 Found 681 hits with Last Name = 'revesz' and Initial = 'l'
Found 681 hits with Last Name = 'revesz' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546172

(CHEMBL4762397)Show SMILES CN(Cc1c[nH]c2ncnc(-c3cccc(NC(=O)c4ccc(cc4)C(C)(C)C)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174708

(CHEMBL197375 | N-(2-(3-((2R,5S)-4-(4-fluorobenzyl)...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174703
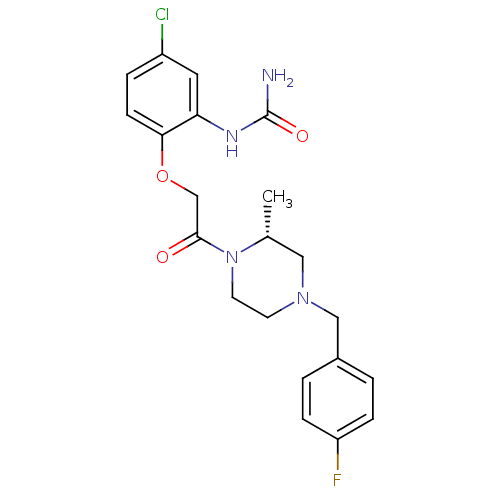
((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50175995
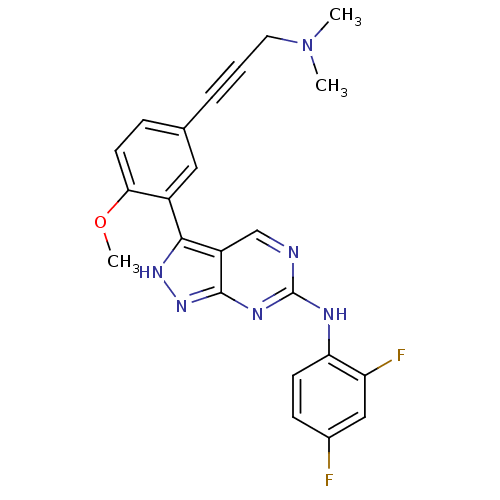
(CHEMBL199986 | N-(2,4-difluorophenyl)-3-(5-(3-(dim...)Show SMILES COc1ccc(cc1-c1[nH]nc2nc(Nc3ccc(F)cc3F)ncc12)C#CCN(C)C Show InChI InChI=1S/C23H20F2N6O/c1-31(2)10-4-5-14-6-9-20(32-3)16(11-14)21-17-13-26-23(28-22(17)30-29-21)27-19-8-7-15(24)12-18(19)25/h6-9,11-13H,10H2,1-3H3,(H2,26,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine p38alpha MAPK |
Bioorg Med Chem Lett 16: 262-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.015
BindingDB Entry DOI: 10.7270/Q2DJ5F6T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546180
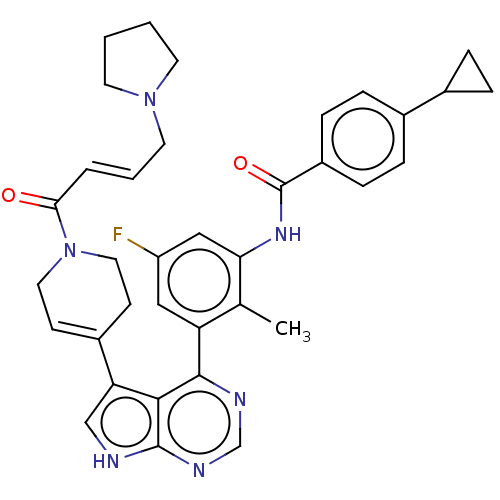
(CHEMBL4749522)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)\C=C\CN3CCCC3)c12 |t:31| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50176013
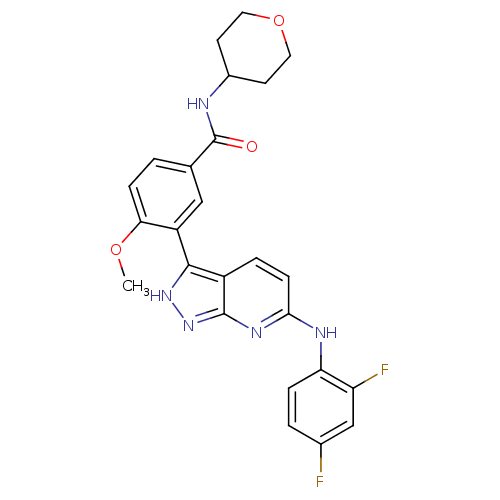
(3-(6-(2,4-difluorophenylamino)-1H-pyrazolo[3,4-b]p...)Show SMILES COc1ccc(cc1-c1[nH]nc2nc(Nc3ccc(F)cc3F)ccc12)C(=O)NC1CCOCC1 Show InChI InChI=1S/C25H23F2N5O3/c1-34-21-6-2-14(25(33)28-16-8-10-35-11-9-16)12-18(21)23-17-4-7-22(30-24(17)32-31-23)29-20-5-3-15(26)13-19(20)27/h2-7,12-13,16H,8-11H2,1H3,(H,28,33)(H2,29,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine p38alpha MAPK |
Bioorg Med Chem Lett 16: 262-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.015
BindingDB Entry DOI: 10.7270/Q2DJ5F6T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514642

