 Found 493 hits with Last Name = 'rhee' and Initial = 'sd'
Found 493 hits with Last Name = 'rhee' and Initial = 'sd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50206821
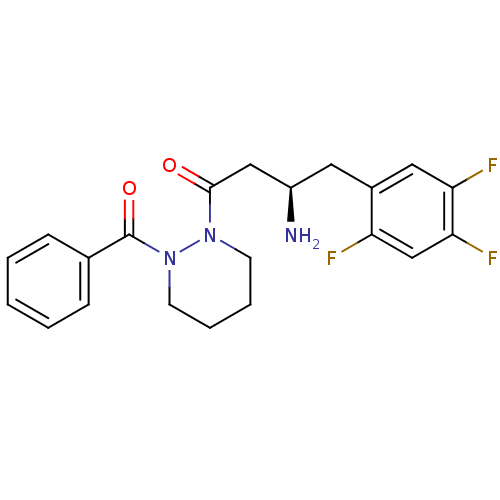
((R)-3-amino-1-(2-benzoylpiperazin-1-yl)-4-(2,4,5-t...)Show SMILES N[C@@H](CC(=O)N1CCCCN1C(=O)c1ccccc1)Cc1cc(F)c(F)cc1F Show InChI InChI=1S/C21H22F3N3O2/c22-17-13-19(24)18(23)11-15(17)10-16(25)12-20(28)26-8-4-5-9-27(26)21(29)14-6-2-1-3-7-14/h1-3,6-7,11,13,16H,4-5,8-10,12,25H2/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney DPP4 |
Bioorg Med Chem Lett 17: 2622-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.111
BindingDB Entry DOI: 10.7270/Q21V5FS4 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50206820

((R)-3-amino-1-(2-benzoyl-1,2-diazepan-1-yl)-4-(2,4...)Show SMILES N[C@@H](CC(=O)N1CCCCCN1C(=O)c1ccccc1)Cc1cc(F)c(F)cc1F Show InChI InChI=1S/C22H24F3N3O2/c23-18-14-20(25)19(24)12-16(18)11-17(26)13-21(29)27-9-5-2-6-10-28(27)22(30)15-7-3-1-4-8-15/h1,3-4,7-8,12,14,17H,2,5-6,9-11,13,26H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 56.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney DPP4 |
Bioorg Med Chem Lett 17: 2622-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.111
BindingDB Entry DOI: 10.7270/Q21V5FS4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167443
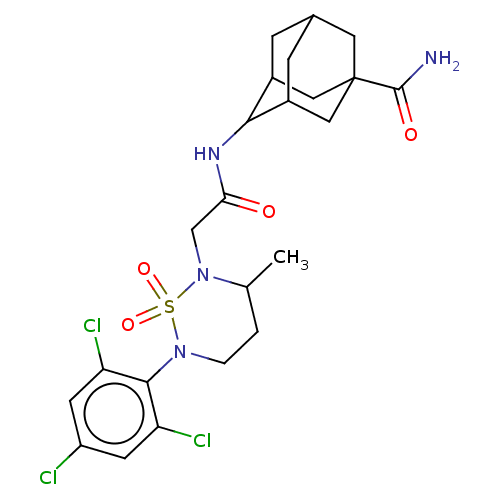
(US9073906, 128)Show SMILES CC1CCN(c2c(Cl)cc(Cl)cc2Cl)S(=O)(=O)N1CC(=O)NC1C2CC3CC1CC(C3)(C2)C(N)=O |TLB:21:22:24:29.30.31,21:22:29.28.31:24.25.26,32:29:24:22.27.26,THB:30:29:22:24.25.26,30:25:22:29.28.31,28:29:24:22.27.26,28:27:24:29.30.31,(.07,1.54,;-1.26,.77,;-2.6,1.54,;-3.93,.77,;-3.93,-.77,;-5.27,-1.54,;-6.6,-.77,;-6.6,.77,;-7.93,-1.54,;-7.93,-3.08,;-9.27,-3.85,;-6.6,-3.85,;-5.27,-3.08,;-3.93,-3.85,;-2.6,-1.54,;-3.37,-2.88,;-1.83,-2.88,;-1.26,-.77,;.07,-1.54,;1.4,-.77,;1.4,.77,;2.74,-1.54,;4.07,-.77,;4.39,1.28,;3.99,2.77,;5.08,3.85,;4.65,2.53,;5.25,.21,;6.59,-.13,;6.96,1.97,;6.56,3.45,;5.87,.88,;8.5,1.97,;9.27,3.3,;9.27,.63,)| Show InChI InChI=1S/C23H29Cl3N4O4S/c1-12-2-3-29(21-17(25)6-16(24)7-18(21)26)35(33,34)30(12)11-19(31)28-20-14-4-13-5-15(20)10-23(8-13,9-14)22(27)32/h6-7,12-15,20H,2-5,8-11H2,1H3,(H2,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167438
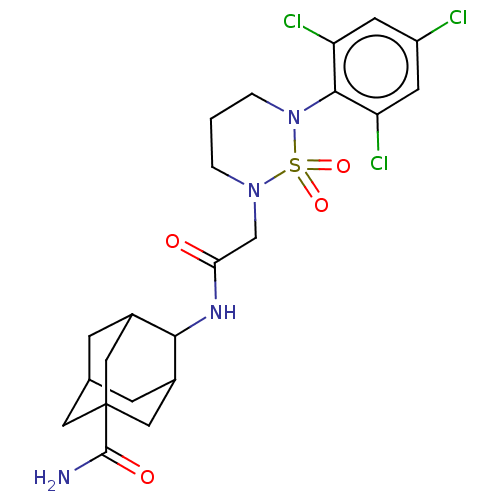
(US9073906, 88)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)CN1CCCN(c4c(Cl)cc(Cl)cc4Cl)S1(=O)=O)C(C3)C2 |TLB:10:9:6:3.4.8,10:9:3.33.8:6.5.32,1:3:9:6.5.32,THB:33:3:6:9.31.32,33:31:6:3.4.8,4:3:9:6.5.32,4:5:9:3.33.8,(9.04,3.66,;8.27,2.32,;9.04,.99,;6.73,2.32,;6.11,3.73,;4.59,3.9,;3.68,2.66,;4.29,1.25,;5.83,1.08,;4.29,-.82,;2.96,-1.59,;1.63,-.82,;1.63,.72,;.29,-1.59,;-1.04,-.82,;-1.04,.72,;-2.37,1.49,;-3.71,.72,;-3.71,-.82,;-5.04,-1.59,;-6.37,-.82,;-6.37,.72,;-7.71,-1.59,;-7.71,-3.13,;-9.04,-3.9,;-6.37,-3.9,;-5.04,-3.13,;-3.71,-3.9,;-2.37,-1.59,;-1.6,-2.93,;-3.14,-2.93,;5.31,.33,;4.37,2.53,;6.68,.2,)| Show InChI InChI=1S/C22H27Cl3N4O4S/c23-15-6-16(24)20(17(25)7-15)29-3-1-2-28(34(29,32)33)11-18(30)27-19-13-4-12-5-14(19)10-22(8-12,9-13)21(26)31/h6-7,12-14,19H,1-5,8-11H2,(H2,26,31)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167440
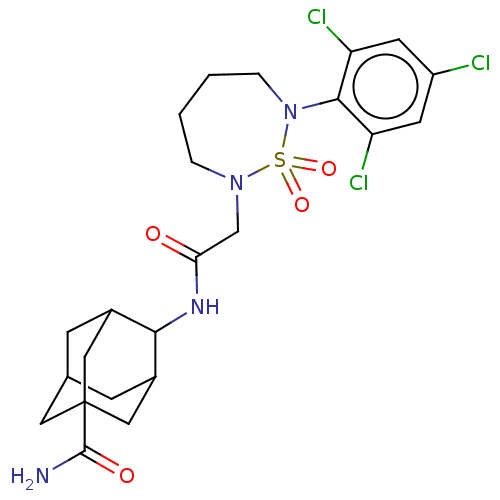
(US9073906, 122)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)CN1CCCCN(c4c(Cl)cc(Cl)cc4Cl)S1(=O)=O)C(C3)C2 |TLB:1:3:6:9.32.33,10:9:6:3.4.8,10:9:3.34.8:6.5.33,THB:4:3:9:6.5.33,4:5:9:3.34.8,34:3:6:9.32.33,34:32:6:3.4.8,(9.32,3.3,;8.55,1.97,;9.32,.63,;7.01,1.97,;6.61,3.45,;5.13,3.85,;4.04,2.77,;4.44,1.28,;5.93,.88,;4.12,-.77,;2.79,-1.54,;1.46,-.77,;1.46,.77,;.12,-1.54,;-1.21,-.77,;-.87,.73,;-1.83,1.93,;-3.37,1.93,;-4.33,.73,;-3.99,-.77,;-5.32,-1.54,;-6.65,-.77,;-6.65,.77,;-7.99,-1.54,;-7.99,-3.08,;-9.32,-3.85,;-6.65,-3.85,;-5.32,-3.08,;-3.99,-3.85,;-2.6,-1.44,;-3.37,-2.77,;-1.83,-2.77,;5.31,.21,;4.7,2.53,;6.64,-.13,)| Show InChI InChI=1S/C23H29Cl3N4O4S/c24-16-7-17(25)21(18(26)8-16)30-4-2-1-3-29(35(30,33)34)12-19(31)28-20-14-5-13-6-15(20)11-23(9-13,10-14)22(27)32/h7-8,13-15,20H,1-6,9-12H2,(H2,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167441

