | US Patent US10092529 (2018) BindingDB Entry DOI: 10.7270/Q26W9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370555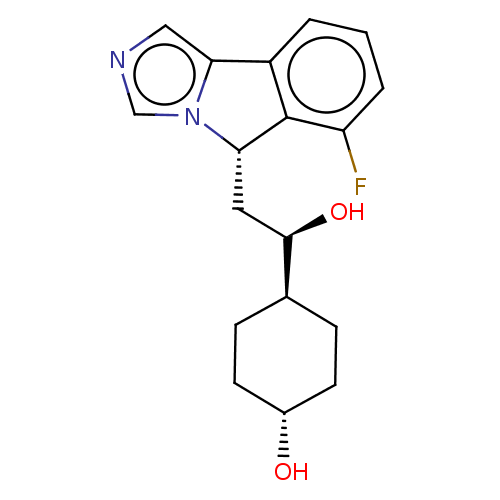 ((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00303 BindingDB Entry DOI: 10.7270/Q2474G0K | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM288691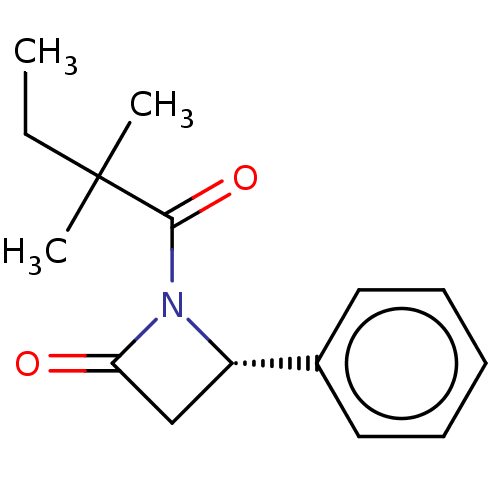 (TC001207 | US10092529, Compound 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 19.8 | -44.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
National Institute of Biological Sciences, Beijing US Patent | Assay Description Materials: Recombinant full-length RIPK1 protein with N-terminal GST-tag (Cat#R07-34G) was purchased from SignalChem. The ADP-Glo kinase assay kit (C... | US Patent US10092529 (2018) BindingDB Entry DOI: 10.7270/Q26W9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM288686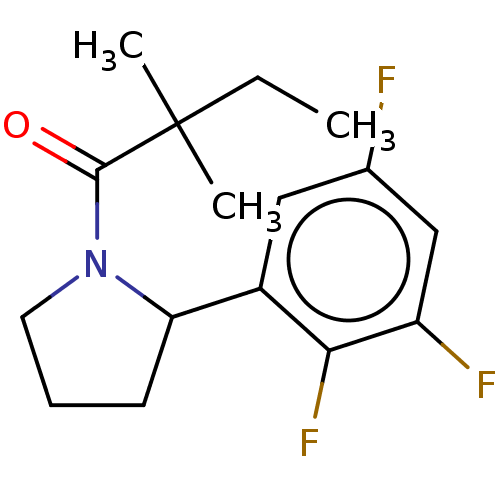 (TC001124 | US10092529, Compound 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 22.5 | -43.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
National Institute of Biological Sciences, Beijing US Patent | Assay Description Materials: Recombinant full-length RIPK1 protein with N-terminal GST-tag (Cat#R07-34G) was purchased from SignalChem. The ADP-Glo kinase assay kit (C... | US Patent US10092529 (2018) BindingDB Entry DOI: 10.7270/Q26W9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM288687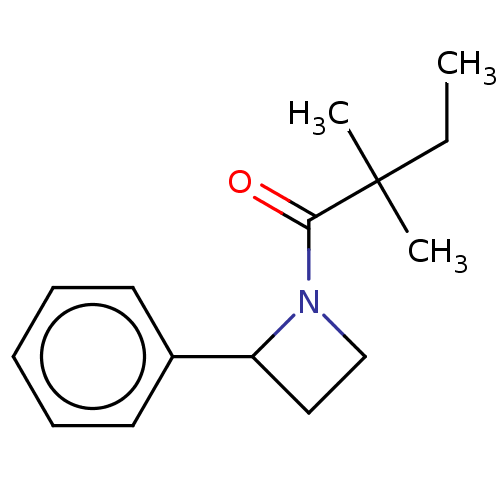 (TC001129 | US10092529, Compound 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 34.8 | -42.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
National Institute of Biological Sciences, Beijing US Patent | Assay Description Materials: Recombinant full-length RIPK1 protein with N-terminal GST-tag (Cat#R07-34G) was purchased from SignalChem. The ADP-Glo kinase assay kit (C... | US Patent US10092529 (2018) BindingDB Entry DOI: 10.7270/Q26W9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM288688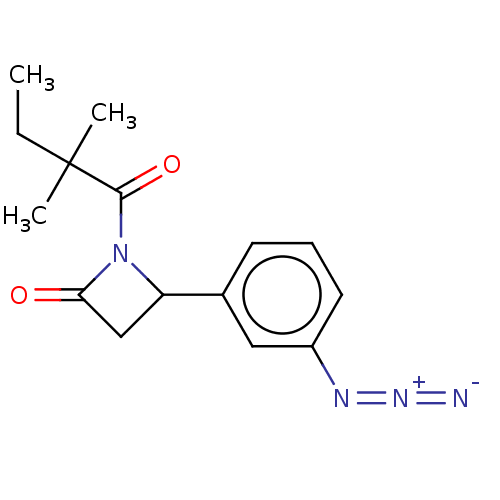 (TC001273 | US10092529, Compound 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 152 | -38.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