(CHEMBL4593663)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)C=C)c12 |t:31| Show InChI InChI=1S/C31H28FN5O2/c1-3-27(38)37-12-10-21(11-13-37)25-16-33-30-28(25)29(34-17-35-30)24-14-23(32)15-26(18(24)2)36-31(39)22-8-6-20(7-9-22)19-4-5-19/h3,6-10,14-17,19H,1,4-5,11-13H2,2H3,(H,36,39)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50176003

(CHEMBL199993 | N-(2,4-difluorophenyl)-3-(2-methoxy...)Show SMILES COc1ccc(cc1-c1[nH]nc2nc(Nc3ccc(F)cc3F)ncc12)C#CCN1CCN(C)CC1 Show InChI InChI=1S/C26H25F2N7O/c1-34-10-12-35(13-11-34)9-3-4-17-5-8-23(36-2)19(14-17)24-20-16-29-26(31-25(20)33-32-24)30-22-7-6-18(27)15-21(22)28/h5-8,14-16H,9-13H2,1-2H3,(H2,29,30,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine p38alpha MAPK |
Bioorg Med Chem Lett 16: 262-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.015
BindingDB Entry DOI: 10.7270/Q2DJ5F6T |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174707

((R)-1-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C22H24ClFN4O2/c1-15-13-27(14-16-2-7-19(24)8-3-16)10-11-28(15)21(29)9-5-17-4-6-18(23)12-20(17)26-22(25)30/h2-9,12,15H,10-11,13-14H2,1H3,(H3,25,26,30)/b9-5+/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348537

(CHEMBL1801376)Show SMILES Fc1cccc(Nc2cc3c4[nH]c5CNC(=O)c5c4ccc3cn2)c1 Show InChI InChI=1S/C19H13FN4O/c20-11-2-1-3-12(6-11)23-16-7-14-10(8-21-16)4-5-13-17-15(24-18(13)14)9-22-19(17)25/h1-8,24H,9H2,(H,21,23)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BMX (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546179

(CHEMBL4739958)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3CCN(CC3)C(=O)C=C)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174711
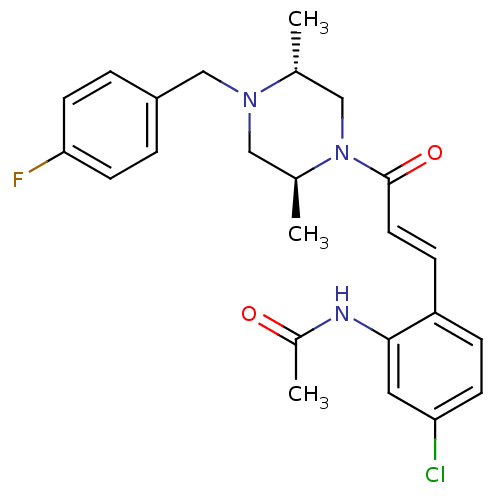
(CHEMBL197345 | N-(2-(3-((2S,5R)-4-(4-fluorobenzyl)...)Show SMILES C[C@@H]1CN([C@@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348545

(CHEMBL1801384)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(\C=C\c5ccc(CN6CCOCC6)cc5)cc34)c12 Show InChI InChI=1S/C27H26N4O2/c32-27-25-22-8-6-20-16-29-21(15-23(20)26(22)30-24(25)9-10-28-27)7-5-18-1-3-19(4-2-18)17-31-11-13-33-14-12-31/h1-8,15-16,30H,9-14,17H2,(H,28,32)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174709
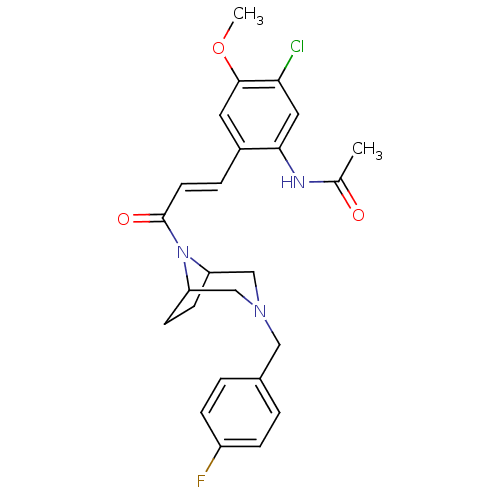
(CHEMBL372807 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES COc1cc(\C=C\C(=O)N2C3CCC2CN(Cc2ccc(F)cc2)C3)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C25H27ClFN3O3/c1-16(31)28-23-12-22(26)24(33-2)11-18(23)5-10-25(32)30-20-8-9-21(30)15-29(14-20)13-17-3-6-19(27)7-4-17/h3-7,10-12,20-21H,8-9,13-15H2,1-2H3,(H,28,31)/b10-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546175