(US9073906, 123)Show SMILES CN(CC(=O)NC1C2CC3CC1CC(C3)(C2)C(N)=O)S(=O)(=O)N(C)c1c(Cl)cc(Cl)cc1Cl |TLB:5:6:8:13.14.15,5:6:13.12.15:8.9.10,16:13:8:6.11.10,THB:14:13:6:8.9.10,14:9:6:13.12.15,12:13:8:6.11.10,12:11:8:13.14.15,(-1.26,.77,;-1.26,-.77,;.07,-1.54,;1.4,-.77,;1.4,.77,;2.74,-1.54,;4.07,-.77,;4.39,1.28,;3.99,2.77,;5.08,3.85,;4.65,2.53,;5.25,.21,;6.59,-.13,;6.96,1.97,;6.56,3.45,;5.87,.88,;8.5,1.97,;9.27,3.3,;9.27,.63,;-2.6,-1.54,;-3.37,-2.88,;-1.83,-2.88,;-3.93,-.77,;-3.93,.77,;-5.27,-1.54,;-6.6,-.77,;-6.6,.77,;-7.93,-1.54,;-7.93,-3.08,;-9.27,-3.85,;-6.6,-3.85,;-5.27,-3.08,;-3.93,-3.85,)| Show InChI InChI=1S/C21H27Cl3N4O4S/c1-27(33(31,32)28(2)19-15(23)5-14(22)6-16(19)24)10-17(29)26-18-12-3-11-4-13(18)9-21(7-11,8-12)20(25)30/h5-6,11-13,18H,3-4,7-10H2,1-2H3,(H2,25,30)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50384522
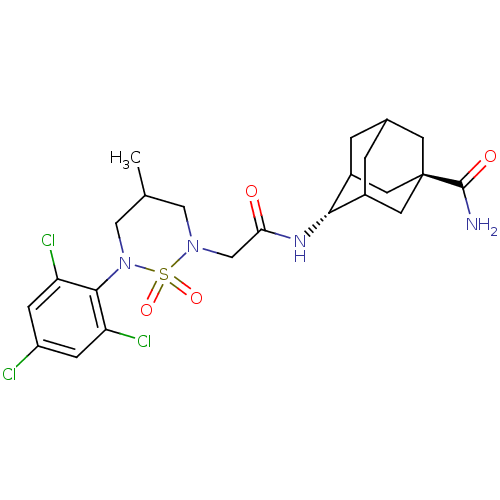
(CHEMBL2036234)Show SMILES CC1CN(CC(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(Cl)cc1Cl |r,wU:8.7,wD:15.20,TLB:17:15:12:10.9.8,7:8:16.15.14:12,18:15:12:10.9.8,8:9:16:14.13.12,THB:17:9:16.15.14:12,8:13:16:10.17.9,(10.39,-6.25,;10.39,-7.79,;11.73,-8.57,;11.73,-10.12,;13.06,-10.89,;14.39,-10.13,;14.4,-8.59,;15.73,-10.9,;15.72,-12.44,;17.09,-13.12,;16.8,-14.53,;15.79,-15.51,;14.44,-14.87,;14.59,-13.45,;15.26,-14.93,;16.69,-15.6,;16.42,-17.14,;17.81,-14.58,;18.17,-16.01,;18.57,-17.5,;19.27,-14.93,;10.38,-10.88,;11.15,-12.22,;9.6,-12.21,;9.05,-10.12,;9.05,-8.57,;7.72,-10.89,;6.39,-10.12,;6.39,-8.58,;5.05,-10.9,;5.06,-12.44,;3.73,-13.22,;6.4,-13.2,;7.72,-12.43,;9.05,-13.2,)| Show InChI InChI=1S/C23H29Cl3N4O4S/c1-12-9-29(35(33,34)30(10-12)21-17(25)4-16(24)5-18(21)26)11-19(31)28-20-14-2-13-3-15(20)8-23(6-13,7-14)22(27)32/h4-5,12-15,20H,2-3,6-11H2,1H3,(H2,27,32)(H,28,31)/t12?,13?,14?,15?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50384520
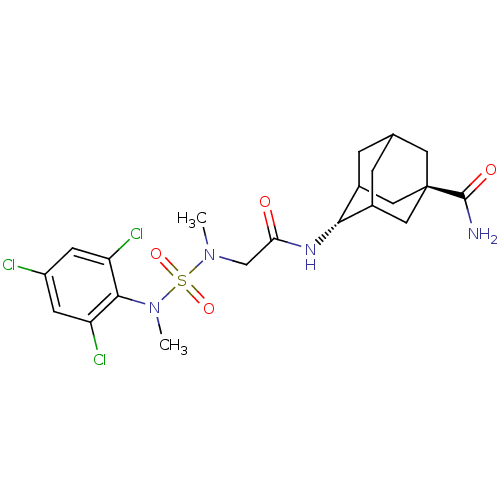
(CHEMBL2036232)Show SMILES CN(CC(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O)S(=O)(=O)N(C)c1c(Cl)cc(Cl)cc1Cl |r,wU:6.5,wD:13.18,TLB:5:6:14.13.12:10,16:13:10:8.7.6,15:13:10:8.7.6,6:7:14:12.11.10,THB:15:7:14.13.12:10,6:11:14:8.15.7,(12.01,-12.22,;12.01,-13.77,;13.34,-14.54,;14.67,-13.78,;14.68,-12.24,;16.01,-14.55,;16,-16.09,;17.37,-16.76,;17.08,-18.18,;16.06,-19.16,;14.72,-18.52,;14.87,-17.1,;15.54,-18.58,;16.97,-19.25,;16.7,-20.79,;18.09,-18.23,;18.45,-19.66,;18.85,-21.15,;19.55,-18.57,;10.66,-14.53,;11.43,-15.87,;9.88,-15.86,;9.33,-13.77,;9.33,-12.22,;8,-14.54,;6.67,-13.77,;6.67,-12.23,;5.33,-14.54,;5.34,-16.09,;4.01,-16.87,;6.68,-16.85,;8,-16.08,;9.33,-16.84,)| Show InChI InChI=1S/C21H27Cl3N4O4S/c1-27(33(31,32)28(2)19-15(23)5-14(22)6-16(19)24)10-17(29)26-18-12-3-11-4-13(18)9-21(7-11,8-12)20(25)30/h5-6,11-13,18H,3-4,7-10H2,1-2H3,(H2,25,30)(H,26,29)/t11?,12?,13?,18-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50384519
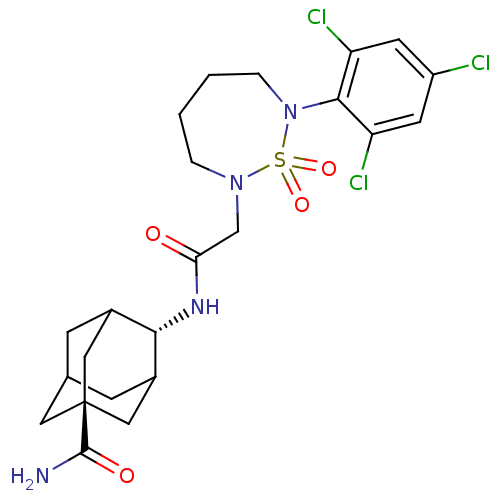
(CHEMBL2036239)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CCCCN(c4c(Cl)cc(Cl)cc4Cl)S1(=O)=O)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:1:3:33:6.7.9,10:9:4.3.34:33,8:3:33:6.7.9,THB:8:7:4.3.34:33,9:7:4:34.32.33,9:32:4:6.8.7,(18.68,-16.84,;18.05,-15.43,;18.96,-14.19,;16.52,-15.27,;16.53,-16.75,;15.65,-15.33,;16.43,-14.23,;16.46,-12.86,;17.39,-14.11,;15.05,-12.47,;15.07,-10.93,;13.86,-9.97,;14.1,-8.44,;12.43,-10.53,;11.22,-9.56,;11.57,-8.05,;10.61,-6.85,;9.07,-6.84,;8.11,-8.04,;8.45,-9.55,;7.24,-10.51,;5.81,-9.93,;5.59,-8.41,;4.6,-10.89,;4.82,-12.42,;3.61,-13.37,;6.26,-12.98,;7.47,-12.03,;8.9,-12.59,;9.82,-10.22,;10.59,-11.55,;9.05,-11.55,;14.17,-13.62,;14.28,-14.97,;15.05,-14.88,)| Show InChI InChI=1S/C23H29Cl3N4O4S/c24-16-7-17(25)21(18(26)8-16)30-4-2-1-3-29(35(30,33)34)12-19(31)28-20-14-5-13-6-15(20)11-23(9-13,10-14)22(27)32/h7-8,13-15,20H,1-6,9-12H2,(H2,27,32)(H,28,31)/t13?,14?,15?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50384518
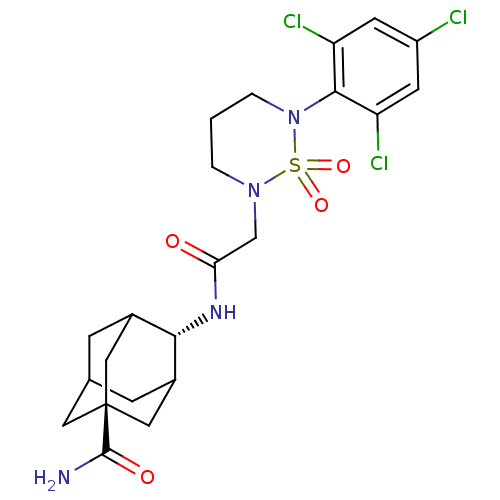
(CHEMBL2036240)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CCCN(c4c(Cl)cc(Cl)cc4Cl)S1(=O)=O)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:8:3:32:6.7.9,10:9:4.3.33:32,1:3:32:6.7.9,THB:8:7:4.3.33:32,9:7:4:33.31.32,9:31:4:6.8.7,(18.57,-16.7,;18.17,-15.21,;19.27,-14.12,;16.69,-14.8,;16.42,-16.34,;15.79,-14.71,;16.8,-13.73,;17.09,-12.31,;17.81,-13.78,;15.72,-11.64,;15.73,-10.1,;14.39,-9.33,;14.4,-7.79,;13.06,-10.09,;11.73,-9.32,;11.73,-7.77,;10.39,-6.98,;9.05,-7.77,;9.05,-9.32,;7.72,-10.09,;6.39,-9.32,;6.39,-7.78,;5.05,-10.09,;5.06,-11.64,;3.73,-12.42,;6.4,-12.4,;7.72,-11.63,;9.05,-12.39,;10.38,-10.08,;11.15,-11.42,;9.6,-11.41,;14.59,-12.65,;14.44,-14.07,;15.26,-14.13,)| Show InChI InChI=1S/C22H27Cl3N4O4S/c23-15-6-16(24)20(17(25)7-15)29-3-1-2-28(34(29,32)33)11-18(30)27-19-13-4-12-5-14(19)10-22(8-12,9-13)21(26)31/h6-7,12-14,19H,1-5,8-11H2,(H2,26,31)(H,27,30)/t12?,13?,14?,19-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167444

(US9073906, 156)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(Cl)cc1Cl |TLB:7:8:10:15.16.17,7:8:15.14.17:10.11.12,18:15:10:8.13.12,THB:16:15:8:10.11.12,16:11:8:15.14.17,14:15:10:8.13.12,14:13:10:15.16.17,18:15:8:10.11.12,(-2.33,2.4,;-2.33,.86,;-.99,.09,;-.99,-1.45,;.34,-2.22,;1.68,-1.45,;1.68,.09,;3.01,-2.22,;4.34,-1.45,;4.11,.62,;3.34,1.95,;4.11,3.28,;4.04,1.9,;5.23,-.19,;6.61,-.17,;6.42,1.95,;5.65,3.28,;5.65,.62,;7.51,3.04,;8.99,2.64,;7.11,4.53,;-2.33,-2.22,;-3.1,-3.55,;-1.56,-3.55,;-3.66,-1.45,;-3.66,.09,;-4.99,-2.22,;-6.33,-1.45,;-6.33,.09,;-7.66,-2.22,;-7.66,-3.76,;-8.99,-4.53,;-6.33,-4.53,;-4.99,-3.76,;-3.66,-4.53,)| Show InChI InChI=1S/C23H29Cl3N4O4S/c1-12-9-29(35(33,34)30(10-12)21-17(25)4-16(24)5-18(21)26)11-19(31)28-20-14-2-13-3-15(20)8-23(6-13,7-14)22(27)32/h4-5,12-15,20H,2-3,6-11H2,1H3,(H2,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50337787
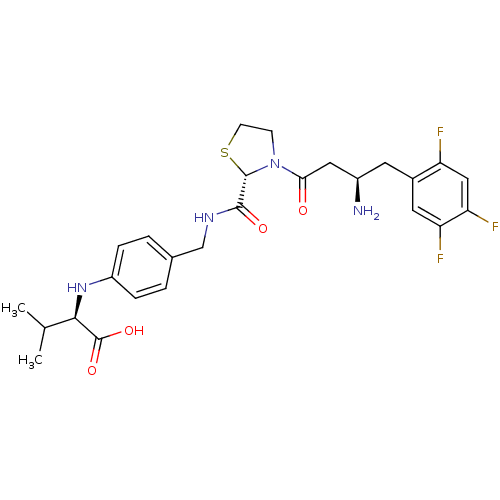
((R)-2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifluoroph...)Show SMILES CC(C)[C@@H](Nc1ccc(CNC(=O)[C@@H]2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C26H31F3N4O4S/c1-14(2)23(26(36)37)32-18-5-3-15(4-6-18)13-31-24(35)25-33(7-8-38-25)22(34)11-17(30)9-16-10-20(28)21(29)12-19(16)27/h3-6,10,12,14,17,23,25,32H,7-9,11,13,30H2,1-2H3,(H,31,35)(H,36,37)/t17-,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50384519