National Institute of Biological Sciences, Beijing US Patent | Assay Description Materials: Recombinant full-length RIPK1 protein with N-terminal GST-tag (Cat#R07-34G) was purchased from SignalChem. The ADP-Glo kinase assay kit (C... | US Patent US10092529 (2018) BindingDB Entry DOI: 10.7270/Q26W9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50606592 (CHEMBL5219838) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00303 BindingDB Entry DOI: 10.7270/Q2474G0K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50200540 (CHEMBL3972619) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00303 BindingDB Entry DOI: 10.7270/Q2474G0K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM288690 (TC001287 | US10092529, Compound 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 460 | -36.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
National Institute of Biological Sciences, Beijing US Patent | Assay Description Materials: Recombinant full-length RIPK1 protein with N-terminal GST-tag (Cat#R07-34G) was purchased from SignalChem. The ADP-Glo kinase assay kit (C... | US Patent US10092529 (2018) BindingDB Entry DOI: 10.7270/Q26W9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531933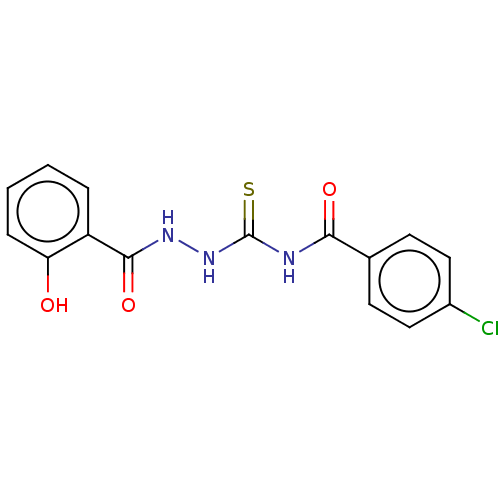 (CHEMBL4448356) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531928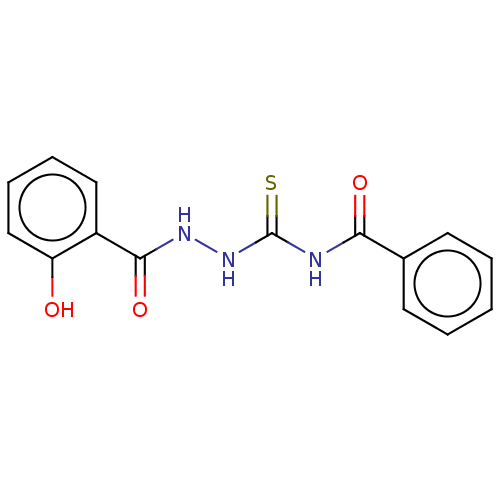 (CHEMBL1551854) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM75654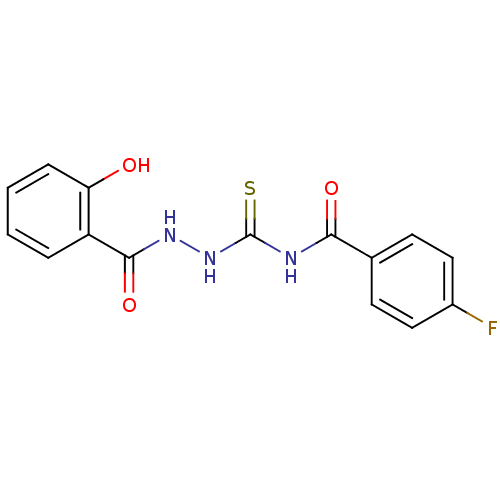 (4-Fluoro-N-[N'-(2-hydroxy-benzoyl)-hydrazinoca...) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531928 (CHEMBL1551854) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 S55A mutant expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5... | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM288692 (TC001265 | US10092529, Compound 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.07E+3 | -34.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