(CHEMBL4795673)Show SMILES CN(C(=O)Cc1c[nH]c2ncnc(-c3cc(F)cc(NC(=O)c4ccc(cc4)C4CC4)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546176

(CHEMBL4746262)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(c12)C1(O)CN(C1)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174714
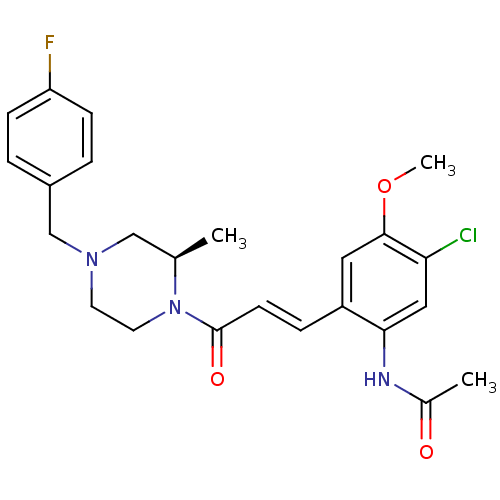
((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES COc1cc(\C=C\C(=O)N2CCN(Cc3ccc(F)cc3)C[C@H]2C)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C24H27ClFN3O3/c1-16-14-28(15-18-4-7-20(26)8-5-18)10-11-29(16)24(31)9-6-19-12-23(32-3)21(25)13-22(19)27-17(2)30/h4-9,12-13,16H,10-11,14-15H2,1-3H3,(H,27,30)/b9-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348534
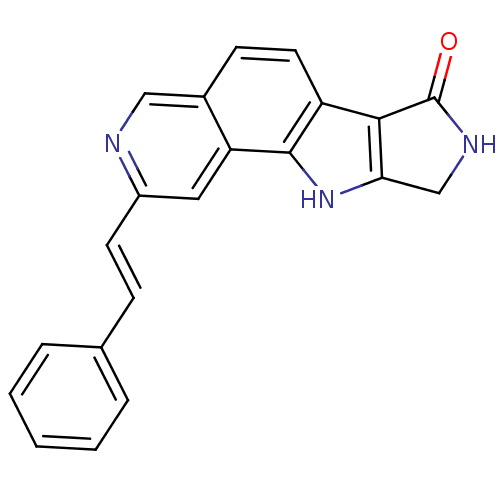
(CHEMBL1801373)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccccc5)cc34)c12 Show InChI InChI=1S/C21H15N3O/c25-21-19-16-9-7-14-11-22-15(8-6-13-4-2-1-3-5-13)10-17(14)20(16)24-18(19)12-23-21/h1-11,24H,12H2,(H,23,25)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148700
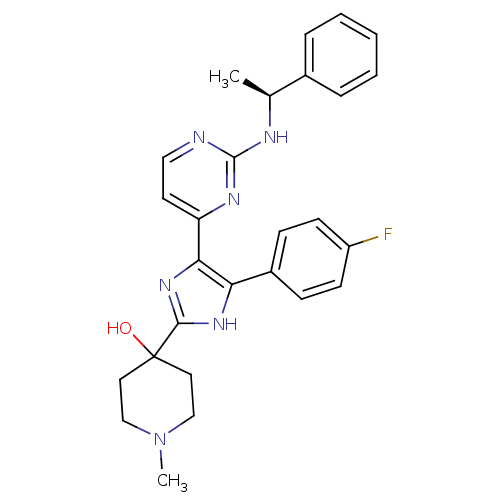
(4-{4-(4-Fluoro-phenyl)-5-[2-((S)-1-phenyl-ethylami...)Show SMILES C[C@H](Nc1nccc(n1)-c1nc([nH]c1-c1ccc(F)cc1)C1(O)CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C27H29FN6O/c1-18(19-6-4-3-5-7-19)30-26-29-15-12-22(31-26)24-23(20-8-10-21(28)11-9-20)32-25(33-24)27(35)13-16-34(2)17-14-27/h3-12,15,18,35H,13-14,16-17H2,1-2H3,(H,32,33)(H,29,30,31)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174720
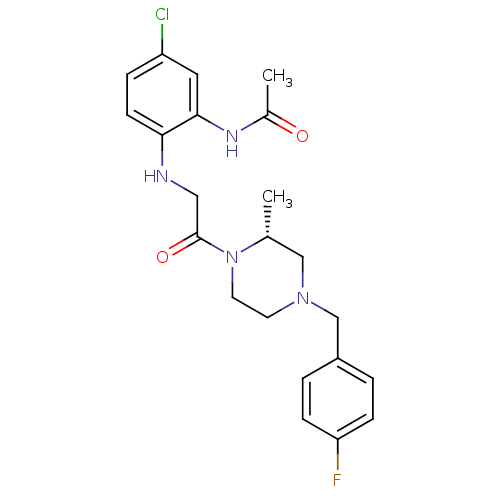
((R)-N-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)CNc1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C22H26ClFN4O2/c1-15-13-27(14-17-3-6-19(24)7-4-17)9-10-28(15)22(30)12-25-20-8-5-18(23)11-21(20)26-16(2)29/h3-8,11,15,25H,9-10,12-14H2,1-2H3,(H,26,29)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174703