(CHEMBL2036239)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CCCCN(c4c(Cl)cc(Cl)cc4Cl)S1(=O)=O)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:1:3:33:6.7.9,10:9:4.3.34:33,8:3:33:6.7.9,THB:8:7:4.3.34:33,9:7:4:34.32.33,9:32:4:6.8.7,(18.68,-16.84,;18.05,-15.43,;18.96,-14.19,;16.52,-15.27,;16.53,-16.75,;15.65,-15.33,;16.43,-14.23,;16.46,-12.86,;17.39,-14.11,;15.05,-12.47,;15.07,-10.93,;13.86,-9.97,;14.1,-8.44,;12.43,-10.53,;11.22,-9.56,;11.57,-8.05,;10.61,-6.85,;9.07,-6.84,;8.11,-8.04,;8.45,-9.55,;7.24,-10.51,;5.81,-9.93,;5.59,-8.41,;4.6,-10.89,;4.82,-12.42,;3.61,-13.37,;6.26,-12.98,;7.47,-12.03,;8.9,-12.59,;9.82,-10.22,;10.59,-11.55,;9.05,-11.55,;14.17,-13.62,;14.28,-14.97,;15.05,-14.88,)| Show InChI InChI=1S/C23H29Cl3N4O4S/c24-16-7-17(25)21(18(26)8-16)30-4-2-1-3-29(35(30,33)34)12-19(31)28-20-14-5-13-6-15(20)11-23(9-13,10-14)22(27)32/h7-8,13-15,20H,1-6,9-12H2,(H2,27,32)(H,28,31)/t13?,14?,15?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50384521
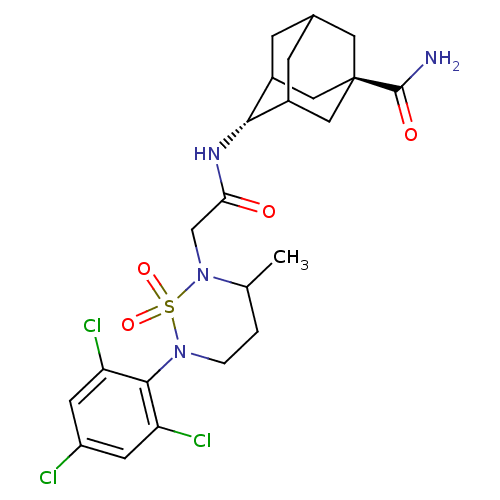
(CHEMBL2036233)Show SMILES CC1CCN(c2c(Cl)cc(Cl)cc2Cl)S(=O)(=O)N1CC(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |r,wU:22.23,wD:29.36,TLB:31:29:26:24.23.22,21:22:30.29.28:26,32:29:26:24.23.22,22:23:30:28.27.26,THB:31:23:30.29.28:26,22:27:30:24.31.23,(13.06,-7.03,;11.73,-7.79,;10.39,-7.01,;9.05,-7.79,;9.05,-9.34,;7.72,-10.11,;6.39,-9.35,;6.39,-7.81,;5.05,-10.12,;5.06,-11.67,;3.73,-12.44,;6.4,-12.43,;7.72,-11.65,;9.05,-12.42,;10.38,-10.1,;11.15,-11.44,;9.6,-11.44,;11.73,-9.34,;13.06,-10.12,;14.39,-9.35,;14.4,-7.81,;15.73,-10.13,;15.72,-11.67,;17.09,-12.34,;16.8,-13.75,;15.78,-14.73,;14.44,-14.1,;14.59,-12.67,;15.26,-14.15,;16.69,-14.83,;16.42,-16.36,;17.81,-13.8,;18.17,-15.23,;18.57,-16.72,;19.27,-14.15,)| Show InChI InChI=1S/C23H29Cl3N4O4S/c1-12-2-3-29(21-17(25)6-16(24)7-18(21)26)35(33,34)30(12)11-19(31)28-20-14-4-13-5-15(20)10-23(8-13,9-14)22(27)32/h6-7,12-15,20H,2-5,8-11H2,1H3,(H2,27,32)(H,28,31)/t12?,13?,14?,15?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50337789
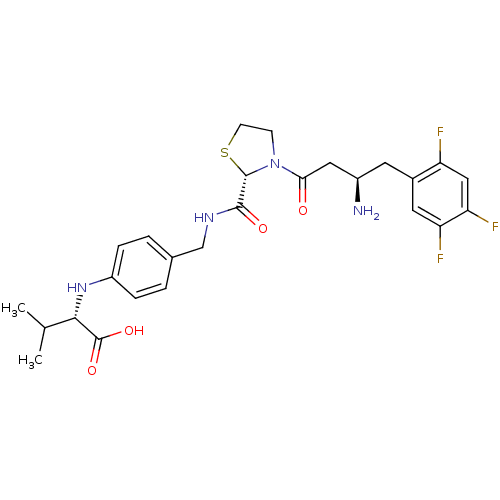
((S)-2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifluoroph...)Show SMILES CC(C)[C@H](Nc1ccc(CNC(=O)[C@@H]2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C26H31F3N4O4S/c1-14(2)23(26(36)37)32-18-5-3-15(4-6-18)13-31-24(35)25-33(7-8-38-25)22(34)11-17(30)9-16-10-20(28)21(29)12-19(16)27/h3-6,10,12,14,17,23,25,32H,7-9,11,13,30H2,1-2H3,(H,31,35)(H,36,37)/t17-,23+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167451
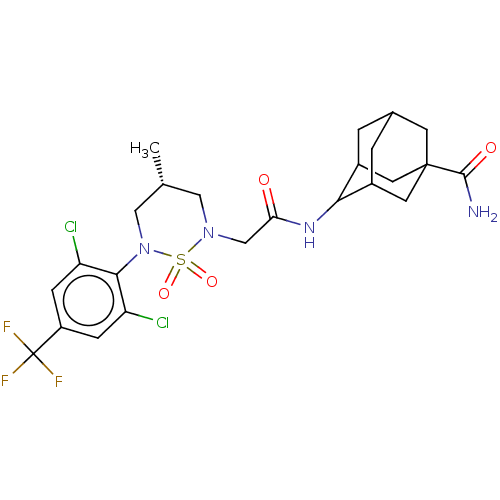
(US9073906, 196)Show SMILES C[C@@H]1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(cc1Cl)C(F)(F)F |r,wD:1.0,TLB:18:15:10:13.12.8,7:8:10:15.16.17,7:8:15.14.17:10.11.12,THB:18:15:10.11.12:8,16:15:10.11.12:8,16:11:15.14.17:8,14:15:10:13.12.8,14:13:10:15.16.17,(-2,3.49,;-2,1.95,;-.67,1.18,;-.67,-.36,;.67,-1.13,;2,-.36,;2,1.18,;3.33,-1.13,;4.67,-.36,;5.12,1.07,;4.35,2.4,;5.12,3.73,;5.25,1.73,;6.04,.43,;7.42,.77,;7.43,2.4,;6.66,3.73,;6.66,1.07,;8.51,3.49,;10,3.09,;8.12,4.98,;-2,-1.13,;-2.77,-2.46,;-1.23,-2.46,;-3.33,-.36,;-3.33,1.18,;-4.67,-1.13,;-6,-.36,;-6,1.18,;-7.34,-1.13,;-7.34,-2.67,;-6,-3.44,;-4.67,-2.67,;-3.33,-3.44,;-8.67,-3.44,;-10,-2.67,;-8.67,-4.98,;-10,-4.21,)| Show InChI InChI=1S/C24H29Cl2F3N4O4S/c1-12-9-32(38(36,37)33(10-12)21-17(25)4-16(5-18(21)26)24(27,28)29)11-19(34)31-20-14-2-13-3-15(20)8-23(6-13,7-14)22(30)35/h4-5,12-15,20H,2-3,6-11H2,1H3,(H2,30,35)(H,31,34)/t12-,13?,14?,15?,20?,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50384520

(CHEMBL2036232)Show SMILES CN(CC(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O)S(=O)(=O)N(C)c1c(Cl)cc(Cl)cc1Cl |r,wU:6.5,wD:13.18,TLB:5:6:14.13.12:10,16:13:10:8.7.6,15:13:10:8.7.6,6:7:14:12.11.10,THB:15:7:14.13.12:10,6:11:14:8.15.7,(12.01,-12.22,;12.01,-13.77,;13.34,-14.54,;14.67,-13.78,;14.68,-12.24,;16.01,-14.55,;16,-16.09,;17.37,-16.76,;17.08,-18.18,;16.06,-19.16,;14.72,-18.52,;14.87,-17.1,;15.54,-18.58,;16.97,-19.25,;16.7,-20.79,;18.09,-18.23,;18.45,-19.66,;18.85,-21.15,;19.55,-18.57,;10.66,-14.53,;11.43,-15.87,;9.88,-15.86,;9.33,-13.77,;9.33,-12.22,;8,-14.54,;6.67,-13.77,;6.67,-12.23,;5.33,-14.54,;5.34,-16.09,;4.01,-16.87,;6.68,-16.85,;8,-16.08,;9.33,-16.84,)| Show InChI InChI=1S/C21H27Cl3N4O4S/c1-27(33(31,32)28(2)19-15(23)5-14(22)6-16(19)24)10-17(29)26-18-12-3-11-4-13(18)9-21(7-11,8-12)20(25)30/h5-6,11-13,18H,3-4,7-10H2,1-2H3,(H2,25,30)(H,26,29)/t11?,12?,13?,18-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50384522