National Institute of Biological Sciences, Beijing US Patent | Assay Description Materials: Recombinant full-length RIPK1 protein with N-terminal GST-tag (Cat#R07-34G) was purchased from SignalChem. The ADP-Glo kinase assay kit (C... | US Patent US10092529 (2018) BindingDB Entry DOI: 10.7270/Q26W9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531934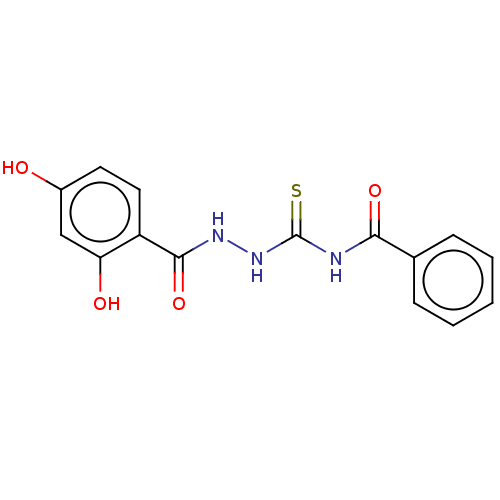 (CHEMBL4520626) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531939 (CHEMBL1424907) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531927 (CHEMBL4476697) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531930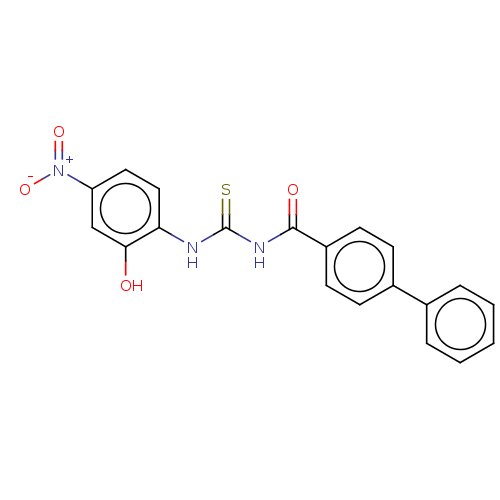 (CHEMBL4450278) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336257 (4-(3-fluorobenzylidene)-1,7-bis(4-hydroxy-3-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM58344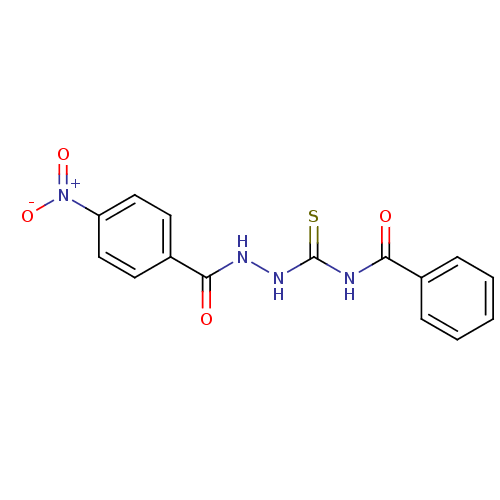 (MLS001211194 | N-[N'-(4-Nitro-benzoyl)-hydrazi...) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50200540 (CHEMBL3972619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00303 BindingDB Entry DOI: 10.7270/Q2474G0K | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531935 (CHEMBL4562397) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336258 (4-(4-fluorobenzylidene)-1,7-bis(4-hydroxy-3-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531927 (CHEMBL4476697) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 R51A mutant expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5... | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531927 (CHEMBL4476697) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 S55A mutant expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5... | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531936 (CHEMBL4566399) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336259 (4-(5-(3,4-dimethoxyphenyl)-2-(-3-(3,4-dimethoxyphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336261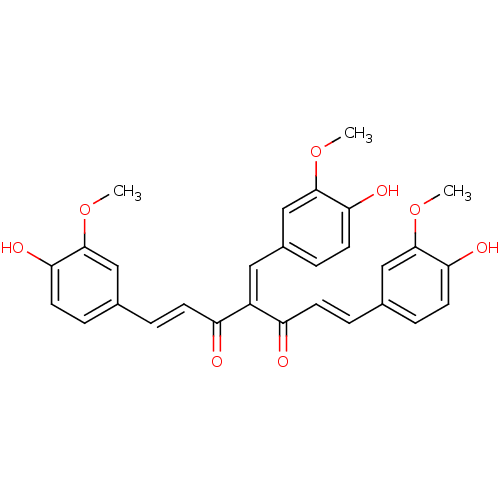 (4-(4-hydroxy-3-methoxybenzylidene)-1,7-bis(4-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531928 (CHEMBL1551854) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 R51A mutant expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5... | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531927 (CHEMBL4476697) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 S50A mutant expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5... | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531928 (CHEMBL1551854) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 T278A mutant expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over ... | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50606592 (CHEMBL5219838) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 4.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00303 BindingDB Entry DOI: 10.7270/Q2474G0K | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531927 (CHEMBL4476697) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 D277A mutant expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over ... | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336256 (4-(3,4-dimethoxybenzylidene)-1,7-bis(4-hydroxy-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336251 (1,7-bis(4-fluorophenyl)-5-hydroxyhepta-1,4,6-trien...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531940 (CHEMBL4469784) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531938 (CHEMBL4455886) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531929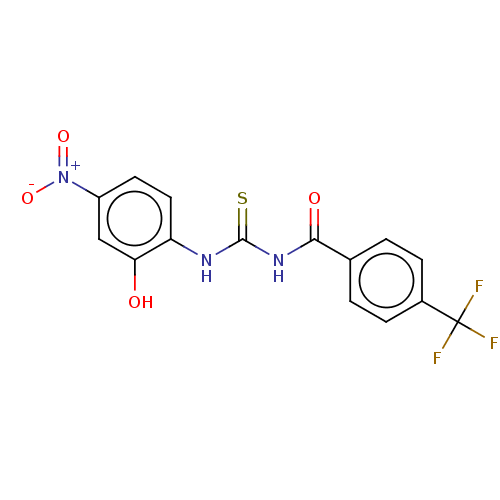 (CHEMBL4456429) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531928 (CHEMBL1551854) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 D277A mutant expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over ... | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531931 (CHEMBL4468799) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase class 2 (Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531932 (CHEMBL4439217) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei) Curated by ChEMBL | Assay Description Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins | Bioorg Med Chem 27: 805-812 (2019) Article DOI: 10.1016/j.bmc.2019.01.023 BindingDB Entry DOI: 10.7270/Q20P13HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2380 total ) | Next | Last >> |