((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory effect on transwell chemotaxis induced by 1 nM MIP-1alpha in mouse pre-B cells transfected with human CCR1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465455
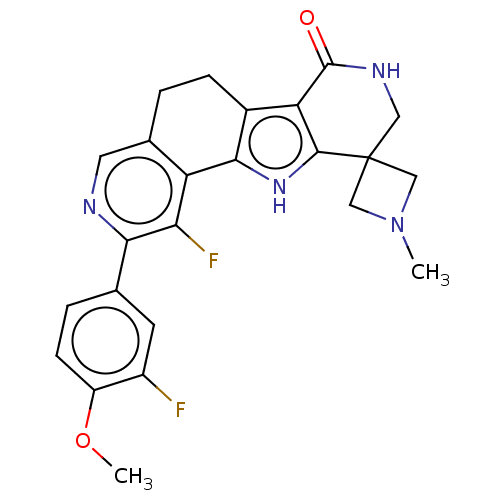
(CHEMBL4282514)Show SMILES COc1ccc(cc1F)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2c1F Show InChI InChI=1S/C24H22F2N4O2/c1-30-10-24(11-30)9-28-23(31)18-14-5-3-13-8-27-20(12-4-6-16(32-2)15(25)7-12)19(26)17(13)21(14)29-22(18)24/h4,6-8,29H,3,5,9-11H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348515
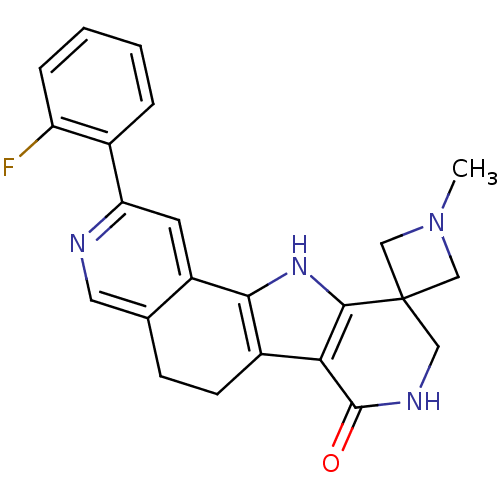
(CHEMBL1233942)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(cc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C23H21FN4O/c1-28-11-23(12-28)10-26-22(29)19-15-7-6-13-9-25-18(14-4-2-3-5-17(14)24)8-16(13)20(15)27-21(19)23/h2-5,8-9,27H,6-7,10-12H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MK2 mediated anisomycin-stimulated hsp27 phosphorylation in human THP-1 cells by fluorometric analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465454

(CHEMBL4293121)Show SMILES COc1ccc(cn1)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2c1F Show InChI InChI=1S/C23H22FN5O2/c1-29-10-23(11-29)9-27-22(30)17-14-5-3-12-7-26-19(13-4-6-15(31-2)25-8-13)18(24)16(12)20(14)28-21(17)23/h4,6-8,28H,3,5,9-11H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465453

(CHEMBL4277929)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(c(F)c4-c3[nH]c21)-c1cccc(F)c1 Show InChI InChI=1S/C23H20F2N4O/c1-29-10-23(11-29)9-27-22(30)17-15-6-5-13-8-26-19(12-3-2-4-14(24)7-12)18(25)16(13)20(15)28-21(17)23/h2-4,7-8,28H,5-6,9-11H2,1H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174709

(CHEMBL372807 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES COc1cc(\C=C\C(=O)N2C3CCC2CN(Cc2ccc(F)cc2)C3)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C25H27ClFN3O3/c1-16(31)28-23-12-22(26)24(33-2)11-18(23)5-10-25(32)30-20-8-9-21(30)15-29(14-20)13-17-3-6-19(27)7-4-17/h3-7,10-12,20-21H,8-9,13-15H2,1-2H3,(H,28,31)/b10-5+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Mucosa-associated lymphoid tissue lymphoma translocation protein 1
(Homo sapiens (Human)) | BDBM50273330
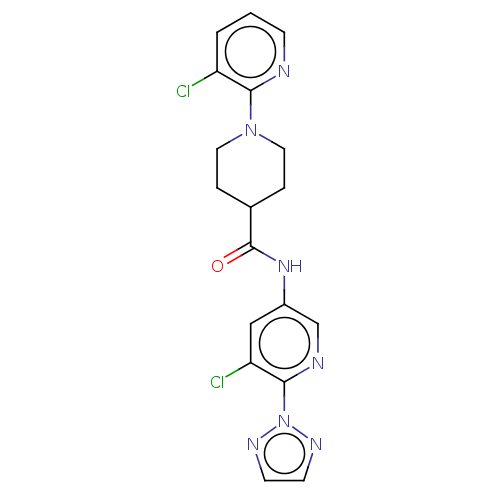
(CHEMBL4128769)Show SMILES Clc1cccnc1N1CCC(CC1)C(=O)Nc1cnc(c(Cl)c1)-n1nccn1 Show InChI InChI=1S/C18H17Cl2N7O/c19-14-2-1-5-21-16(14)26-8-3-12(4-9-26)18(28)25-13-10-15(20)17(22-11-13)27-23-6-7-24-27/h1-2,5-7,10-12H,3-4,8-9H2,(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MALT1 (unknown origin) using Ac-Trp-Leu-Arg-Ser-Arg'Cys(PT14)-NH2 as substrate preincubated for 60 mins followed by substrate addition ... |
Bioorg Med Chem Lett 28: 2153-2158 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.017
BindingDB Entry DOI: 10.7270/Q2G73H89 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148703