(CHEMBL2036234)Show SMILES CC1CN(CC(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(Cl)cc1Cl |r,wU:8.7,wD:15.20,TLB:17:15:12:10.9.8,7:8:16.15.14:12,18:15:12:10.9.8,8:9:16:14.13.12,THB:17:9:16.15.14:12,8:13:16:10.17.9,(10.39,-6.25,;10.39,-7.79,;11.73,-8.57,;11.73,-10.12,;13.06,-10.89,;14.39,-10.13,;14.4,-8.59,;15.73,-10.9,;15.72,-12.44,;17.09,-13.12,;16.8,-14.53,;15.79,-15.51,;14.44,-14.87,;14.59,-13.45,;15.26,-14.93,;16.69,-15.6,;16.42,-17.14,;17.81,-14.58,;18.17,-16.01,;18.57,-17.5,;19.27,-14.93,;10.38,-10.88,;11.15,-12.22,;9.6,-12.21,;9.05,-10.12,;9.05,-8.57,;7.72,-10.89,;6.39,-10.12,;6.39,-8.58,;5.05,-10.9,;5.06,-12.44,;3.73,-13.22,;6.4,-13.2,;7.72,-12.43,;9.05,-13.2,)| Show InChI InChI=1S/C23H29Cl3N4O4S/c1-12-9-29(35(33,34)30(10-12)21-17(25)4-16(24)5-18(21)26)11-19(31)28-20-14-2-13-3-15(20)8-23(6-13,7-14)22(27)32/h4-5,12-15,20H,2-3,6-11H2,1H3,(H2,27,32)(H,28,31)/t12?,13?,14?,15?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117151

(CHEMBL3613351)Show SMILES Cc1ccccc1-c1ccc(cc1)C(=O)NCCNC(=O)c1ccc(O[C@H]2C3CC4CC2C[C@](C4)(C3)C(O)=O)cc1 |r,wU:26.27,wD:33.40,TLB:26:27:34:30.31.32,25:26:34.29.30:32,THB:28:29:32:35.27.26,28:27:34.29.30:32,26:31:34:35.28.27,(-17.57,11.45,;-18.54,10.69,;-19.97,11.26,;-21.18,10.31,;-20.96,8.79,;-19.53,8.21,;-18.32,9.17,;-16.89,8.59,;-15.68,9.54,;-14.25,8.97,;-14.03,7.44,;-15.25,6.49,;-16.67,7.07,;-12.61,6.87,;-11.63,7.62,;-12.39,5.34,;-10.96,4.76,;-10.75,3.24,;-9.32,2.66,;-9.1,1.13,;-10.07,.37,;-7.68,.56,;-7.46,-.97,;-6.03,-1.54,;-4.82,-.59,;-3.39,-1.17,;-2.19,-.22,;-1.2,1.02,;-1.2,2.69,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;1.56,1.02,;1.56,2.59,;.34,.44,;3.07,1.15,;3.59,2.27,;3.78,.15,;-5.03,.93,;-6.46,1.51,)| Show InChI InChI=1S/C34H36N2O5/c1-21-4-2-3-5-29(21)23-6-8-24(9-7-23)31(37)35-14-15-36-32(38)25-10-12-28(13-11-25)41-30-26-16-22-17-27(30)20-34(18-22,19-26)33(39)40/h2-13,22,26-27,30H,14-20H2,1H3,(H,35,37)(H,36,38)(H,39,40)/t22?,26?,27?,30-,34- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50337785

(2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...)Show SMILES CC(C)C(Nc1ccc(CNC(=O)C2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C26H31F3N4O4S/c1-14(2)23(26(36)37)32-18-5-3-15(4-6-18)13-31-24(35)25-33(7-8-38-25)22(34)11-17(30)9-16-10-20(28)21(29)12-19(16)27/h3-6,10,12,14,17,23,25,32H,7-9,11,13,30H2,1-2H3,(H,31,35)(H,36,37)/t17-,23?,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167437

(US9073906, 69)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)CN1CCCN(c4cccc(F)c4)S1(=O)=O)C(C3)C2 |TLB:10:9:6:3.4.8,10:9:3.31.8:6.5.30,1:3:9:6.5.30,THB:31:3:6:9.29.30,31:29:6:3.4.8,4:3:9:6.5.30,4:5:9:3.31.8,(9.04,3.66,;8.27,2.32,;9.04,.99,;6.73,2.32,;6.11,3.73,;4.59,3.9,;3.68,2.66,;4.29,1.25,;5.83,1.08,;4.29,-.82,;2.96,-1.59,;1.63,-.82,;1.63,.72,;.29,-1.59,;-1.04,-.82,;-1.04,.72,;-2.37,1.49,;-3.71,.72,;-3.71,-.82,;-5.04,-1.59,;-5.04,-3.13,;-6.37,-3.9,;-7.71,-3.13,;-7.71,-1.59,;-9.04,-.82,;-6.37,-.82,;-2.37,-1.59,;-1.6,-2.93,;-3.14,-2.93,;5.31,.33,;4.37,2.53,;6.68,.2,)| Show InChI InChI=1S/C22H29FN4O4S/c23-17-3-1-4-18(9-17)27-6-2-5-26(32(27,30)31)13-19(28)25-20-15-7-14-8-16(20)12-22(10-14,11-15)21(24)29/h1,3-4,9,14-16,20H,2,5-8,10-13H2,(H2,24,29)(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50384518

(CHEMBL2036240)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CCCN(c4c(Cl)cc(Cl)cc4Cl)S1(=O)=O)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:8:3:32:6.7.9,10:9:4.3.33:32,1:3:32:6.7.9,THB:8:7:4.3.33:32,9:7:4:33.31.32,9:31:4:6.8.7,(18.57,-16.7,;18.17,-15.21,;19.27,-14.12,;16.69,-14.8,;16.42,-16.34,;15.79,-14.71,;16.8,-13.73,;17.09,-12.31,;17.81,-13.78,;15.72,-11.64,;15.73,-10.1,;14.39,-9.33,;14.4,-7.79,;13.06,-10.09,;11.73,-9.32,;11.73,-7.77,;10.39,-6.98,;9.05,-7.77,;9.05,-9.32,;7.72,-10.09,;6.39,-9.32,;6.39,-7.78,;5.05,-10.09,;5.06,-11.64,;3.73,-12.42,;6.4,-12.4,;7.72,-11.63,;9.05,-12.39,;10.38,-10.08,;11.15,-11.42,;9.6,-11.41,;14.59,-12.65,;14.44,-14.07,;15.26,-14.13,)| Show InChI InChI=1S/C22H27Cl3N4O4S/c23-15-6-16(24)20(17(25)7-15)29-3-1-2-28(34(29,32)33)11-18(30)27-19-13-4-12-5-14(19)10-22(8-12,9-13)21(26)31/h6-7,12-14,19H,1-5,8-11H2,(H2,26,31)(H,27,30)/t12?,13?,14?,19-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167449

(US9073906, 183)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2C[C@@](C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(cc1Cl)C(F)(F)F |r,wD:15.20,TLB:7:8:12:15.16.14,7:8:15.17.14:12.11.10,17:15:12:9.10.8,THB:18:15:12:9.10.8,18:15:12.11.10:8,16:15:12.11.10:8,16:11:15.17.14:8,17:9:12:15.16.14,(-2,3.49,;-2,1.95,;-.67,1.18,;-.67,-.36,;.67,-1.13,;2,-.36,;2,1.18,;3.33,-1.13,;4.67,-.36,;6.04,.43,;5.25,1.73,;5.12,3.73,;4.35,2.4,;5.12,1.07,;6.66,1.07,;7.43,2.4,;6.66,3.73,;7.42,.77,;8.51,3.49,;10,3.09,;8.12,4.98,;-2,-1.13,;-2.77,-2.46,;-1.23,-2.46,;-3.33,-.36,;-3.33,1.18,;-4.67,-1.13,;-6,-.36,;-6,1.18,;-7.34,-1.13,;-7.34,-2.67,;-6,-3.44,;-4.67,-2.67,;-3.33,-3.44,;-8.67,-3.44,;-10,-2.67,;-8.67,-4.98,;-10,-4.21,)| Show InChI InChI=1S/C24H29Cl2F3N4O4S/c1-12-9-32(38(36,37)33(10-12)21-17(25)4-16(5-18(21)26)24(27,28)29)11-19(34)31-20-14-2-13-3-15(20)8-23(6-13,7-14)22(30)35/h4-5,12-15,20H,2-3,6-11H2,1H3,(H2,30,35)(H,31,34)/t12?,13?,14?,15?,20?,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117187
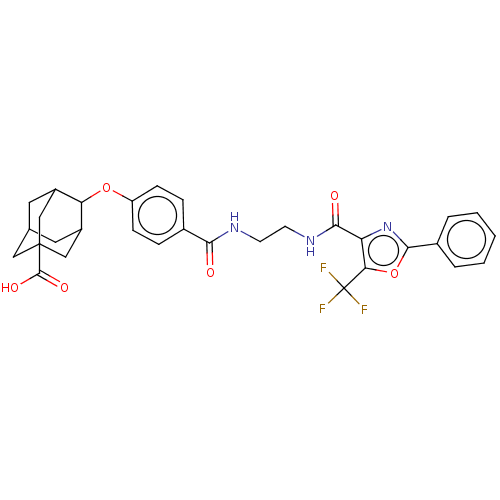
(CHEMBL3613341)Show SMILES OC(=O)C12CC3CC(C1)C(Oc1ccc(cc1)C(=O)NCCNC(=O)c1nc(oc1C(F)(F)F)-c1ccccc1)C(C3)C2 |TLB:6:5:42:8.7.9,6:7:4.5.41:42,THB:9:7:4:41.40.42,9:40:4:8.6.7,10:9:4.5.41:42,(3.79,.17,;3.07,1.17,;3.58,2.29,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;-2.19,-.22,;-3.39,-1.16,;-3.17,-2.69,;-4.38,-3.64,;-4.16,-5.17,;-2.73,-5.74,;-1.52,-4.78,;-1.74,-3.26,;-2.5,-7.26,;-3.47,-8.03,;-1.07,-7.83,;-.85,-9.36,;.59,-9.92,;.81,-11.45,;2.24,-12.02,;3.21,-11.25,;2.47,-13.54,;1.36,-14.59,;2.04,-15.98,;3.57,-15.75,;3.83,-14.24,;5.2,-13.55,;5.27,-12.32,;6.23,-14.22,;6.3,-12.99,;1.32,-17.34,;2.04,-18.7,;1.23,-20,;-.31,-19.94,;-1.03,-18.58,;-.22,-17.28,;-.95,.32,;-1,2.05,;.56,-.22,)| Show InChI InChI=1S/C31H30F3N3O6/c32-31(33,34)25-23(37-28(43-25)19-4-2-1-3-5-19)27(39)36-11-10-35-26(38)18-6-8-22(9-7-18)42-24-20-12-17-13-21(24)16-30(14-17,15-20)29(40)41/h1-9,17,20-21,24H,10-16H2,(H,35,38)(H,36,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117150