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(s1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4OS/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148699
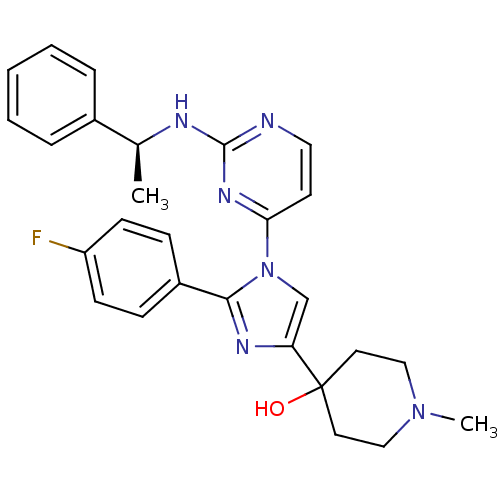
(4-{2-(4-Fluoro-phenyl)-1-[2-((S)-1-phenyl-ethylami...)Show SMILES C[C@H](Nc1nccc(n1)-n1cc(nc1-c1ccc(F)cc1)C1(O)CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C27H29FN6O/c1-19(20-6-4-3-5-7-20)30-26-29-15-12-24(32-26)34-18-23(27(35)13-16-33(2)17-14-27)31-25(34)21-8-10-22(28)11-9-21/h3-12,15,18-19,35H,13-14,16-17H2,1-2H3,(H,29,30,32)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174714

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES COc1cc(\C=C\C(=O)N2CCN(Cc3ccc(F)cc3)C[C@H]2C)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C24H27ClFN3O3/c1-16-14-28(15-18-4-7-20(26)8-5-18)10-11-29(16)24(31)9-6-19-12-23(32-3)21(25)13-22(19)27-17(2)30/h4-9,12-13,16H,10-11,14-15H2,1-3H3,(H,27,30)/b9-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50116031
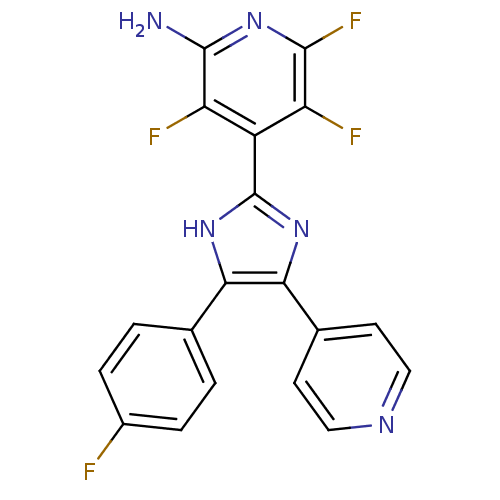
(3,5,6-Trifluoro-4-[4-(4-fluoro-phenyl)-5-pyridin-4...)Show SMILES Nc1nc(F)c(F)c(-c2nc(c([nH]2)-c2ccc(F)cc2)-c2ccncc2)c1F Show InChI InChI=1S/C19H11F4N5/c20-11-3-1-9(2-4-11)15-16(10-5-7-25-8-6-10)27-19(26-15)12-13(21)17(23)28-18(24)14(12)22/h1-8H,(H2,24,28)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vivo inhibition of murine Mitogen-activated protein kinase p38 alpha activity, GST-ATF-2 as substrate in the presence of 120 microM ATP |
Bioorg Med Chem Lett 12: 2109-12 (2002)
BindingDB Entry DOI: 10.7270/Q2610ZNX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50116037