(CHEMBL3613355)Show SMILES Cc1cccc(C)c1-c1ccc(cc1)C(=O)NCCNC(=O)c1ccc(O[C@H]2C3CC4CC2C[C@](C4)(C3)C(O)=O)cc1 |r,wU:27.28,wD:34.41,TLB:27:28:35:31.32.33,26:27:35.30.31:33,THB:29:30:33:36.28.27,29:28:35.30.31:33,27:32:35:36.29.28,(-19.36,6.99,;-19.53,8.21,;-20.96,8.79,;-21.18,10.31,;-19.97,11.26,;-18.54,10.69,;-17.57,11.45,;-18.32,9.17,;-16.89,8.59,;-15.68,9.54,;-14.25,8.97,;-14.03,7.44,;-15.25,6.49,;-16.67,7.07,;-12.61,6.87,;-11.63,7.62,;-12.39,5.34,;-10.96,4.76,;-10.75,3.24,;-9.32,2.66,;-9.1,1.13,;-10.07,.37,;-7.68,.56,;-7.46,-.97,;-6.03,-1.54,;-4.82,-.59,;-3.39,-1.17,;-2.19,-.22,;-1.2,1.02,;-1.2,2.69,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;1.56,1.02,;1.56,2.59,;.34,.44,;3.07,1.15,;3.59,2.27,;3.78,.15,;-5.03,.93,;-6.46,1.51,)| Show InChI InChI=1S/C35H38N2O5/c1-21-4-3-5-22(2)30(21)24-6-8-25(9-7-24)32(38)36-14-15-37-33(39)26-10-12-29(13-11-26)42-31-27-16-23-17-28(31)20-35(18-23,19-27)34(40)41/h3-13,23,27-28,31H,14-20H2,1-2H3,(H,36,38)(H,37,39)(H,40,41)/t23?,27?,28?,31-,35- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167447
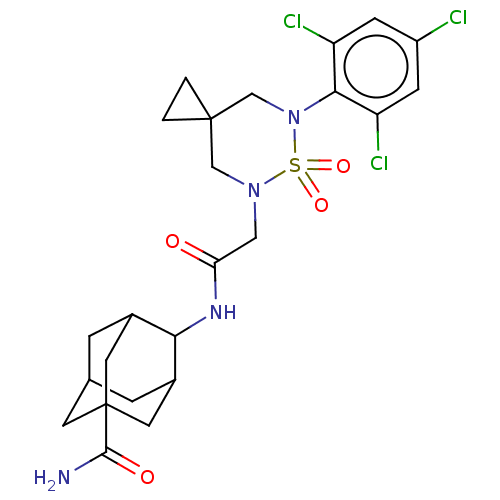
(US9073906, 176)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)CN1CC4(CC4)CN(c4c(Cl)cc(Cl)cc4Cl)S1(=O)=O)C(C3)C2 |TLB:10:9:6:3.4.8,10:9:3.35.8:6.5.34,1:3:6:33.34.9,THB:1:3:6.5.34:9,4:3:6.5.34:9,4:5:3.35.8:9,35:3:6:33.34.9,35:33:6:3.4.8,(9.34,2.32,;7.85,2.72,;7.45,4.21,;6.76,1.63,;5.99,2.96,;4.45,2.96,;3.68,1.63,;4.45,.3,;5.99,.3,;4,-1.13,;2.67,-1.9,;1.33,-1.13,;1.33,.41,;,-1.9,;-1.33,-1.13,;-1.33,.41,;-2.67,1.18,;-1.9,2.52,;-3.44,2.52,;-4,.41,;-4,-1.13,;-5.33,-1.9,;-6.67,-1.13,;-6.67,.41,;-8,-1.9,;-8,-3.44,;-9.34,-4.21,;-6.67,-4.21,;-5.33,-3.44,;-4,-4.21,;-2.67,-1.9,;-3.44,-3.23,;-1.9,-3.23,;5.37,-.34,;4.58,.96,;6.76,0,)| Show InChI InChI=1S/C24H29Cl3N4O4S/c25-16-5-17(26)21(18(27)6-16)31-12-23(1-2-23)11-30(36(31,34)35)10-19(32)29-20-14-3-13-4-15(20)9-24(7-13,8-14)22(28)33/h5-6,13-15,20H,1-4,7-12H2,(H2,28,33)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117149
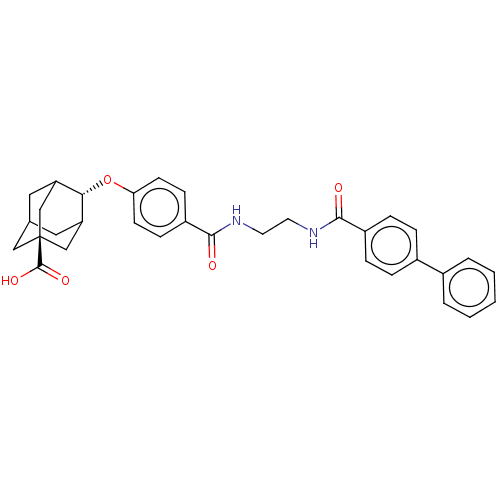
(CHEMBL3613349)Show SMILES OC(=O)[C@@]12CC3CC(C1)[C@H](Oc1ccc(cc1)C(=O)NCCNC(=O)c1ccc(cc1)-c1ccccc1)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:6:5:39:8.7.9,6:7:4.5.38:39,THB:9:7:4:38.37.39,9:37:4:8.6.7,10:9:4.5.38:39,(3.59,2.27,;3.07,1.15,;3.78,.15,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;-2.19,-.22,;-3.39,-1.17,;-4.82,-.59,;-6.03,-1.54,;-7.46,-.97,;-7.68,.56,;-6.46,1.51,;-5.03,.93,;-9.1,1.13,;-10.07,.37,;-9.32,2.66,;-10.75,3.24,;-10.96,4.76,;-12.39,5.34,;-12.61,6.87,;-11.63,7.62,;-14.03,7.44,;-14.25,8.97,;-15.68,9.54,;-16.89,8.59,;-16.67,7.07,;-15.25,6.49,;-18.32,9.17,;-18.54,10.69,;-19.97,11.26,;-21.18,10.31,;-20.96,8.79,;-19.53,8.21,;-.95,.32,;-1,2.05,;.56,-.22,)| Show InChI InChI=1S/C33H34N2O5/c36-30(24-8-6-23(7-9-24)22-4-2-1-3-5-22)34-14-15-35-31(37)25-10-12-28(13-11-25)40-29-26-16-21-17-27(29)20-33(18-21,19-26)32(38)39/h1-13,21,26-27,29H,14-20H2,(H,34,36)(H,35,37)(H,38,39)/t21?,26?,27?,29-,33- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50117149

(CHEMBL3613349)Show SMILES OC(=O)[C@@]12CC3CC(C1)[C@H](Oc1ccc(cc1)C(=O)NCCNC(=O)c1ccc(cc1)-c1ccccc1)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:6:5:39:8.7.9,6:7:4.5.38:39,THB:9:7:4:38.37.39,9:37:4:8.6.7,10:9:4.5.38:39,(3.59,2.27,;3.07,1.15,;3.78,.15,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;-2.19,-.22,;-3.39,-1.17,;-4.82,-.59,;-6.03,-1.54,;-7.46,-.97,;-7.68,.56,;-6.46,1.51,;-5.03,.93,;-9.1,1.13,;-10.07,.37,;-9.32,2.66,;-10.75,3.24,;-10.96,4.76,;-12.39,5.34,;-12.61,6.87,;-11.63,7.62,;-14.03,7.44,;-14.25,8.97,;-15.68,9.54,;-16.89,8.59,;-16.67,7.07,;-15.25,6.49,;-18.32,9.17,;-18.54,10.69,;-19.97,11.26,;-21.18,10.31,;-20.96,8.79,;-19.53,8.21,;-.95,.32,;-1,2.05,;.56,-.22,)| Show InChI InChI=1S/C33H34N2O5/c36-30(24-8-6-23(7-9-24)22-4-2-1-3-5-22)34-14-15-35-31(37)25-10-12-28(13-11-25)40-29-26-16-21-17-27(29)20-33(18-21,19-26)32(38)39/h1-13,21,26-27,29H,14-20H2,(H,34,36)(H,35,37)(H,38,39)/t21?,26?,27?,29-,33- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 expressed in human Hep3B cells incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid sc... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50384524
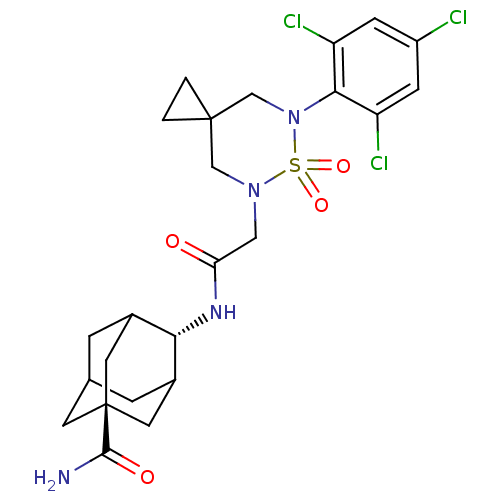
(CHEMBL2036236)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC4(CC4)CN(c4c(Cl)cc(Cl)cc4Cl)S1(=O)=O)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:10:9:4.3.35:34,8:3:34:6.7.9,1:3:34:6.7.9,THB:9:7:4:35.33.34,9:33:4:6.8.7,8:7:4.3.35:34,(18.57,-17.37,;18.17,-15.89,;19.27,-14.8,;16.69,-15.48,;16.42,-17.02,;15.79,-15.38,;16.8,-14.41,;17.09,-12.99,;17.81,-14.46,;15.72,-12.32,;15.73,-10.78,;14.39,-10,;14.4,-8.46,;13.06,-10.77,;11.73,-9.99,;11.73,-8.45,;10.38,-7.66,;9.61,-6.32,;11.15,-6.32,;9.05,-8.45,;9.05,-9.99,;7.72,-10.77,;6.39,-10,;6.39,-8.46,;5.05,-10.77,;5.06,-12.32,;3.73,-13.1,;6.4,-13.08,;7.72,-12.3,;9.05,-13.07,;10.38,-10.76,;11.15,-12.09,;9.6,-12.09,;14.59,-13.33,;14.44,-14.75,;15.26,-14.81,)| Show InChI InChI=1S/C24H29Cl3N4O4S/c25-16-5-17(26)21(18(27)6-16)31-12-23(1-2-23)11-30(36(31,34)35)10-19(32)29-20-14-3-13-4-15(20)9-24(7-13,8-14)22(28)33/h5-6,13-15,20H,1-4,7-12H2,(H2,28,33)(H,29,32)/t13?,14?,15?,20-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50384525
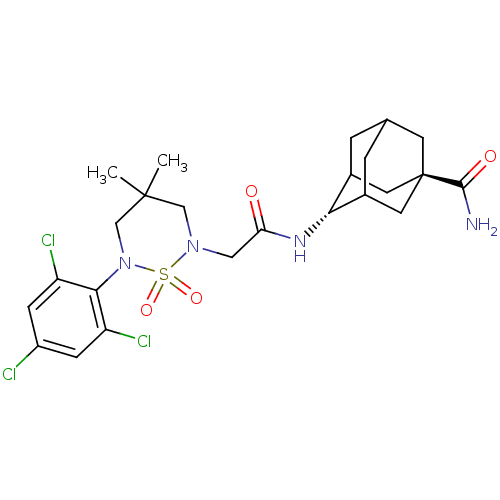
(CHEMBL2036237)Show SMILES CC1(C)CN(CC(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(Cl)cc1Cl |r,wU:9.8,wD:16.21,TLB:9:10:17:15.14.13,8:9:17.16.15:13,18:16:13:11.10.9,19:16:13:11.10.9,THB:9:14:17:11.18.10,18:10:17.16.15:13,(9.61,-6.35,;10.38,-7.68,;11.15,-6.34,;11.73,-8.47,;11.73,-10.02,;13.06,-10.79,;14.39,-10.03,;14.4,-8.49,;15.73,-10.8,;15.72,-12.34,;17.09,-13.01,;16.8,-14.43,;15.78,-15.41,;14.44,-14.77,;14.59,-13.35,;15.26,-14.83,;16.69,-15.5,;16.42,-17.04,;17.81,-14.48,;18.17,-15.91,;18.57,-17.4,;19.27,-14.82,;10.38,-10.78,;11.15,-12.12,;9.6,-12.11,;9.05,-10.02,;9.05,-8.47,;7.72,-10.79,;6.39,-10.02,;6.39,-8.48,;5.05,-10.79,;5.06,-12.34,;3.73,-13.12,;6.4,-13.1,;7.72,-12.33,;9.05,-13.09,)| Show InChI InChI=1S/C24H31Cl3N4O4S/c1-23(2)11-30(36(34,35)31(12-23)21-17(26)5-16(25)6-18(21)27)10-19(32)29-20-14-3-13-4-15(20)9-24(7-13,8-14)22(28)33/h5-6,13-15,20H,3-4,7-12H2,1-2H3,(H2,28,33)(H,29,32)/t13?,14?,15?,20-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167448
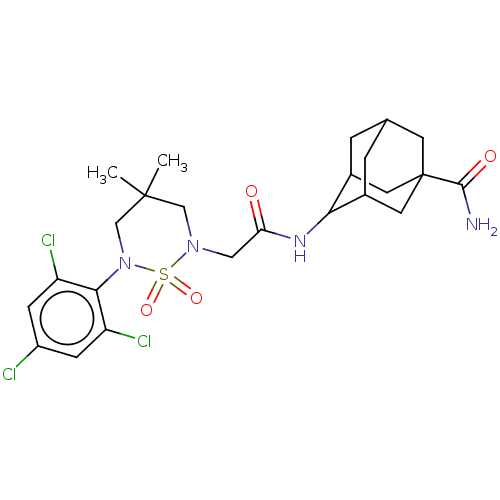
(US9073906, 177)Show SMILES CC1(C)CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(Cl)cc1Cl |TLB:8:9:11:16.17.18,8:9:16.15.18:11.12.13,19:16:11:14.13.9,THB:19:16:11.12.13:9,17:16:11.12.13:9,17:12:16.15.18:9,15:16:11:14.13.9,15:14:11:16.17.18,(-1.9,2.52,;-2.67,1.18,;-3.44,2.52,;-1.33,.41,;-1.33,-1.13,;,-1.9,;1.33,-1.13,;1.33,.41,;2.67,-1.9,;4,-1.13,;4.45,.3,;3.68,1.63,;4.45,2.96,;4.58,.96,;5.37,-.34,;6.76,0,;6.76,1.63,;5.99,2.96,;5.99,.3,;7.85,2.72,;9.34,2.32,;7.45,4.21,;-2.67,-1.9,;-3.44,-3.23,;-1.9,-3.23,;-4,-1.13,;-4,.41,;-5.33,-1.9,;-6.67,-1.13,;-6.67,.41,;-8,-1.9,;-8,-3.44,;-9.34,-4.21,;-6.67,-4.21,;-5.33,-3.44,;-4,-4.21,)| Show InChI InChI=1S/C24H31Cl3N4O4S/c1-23(2)11-30(36(34,35)31(12-23)21-17(26)5-16(25)6-18(21)27)10-19(32)29-20-14-3-13-4-15(20)9-24(7-13,8-14)22(28)33/h5-6,13-15,20H,3-4,7-12H2,1-2H3,(H2,28,33)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50337779