(3,5-Difluoro-4-[5-(4-fluoro-phenyl)-4-pyridin-4-yl...)Show SMILES Nc1nc(N)c(F)c(-c2cc(c([nH]2)-c2ccc(F)cc2)-c2ccncc2)c1F Show InChI InChI=1S/C20H14F3N5/c21-12-3-1-11(2-4-12)18-13(10-5-7-26-8-6-10)9-14(27-18)15-16(22)19(24)28-20(25)17(15)23/h1-9,27H,(H4,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vivo inhibition of murine Mitogen-activated protein kinase p38 alpha activity, GST-ATF-2 as substrate in the presence of 120 microM ATP |
Bioorg Med Chem Lett 12: 2109-12 (2002)
BindingDB Entry DOI: 10.7270/Q2610ZNX |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348492
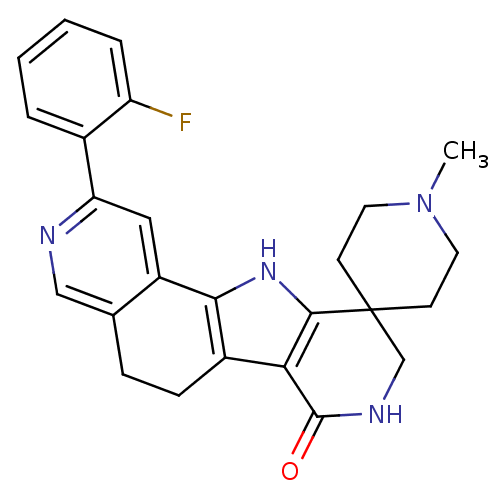
(CHEMBL1801296)Show SMILES CN1CCC2(CC1)CNC(=O)c1c3CCc4cnc(cc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C25H25FN4O/c1-30-10-8-25(9-11-30)14-28-24(31)21-17-7-6-15-13-27-20(16-4-2-3-5-19(16)26)12-18(15)22(17)29-23(21)25/h2-5,12-13,29H,6-11,14H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by im... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546177
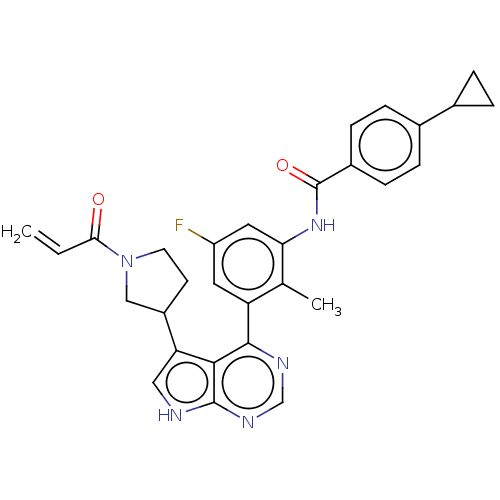
(CHEMBL4740933)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3CCN(C3)C(=O)C=C)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148702
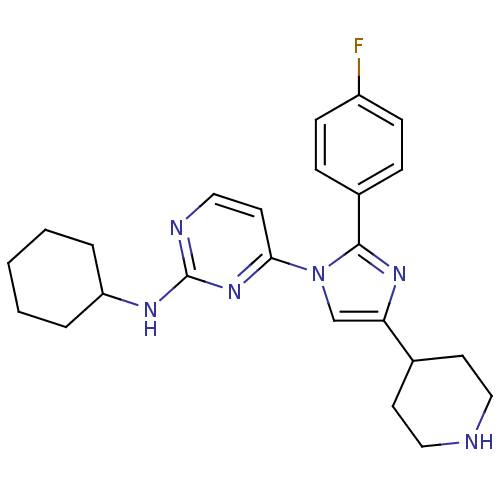
(CHEMBL415712 | Cyclohexyl-{4-[2-(4-fluoro-phenyl)-...)Show SMILES Fc1ccc(cc1)-c1nc(cn1-c1ccnc(NC2CCCCC2)n1)C1CCNCC1 Show InChI InChI=1S/C24H29FN6/c25-19-8-6-18(7-9-19)23-29-21(17-10-13-26-14-11-17)16-31(23)22-12-15-27-24(30-22)28-20-4-2-1-3-5-20/h6-9,12,15-17,20,26H,1-5,10-11,13-14H2,(H,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148691
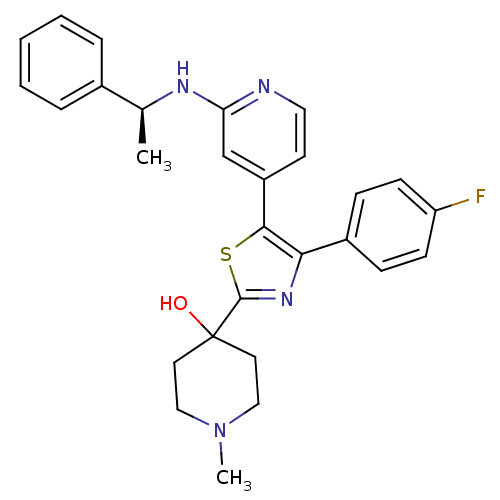
(4-{4-(4-Fluoro-phenyl)-5-[2-((S)-1-phenyl-ethylami...)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1(O)CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4OS/c1-19(20-6-4-3-5-7-20)31-24-18-22(12-15-30-24)26-25(21-8-10-23(29)11-9-21)32-27(35-26)28(34)13-16-33(2)17-14-28/h3-12,15,18-19,34H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348542
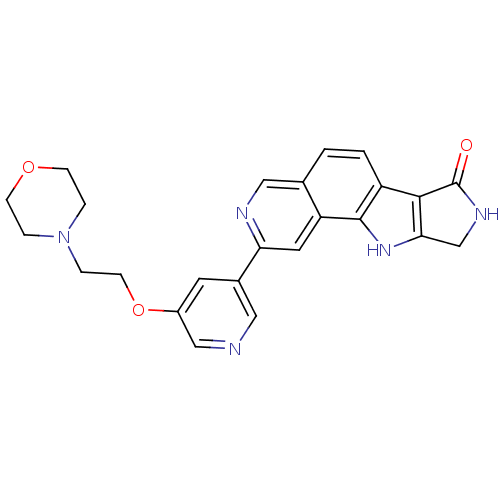
(CHEMBL1801381)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(cc34)-c3cncc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C24H23N5O3/c30-24-22-18-2-1-15-12-26-20(10-19(15)23(18)28-21(22)14-27-24)16-9-17(13-25-11-16)32-8-5-29-3-6-31-7-4-29/h1-2,9-13,28H,3-8,14H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348543