(2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...)Show SMILES CC(C)C(Oc1ccc(CNC(=O)C2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C26H30F3N3O5S/c1-14(2)23(26(35)36)37-18-5-3-15(4-6-18)13-31-24(34)25-32(7-8-38-25)22(33)11-17(30)9-16-10-20(28)21(29)12-19(16)27/h3-6,10,12,14,17,23,25H,7-9,11,13,30H2,1-2H3,(H,31,34)(H,35,36)/t17-,23?,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117189

(CHEMBL3613340)Show SMILES OC(=O)C12CC3CC(C1)C(Oc1ccc(cc1)C(=O)NCCNC(=O)c1ccc(cc1)-c1ccccc1)C(C3)C2 |TLB:6:5:39:8.7.9,6:7:4.5.38:39,THB:9:7:4:38.37.39,9:37:4:8.6.7,10:9:4.5.38:39,(3.79,.17,;3.07,1.17,;3.58,2.29,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;-2.19,-.22,;-3.39,-1.16,;-3.17,-2.69,;-4.38,-3.64,;-4.16,-5.17,;-2.73,-5.74,;-1.52,-4.78,;-1.74,-3.26,;-2.5,-7.26,;-3.47,-8.03,;-1.07,-7.83,;-.85,-9.36,;.59,-9.92,;.81,-11.45,;2.24,-12.02,;3.21,-11.25,;2.47,-13.54,;3.9,-14.11,;4.12,-15.64,;2.91,-16.59,;1.48,-16.02,;1.26,-14.5,;3.13,-18.12,;4.56,-18.69,;4.78,-20.21,;3.57,-21.16,;2.14,-20.59,;1.92,-19.07,;-.95,.32,;-1,2.05,;.56,-.22,)| Show InChI InChI=1S/C33H34N2O5/c36-30(24-8-6-23(7-9-24)22-4-2-1-3-5-22)34-14-15-35-31(37)25-10-12-28(13-11-25)40-29-26-16-21-17-27(29)20-33(18-21,19-26)32(38)39/h1-13,21,26-27,29H,14-20H2,(H,34,36)(H,35,37)(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50384523
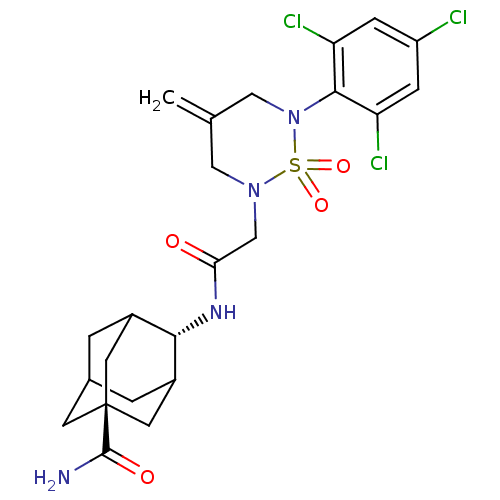
(CHEMBL2036235)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC(=C)CN(c4c(Cl)cc(Cl)cc4Cl)S1(=O)=O)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:8:3:33:6.7.9,10:9:4.3.34:33,1:3:33:6.7.9,THB:8:7:4.3.34:33,9:7:4:34.32.33,9:32:4:6.8.7,(18.57,-17.5,;18.17,-16.01,;19.27,-14.93,;16.69,-15.6,;16.42,-17.14,;15.79,-15.51,;16.8,-14.53,;17.09,-13.12,;17.81,-14.58,;15.72,-12.44,;15.73,-10.9,;14.39,-10.13,;14.4,-8.59,;13.06,-10.89,;11.73,-10.12,;11.73,-8.57,;10.39,-7.79,;10.39,-6.25,;9.05,-8.57,;9.05,-10.12,;7.72,-10.89,;6.39,-10.12,;6.39,-8.58,;5.05,-10.9,;5.06,-12.44,;3.73,-13.22,;6.4,-13.2,;7.72,-12.43,;9.05,-13.2,;10.38,-10.88,;11.15,-12.22,;9.6,-12.21,;14.59,-13.45,;14.44,-14.87,;15.26,-14.93,)| Show InChI InChI=1S/C23H27Cl3N4O4S/c1-12-9-29(35(33,34)30(10-12)21-17(25)4-16(24)5-18(21)26)11-19(31)28-20-14-2-13-3-15(20)8-23(6-13,7-14)22(27)32/h4-5,13-15,20H,1-3,6-11H2,(H2,27,32)(H,28,31)/t13?,14?,15?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117184

(CHEMBL3613347)Show SMILES OC(=O)[C@@]12CC3CC(C1)[C@H](Oc1ccc(cc1)C(=O)NCCNC(=O)c1ccc4ccccc4c1)C(C3)C2 |r,wU:3.2,wD:9.10,TLB:8:3:6.7.9:36,10:9:4.37.3:36,THB:1:3:6.7.9:36,8:7:4.37.3:36,9:7:4:37.36.35,9:35:4:6.7.8,(3.58,2.29,;3.07,1.17,;3.79,.17,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;-2.19,-.22,;-3.39,-1.17,;-4.82,-.59,;-6.03,-1.54,;-7.46,-.97,;-7.68,.56,;-6.46,1.51,;-5.03,.93,;-9.11,1.13,;-9.28,2.35,;-10.32,.17,;-11.75,.75,;-12.96,-.21,;-14.39,.37,;-15.6,-.59,;-15.43,-1.81,;-17.03,-.02,;-17.25,1.51,;-18.69,2.08,;-19.9,1.13,;-21.33,1.7,;-22.54,.75,;-22.32,-.77,;-20.89,-1.35,;-19.68,-.4,;-18.26,-.97,;-1.2,1.02,;-1.2,2.69,;.34,.44,)| Show InChI InChI=1S/C31H32N2O5/c34-28(32-11-12-33-29(35)23-6-5-20-3-1-2-4-22(20)15-23)21-7-9-26(10-8-21)38-27-24-13-19-14-25(27)18-31(16-19,17-24)30(36)37/h1-10,15,19,24-25,27H,11-14,16-18H2,(H,32,34)(H,33,35)(H,36,37)/t19?,24?,25?,27-,31- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117152
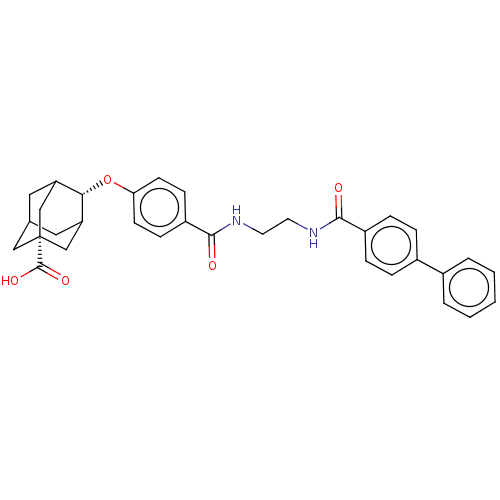
(CHEMBL3613350)Show SMILES OC(=O)[C@]12CC3CC(C1)[C@H](Oc1ccc(cc1)C(=O)NCCNC(=O)c1ccc(cc1)-c1ccccc1)C(C3)C2 |r,wD:9.10,3.2,TLB:38:37:4.5.6:8,THB:38:5:8:39.37.9,9:37:4:6.7.8,9:7:4:39.38.37,10:9:4.5.6:8,(3.59,2.27,;3.07,1.15,;3.78,.15,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;-2.19,-.22,;-3.39,-1.17,;-4.82,-.59,;-6.03,-1.54,;-7.46,-.97,;-7.68,.56,;-6.46,1.51,;-5.03,.93,;-9.1,1.13,;-10.07,.37,;-9.32,2.66,;-10.75,3.24,;-10.96,4.76,;-12.39,5.34,;-12.61,6.87,;-11.63,7.62,;-14.03,7.44,;-14.25,8.97,;-15.68,9.54,;-16.89,8.59,;-16.67,7.07,;-15.25,6.49,;-18.32,9.17,;-18.54,10.69,;-19.97,11.26,;-21.18,10.31,;-20.96,8.79,;-19.53,8.21,;-1.2,1.02,;-1.2,2.69,;.34,.44,)| Show InChI InChI=1S/C33H34N2O5/c36-30(24-8-6-23(7-9-24)22-4-2-1-3-5-22)34-14-15-35-31(37)25-10-12-28(13-11-25)40-29-26-16-21-17-27(29)20-33(18-21,19-26)32(38)39/h1-13,21,26-27,29H,14-20H2,(H,34,36)(H,35,37)(H,38,39)/t21?,26?,27?,29-,33+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50337791

((R)-2-(4-(((R)-3-((R)-3-amino-4-(2,4,5-trifluoroph...)Show SMILES CC(C)[C@@H](Nc1ccc(CNC(=O)[C@H]2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C26H31F3N4O4S/c1-14(2)23(26(36)37)32-18-5-3-15(4-6-18)13-31-24(35)25-33(7-8-38-25)22(34)11-17(30)9-16-10-20(28)21(29)12-19(16)27/h3-6,10,12,14,17,23,25,32H,7-9,11,13,30H2,1-2H3,(H,31,35)(H,36,37)/t17-,23-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50384529
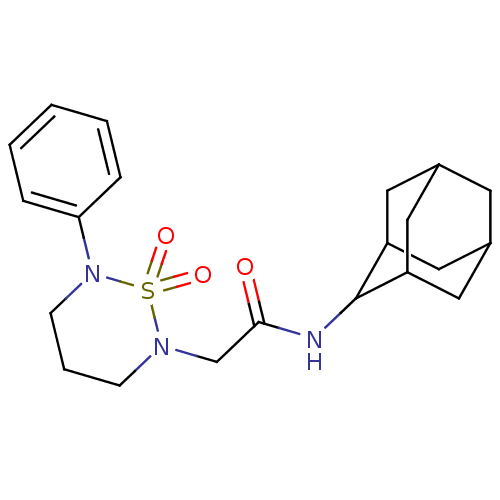
(CHEMBL2036424 | US9073906, 2)Show SMILES O=C(CN1CCCN(c2ccccc2)S1(=O)=O)NC1C2CC3CC(C2)CC1C3 |TLB:24:23:27:20.19.18,24:19:22.23.25:27,THB:17:18:22.23.25:27,18:19:22:25.26.27,18:26:22:20.24.19,(20.37,-7.6,;20.37,-9.14,;19.03,-9.91,;17.7,-9.13,;17.7,-7.58,;16.36,-6.8,;15.03,-7.58,;15.03,-9.13,;13.69,-9.9,;12.36,-9.14,;11.03,-9.91,;11.03,-11.46,;12.37,-12.22,;13.69,-11.44,;16.36,-9.89,;17.12,-11.23,;15.58,-11.23,;21.7,-9.92,;21.69,-11.46,;23.07,-12.13,;22.77,-13.54,;21.76,-14.52,;22.39,-16.15,;22.66,-14.62,;23.78,-13.59,;21.23,-13.94,;20.57,-12.46,;20.42,-13.89,)| Show InChI InChI=1S/C21H29N3O3S/c25-20(22-21-17-10-15-9-16(12-17)13-18(21)11-15)14-23-7-4-8-24(28(23,26)27)19-5-2-1-3-6-19/h1-3,5-6,15-18,21H,4,7-14H2,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50117189

(CHEMBL3613340)Show SMILES OC(=O)C12CC3CC(C1)C(Oc1ccc(cc1)C(=O)NCCNC(=O)c1ccc(cc1)-c1ccccc1)C(C3)C2 |TLB:6:5:39:8.7.9,6:7:4.5.38:39,THB:9:7:4:38.37.39,9:37:4:8.6.7,10:9:4.5.38:39,(3.79,.17,;3.07,1.17,;3.58,2.29,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;-2.19,-.22,;-3.39,-1.16,;-3.17,-2.69,;-4.38,-3.64,;-4.16,-5.17,;-2.73,-5.74,;-1.52,-4.78,;-1.74,-3.26,;-2.5,-7.26,;-3.47,-8.03,;-1.07,-7.83,;-.85,-9.36,;.59,-9.92,;.81,-11.45,;2.24,-12.02,;3.21,-11.25,;2.47,-13.54,;3.9,-14.11,;4.12,-15.64,;2.91,-16.59,;1.48,-16.02,;1.26,-14.5,;3.13,-18.12,;4.56,-18.69,;4.78,-20.21,;3.57,-21.16,;2.14,-20.59,;1.92,-19.07,;-.95,.32,;-1,2.05,;.56,-.22,)| Show InChI InChI=1S/C33H34N2O5/c36-30(24-8-6-23(7-9-24)22-4-2-1-3-5-22)34-14-15-35-31(37)25-10-12-28(13-11-25)40-29-26-16-21-17-27(29)20-33(18-21,19-26)32(38)39/h1-13,21,26-27,29H,14-20H2,(H,34,36)(H,35,37)(H,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 expressed in human Hep3B cells incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid sc... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50384529

(CHEMBL2036424 | US9073906, 2)Show SMILES O=C(CN1CCCN(c2ccccc2)S1(=O)=O)NC1C2CC3CC(C2)CC1C3 |TLB:24:23:27:20.19.18,24:19:22.23.25:27,THB:17:18:22.23.25:27,18:19:22:25.26.27,18:26:22:20.24.19,(20.37,-7.6,;20.37,-9.14,;19.03,-9.91,;17.7,-9.13,;17.7,-7.58,;16.36,-6.8,;15.03,-7.58,;15.03,-9.13,;13.69,-9.9,;12.36,-9.14,;11.03,-9.91,;11.03,-11.46,;12.37,-12.22,;13.69,-11.44,;16.36,-9.89,;17.12,-11.23,;15.58,-11.23,;21.7,-9.92,;21.69,-11.46,;23.07,-12.13,;22.77,-13.54,;21.76,-14.52,;22.39,-16.15,;22.66,-14.62,;23.78,-13.59,;21.23,-13.94,;20.57,-12.46,;20.42,-13.89,)| Show InChI InChI=1S/C21H29N3O3S/c25-20(22-21-17-10-15-9-16(12-17)13-18(21)11-15)14-23-7-4-8-24(28(23,26)27)19-5-2-1-3-6-19/h1-3,5-6,15-18,21H,4,7-14H2,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
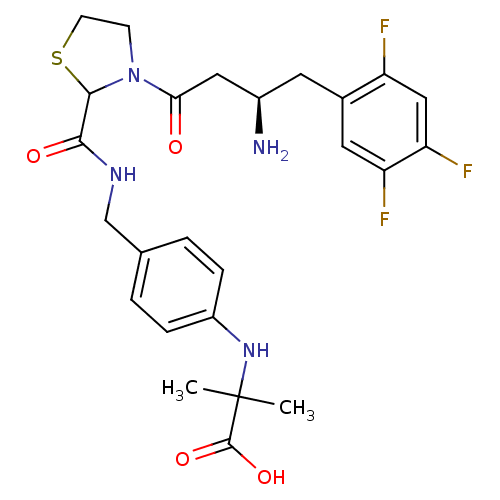
(Homo sapiens (Human)) | BDBM50337783
(2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...)Show SMILES CC(C)(Nc1ccc(CNC(=O)C2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C25H29F3N4O4S/c1-25(2,24(35)36)31-17-5-3-14(4-6-17)13-30-22(34)23-32(7-8-37-23)21(33)11-16(29)9-15-10-19(27)20(28)12-18(15)26/h3-6,10,12,16,23,31H,7-9,11,13,29H2,1-2H3,(H,30,34)(H,35,36)/t16-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
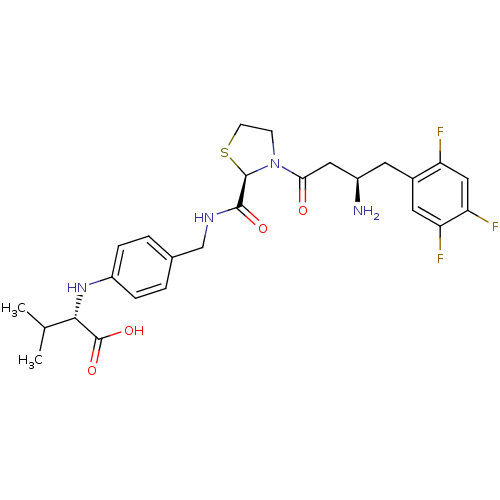
(Homo sapiens (Human)) | BDBM50337793
((S)-2-(4-(((R)-3-((R)-3-amino-4-(2,4,5-trifluoroph...)Show SMILES CC(C)[C@H](Nc1ccc(CNC(=O)[C@H]2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C26H31F3N4O4S/c1-14(2)23(26(36)37)32-18-5-3-15(4-6-18)13-31-24(35)25-33(7-8-38-25)22(34)11-17(30)9-16-10-20(28)21(29)12-19(16)27/h3-6,10,12,14,17,23,25,32H,7-9,11,13,30H2,1-2H3,(H,31,35)(H,36,37)/t17-,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
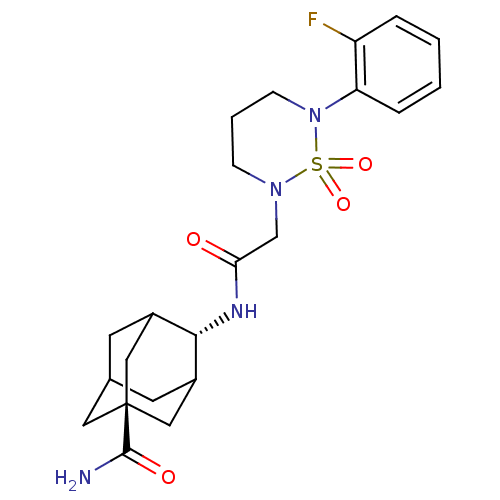
(Homo sapiens (Human)) | BDBM50384532
(CHEMBL2036428)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CCCN(c4ccccc4F)S1(=O)=O)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:8:3:30:6.7.9,10:9:4.3.31:30,1:3:30:6.7.9,THB:8:7:4.3.31:30,9:7:4:31.29.30,9:29:4:6.8.7,(17.67,-16.7,;17.28,-15.21,;18.37,-14.13,;15.79,-14.8,;15.52,-16.34,;14.89,-14.71,;15.9,-13.73,;16.2,-12.31,;16.91,-13.78,;14.82,-11.64,;14.83,-10.1,;13.5,-9.33,;13.51,-7.79,;12.16,-10.09,;10.83,-9.32,;10.83,-7.77,;9.5,-6.98,;8.16,-7.77,;8.16,-9.32,;6.83,-10.09,;6.82,-11.63,;5.5,-12.4,;4.17,-11.64,;4.16,-10.09,;5.49,-9.32,;5.49,-7.78,;9.49,-10.08,;10.25,-11.42,;8.71,-11.41,;13.7,-12.65,;13.55,-14.07,;14.36,-14.13,)| Show InChI InChI=1S/C22H29FN4O4S/c23-17-4-1-2-5-18(17)27-7-3-6-26(32(27,30)31)13-19(28)25-20-15-8-14-9-16(20)12-22(10-14,11-15)21(24)29/h1-2,4-5,14-16,20H,3,6-13H2,(H2,24,29)(H,25,28)/t14?,15?,16?,20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50384523