(CHEMBL1801382)Show SMILES COCCOc1cncc(c1)-c1cc2c3[nH]c4CNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C21H18N4O3/c1-27-4-5-28-14-6-13(8-22-10-14)17-7-16-12(9-23-17)2-3-15-19-18(25-20(15)16)11-24-21(19)26/h2-3,6-10,25H,4-5,11H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348546
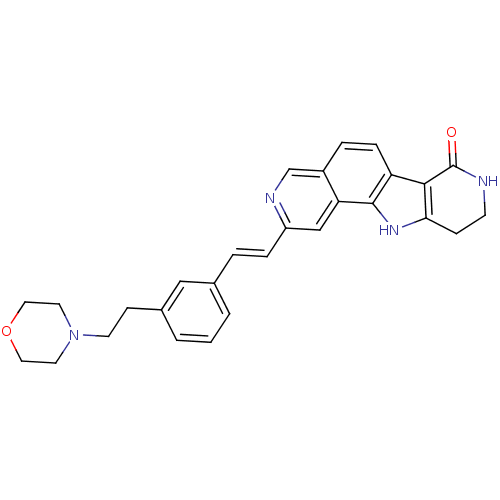
(CHEMBL1801385)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(\C=C\c5cccc(CCN6CCOCC6)c5)cc34)c12 Show InChI InChI=1S/C28H28N4O2/c33-28-26-23-7-5-21-18-30-22(17-24(21)27(23)31-25(26)8-10-29-28)6-4-19-2-1-3-20(16-19)9-11-32-12-14-34-15-13-32/h1-7,16-18,31H,8-15H2,(H,29,33)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50116038
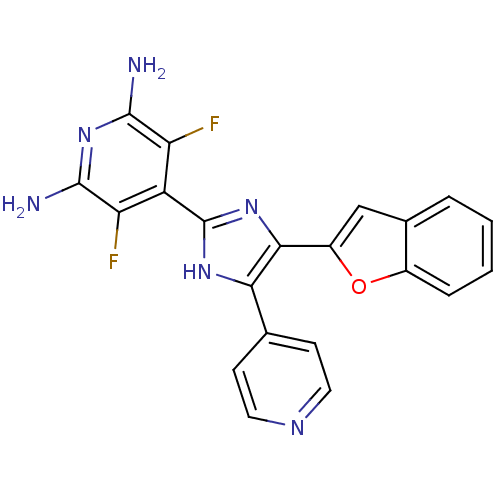
(4-(4-Benzofuran-2-yl-5-pyridin-4-yl-1H-imidazol-2-...)Show SMILES Nc1nc(N)c(F)c(-c2nc(-c3cc4ccccc4o3)c([nH]2)-c2ccncc2)c1F Show InChI InChI=1S/C21H14F2N6O/c22-15-14(16(23)20(25)29-19(15)24)21-27-17(10-5-7-26-8-6-10)18(28-21)13-9-11-3-1-2-4-12(11)30-13/h1-9H,(H,27,28)(H4,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vivo inhibition of murine Mitogen-activated protein kinase p38 alpha activity, GST-ATF-2 as substrate in the presence of 120 microM ATP |
Bioorg Med Chem Lett 12: 2109-12 (2002)
BindingDB Entry DOI: 10.7270/Q2610ZNX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50175994
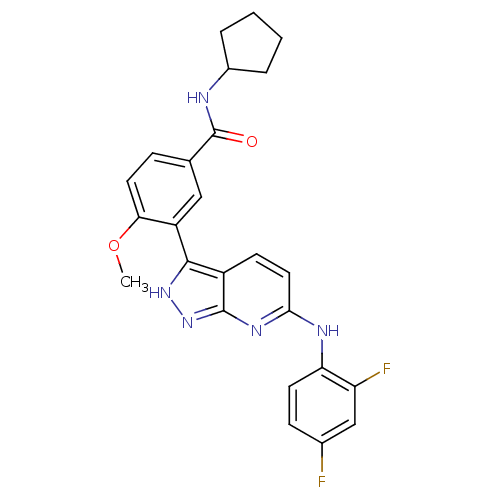
(CHEMBL371756 | N-cyclopentyl-3-(6-(2,4-difluorophe...)Show SMILES COc1ccc(cc1-c1[nH]nc2nc(Nc3ccc(F)cc3F)ccc12)C(=O)NC1CCCC1 Show InChI InChI=1S/C25H23F2N5O2/c1-34-21-10-6-14(25(33)28-16-4-2-3-5-16)12-18(21)23-17-8-11-22(30-24(17)32-31-23)29-20-9-7-15(26)13-19(20)27/h6-13,16H,2-5H2,1H3,(H,28,33)(H2,29,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine p38alpha MAPK |
Bioorg Med Chem Lett 16: 262-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.015
BindingDB Entry DOI: 10.7270/Q2DJ5F6T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546181
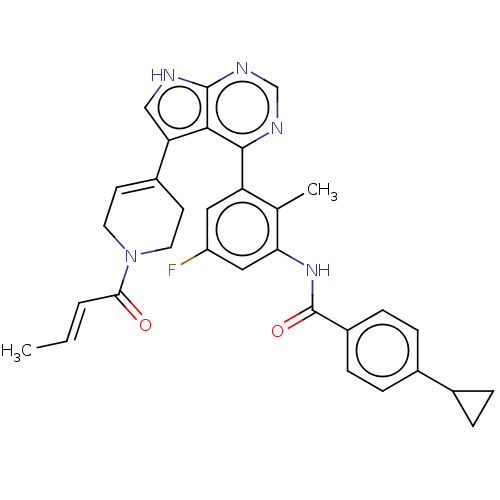
(CHEMBL4789435)Show SMILES C\C=C\C(=O)N1CCC(=CC1)c1c[nH]c2ncnc(-c3cc(F)cc(NC(=O)c4ccc(cc4)C4CC4)c3C)c12 |c:8| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148696