(CHEMBL2036235)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC(=C)CN(c4c(Cl)cc(Cl)cc4Cl)S1(=O)=O)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:8:3:33:6.7.9,10:9:4.3.34:33,1:3:33:6.7.9,THB:8:7:4.3.34:33,9:7:4:34.32.33,9:32:4:6.8.7,(18.57,-17.5,;18.17,-16.01,;19.27,-14.93,;16.69,-15.6,;16.42,-17.14,;15.79,-15.51,;16.8,-14.53,;17.09,-13.12,;17.81,-14.58,;15.72,-12.44,;15.73,-10.9,;14.39,-10.13,;14.4,-8.59,;13.06,-10.89,;11.73,-10.12,;11.73,-8.57,;10.39,-7.79,;10.39,-6.25,;9.05,-8.57,;9.05,-10.12,;7.72,-10.89,;6.39,-10.12,;6.39,-8.58,;5.05,-10.9,;5.06,-12.44,;3.73,-13.22,;6.4,-13.2,;7.72,-12.43,;9.05,-13.2,;10.38,-10.88,;11.15,-12.22,;9.6,-12.21,;14.59,-13.45,;14.44,-14.87,;15.26,-14.93,)| Show InChI InChI=1S/C23H27Cl3N4O4S/c1-12-9-29(35(33,34)30(10-12)21-17(25)4-16(24)5-18(21)26)11-19(31)28-20-14-2-13-3-15(20)8-23(6-13,7-14)22(27)32/h4-5,13-15,20H,1-3,6-11H2,(H2,27,32)(H,28,31)/t13?,14?,15?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167446

(US9073906, 175)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)CN1CC(=C)CN(c4c(Cl)cc(Cl)cc4Cl)S1(=O)=O)C(C3)C2 |TLB:10:9:6:3.4.8,10:9:3.34.8:6.5.33,1:3:6:32.33.9,THB:1:3:6.5.33:9,4:3:6.5.33:9,4:5:3.34.8:9,34:3:6:32.33.9,34:32:6:3.4.8,(9.34,2.32,;7.85,2.72,;7.45,4.21,;6.76,1.63,;5.99,2.96,;4.45,2.96,;3.68,1.63,;4.45,.3,;5.99,.3,;4,-1.13,;2.67,-1.9,;1.33,-1.13,;1.33,.41,;,-1.9,;-1.33,-1.13,;-1.33,.41,;-2.67,1.18,;-2.67,2.72,;-4,.41,;-4,-1.13,;-5.33,-1.9,;-6.67,-1.13,;-6.67,.41,;-8,-1.9,;-8,-3.44,;-9.34,-4.21,;-6.67,-4.21,;-5.33,-3.44,;-4,-4.21,;-2.67,-1.9,;-3.44,-3.23,;-1.9,-3.23,;5.37,-.34,;4.58,.96,;6.76,0,)| Show InChI InChI=1S/C23H27Cl3N4O4S/c1-12-9-29(35(33,34)30(10-12)21-17(25)4-16(24)5-18(21)26)11-19(31)28-20-14-2-13-3-15(20)8-23(6-13,7-14)22(27)32/h4-5,13-15,20H,1-3,6-11H2,(H2,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167435

(US9073906, 59)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)CN1CCCN(c4ccccc4F)S1(=O)=O)C(C3)C2 |TLB:10:9:6:3.4.8,10:9:3.31.8:6.5.30,1:3:9:6.5.30,THB:31:3:6:9.29.30,31:29:6:3.4.8,4:3:9:6.5.30,4:5:9:3.31.8,(8.38,3.66,;7.61,2.32,;8.38,.99,;6.07,2.32,;5.45,3.73,;3.92,3.9,;3.01,2.66,;3.63,1.25,;5.16,1.08,;3.63,-.82,;2.29,-1.59,;.96,-.82,;.96,.72,;-.37,-1.59,;-1.71,-.82,;-1.71,.72,;-3.04,1.49,;-4.37,.72,;-4.37,-.82,;-5.71,-1.59,;-5.71,-3.13,;-7.04,-3.9,;-8.38,-3.13,;-8.38,-1.59,;-7.04,-.82,;-7.04,.72,;-3.04,-1.59,;-2.27,-2.93,;-3.81,-2.93,;4.65,.33,;3.7,2.53,;6.02,.2,)| Show InChI InChI=1S/C22H29FN4O4S/c23-17-4-1-2-5-18(17)27-7-3-6-26(32(27,30)31)13-19(28)25-20-15-8-14-9-16(20)12-22(10-14,11-15)21(24)29/h1-2,4-5,14-16,20H,3,6-13H2,(H2,24,29)(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50337788
((R)-ethyl 2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifl...)Show SMILES CCOC(=O)[C@H](Nc1ccc(CNC(=O)[C@@H]2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(C)C |r| Show InChI InChI=1S/C28H35F3N4O4S/c1-4-39-28(38)25(16(2)3)34-20-7-5-17(6-8-20)15-33-26(37)27-35(9-10-40-27)24(36)13-19(32)11-18-12-22(30)23(31)14-21(18)29/h5-8,12,14,16,19,25,27,34H,4,9-11,13,15,32H2,1-3H3,(H,33,37)/t19-,25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50384531

(CHEMBL2036427)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CCCN(c4ccccc4)S1(=O)=O)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:8:3:29:6.7.9,10:9:4.3.30:29,1:3:29:6.7.9,THB:8:7:4.3.30:29,9:7:4:30.28.29,9:28:4:6.8.7,(17.67,-16.7,;17.28,-15.21,;18.37,-14.13,;15.79,-14.8,;15.52,-16.34,;14.89,-14.71,;15.9,-13.73,;16.2,-12.31,;16.91,-13.78,;14.82,-11.64,;14.83,-10.1,;13.5,-9.33,;13.51,-7.79,;12.16,-10.09,;10.83,-9.32,;10.83,-7.77,;9.5,-6.98,;8.16,-7.77,;8.16,-9.32,;6.83,-10.09,;5.49,-9.32,;4.16,-10.09,;4.17,-11.64,;5.5,-12.4,;6.82,-11.63,;9.49,-10.08,;10.25,-11.42,;8.71,-11.41,;13.7,-12.65,;13.55,-14.07,;14.36,-14.13,)| Show InChI InChI=1S/C22H30N4O4S/c23-21(28)22-11-15-9-16(12-22)20(17(10-15)13-22)24-19(27)14-25-7-4-8-26(31(25,29)30)18-5-2-1-3-6-18/h1-3,5-6,15-17,20H,4,7-14H2,(H2,23,28)(H,24,27)/t15?,16?,17?,20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167436
(US9073906, 60)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)CN1CCCN(c4ccccc4)S1(=O)=O)C(C3)C2 |TLB:10:9:6:3.4.8,10:9:3.30.8:6.5.29,1:3:9:6.5.29,THB:30:3:6:9.28.29,30:28:6:3.4.8,4:3:9:6.5.29,4:5:9:3.30.8,(8.38,3.66,;7.61,2.32,;8.38,.99,;6.07,2.32,;5.45,3.73,;3.92,3.9,;3.01,2.66,;3.63,1.25,;5.16,1.08,;3.63,-.82,;2.29,-1.59,;.96,-.82,;.96,.72,;-.37,-1.59,;-1.71,-.82,;-1.71,.72,;-3.04,1.49,;-4.37,.72,;-4.37,-.82,;-5.71,-1.59,;-7.04,-.82,;-8.38,-1.59,;-8.38,-3.13,;-7.04,-3.9,;-5.71,-3.13,;-3.04,-1.59,;-2.27,-2.93,;-3.81,-2.93,;4.65,.33,;3.7,2.53,;6.02,.2,)| Show InChI InChI=1S/C22H30N4O4S/c23-21(28)22-11-15-9-16(12-22)20(17(10-15)13-22)24-19(27)14-25-7-4-8-26(31(25,29)30)18-5-2-1-3-6-18/h1-3,5-6,15-17,20H,4,7-14H2,(H2,23,28)(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY
US Patent
| Assay Description
The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... |
US Patent US9073906 (2015)
BindingDB Entry DOI: 10.7270/Q2RV0MFB |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117153

(CHEMBL3613348)Show SMILES OC(=O)[C@]12CC3CC(C1)[C@H](Oc1ccc(cc1)C(=O)NCCNC(=O)c1ccc4ccccc4c1)C(C3)C2 |r,wU:3.2,9.10,TLB:1:3:36.35.9:6,10:9:4.8.3:6,THB:37:35:4.8.3:6,37:3:36.35.9:6,9:35:4:8.6.7,9:7:4:36.35.37,(3.58,2.29,;3.07,1.17,;3.79,.17,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;-2.19,-.22,;-3.39,-1.17,;-4.82,-.59,;-6.03,-1.54,;-7.46,-.97,;-7.68,.56,;-6.46,1.51,;-5.03,.93,;-9.11,1.13,;-9.28,2.35,;-10.32,.17,;-11.75,.75,;-12.96,-.21,;-14.39,.37,;-15.6,-.59,;-15.43,-1.81,;-17.03,-.02,;-17.25,1.51,;-18.69,2.08,;-19.9,1.13,;-21.33,1.7,;-22.54,.75,;-22.32,-.77,;-20.89,-1.35,;-19.68,-.4,;-18.26,-.97,;-.95,.32,;-1,2.05,;.56,-.22,)| Show InChI InChI=1S/C31H32N2O5/c34-28(32-11-12-33-29(35)23-6-5-20-3-1-2-4-22(20)15-23)21-7-9-26(10-8-21)38-27-24-13-19-14-25(27)18-31(16-19,17-24)30(36)37/h1-10,15,19,24-25,27H,11-14,16-18H2,(H,32,34)(H,33,35)(H,36,37)/t19?,24?,25?,27-,31+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















