(CHEMBL119952 | {4-[(S)-2-(4-Fluoro-phenyl)-5-(4-me...)Show SMILES C[C@H](Nc1nccc(n1)-n1c(nc2nc(ccc12)N1CCN(C)CC1)-c1ccc(F)cc1)c1ccccc1 Show InChI InChI=1S/C29H29FN8/c1-20(21-6-4-3-5-7-21)32-29-31-15-14-26(34-29)38-24-12-13-25(37-18-16-36(2)17-19-37)33-27(24)35-28(38)22-8-10-23(30)11-9-22/h3-15,20H,16-19H2,1-2H3,(H,31,32,34)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174718

(CHEMBL200680 | N-(6-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CC(=O)Nc1cc2ncccc2cc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C27H27FN4O2/c1-18(33)30-26-14-25-20(3-2-12-29-25)13-21(26)6-11-27(34)32-23-9-10-24(32)17-31(16-23)15-19-4-7-22(28)8-5-19/h2-8,11-14,23-24H,9-10,15-17H2,1H3,(H,30,33)/b11-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348491

(CHEMBL1801305)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(nc4-c3[nH]c21)-c1cccc(F)c1 Show InChI InChI=1S/C22H20FN5O/c1-28-10-22(11-28)9-25-21(29)16-15-6-5-13-8-24-20(12-3-2-4-14(23)7-12)27-17(13)18(15)26-19(16)22/h2-4,7-8,26H,5-6,9-11H2,1H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348493
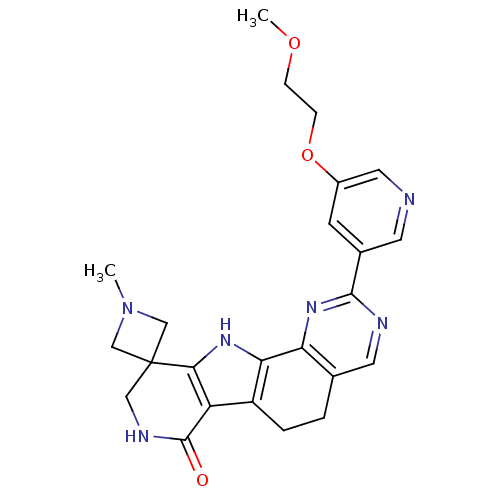
(CHEMBL1801304)Show SMILES COCCOc1cncc(c1)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2n1 Show InChI InChI=1S/C24H26N6O3/c1-30-12-24(13-30)11-27-23(31)18-17-4-3-14-9-26-22(29-19(14)20(17)28-21(18)24)15-7-16(10-25-8-15)33-6-5-32-2/h7-10,28H,3-6,11-13H2,1-2H3,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data