

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258507 (CHEMBL4078345) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258514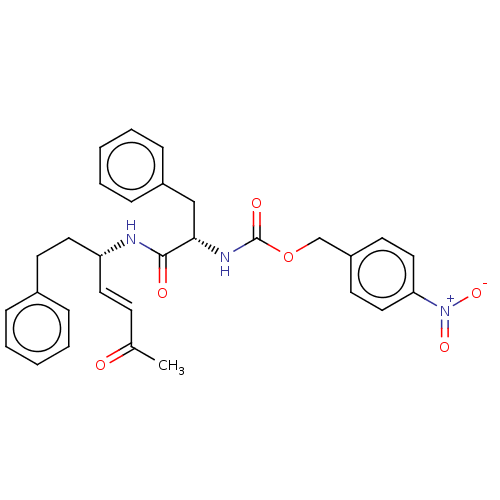 (CHEMBL4062015) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17689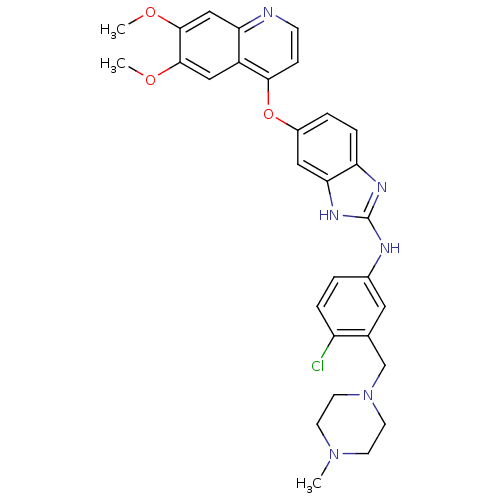 (2-aminobenzimidazole, 14 | N-{4-chloro-3-[(4-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50258507 (CHEMBL4078345) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258506 (CHEMBL4072275) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17703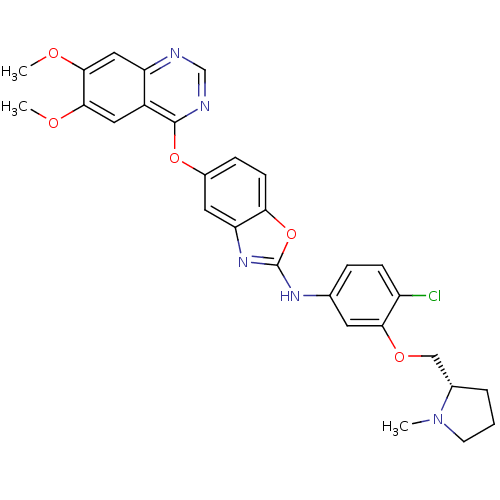 (2-aminobenzoxazole, 28 | N-(4-chloro-3-{[(2S)-1-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17705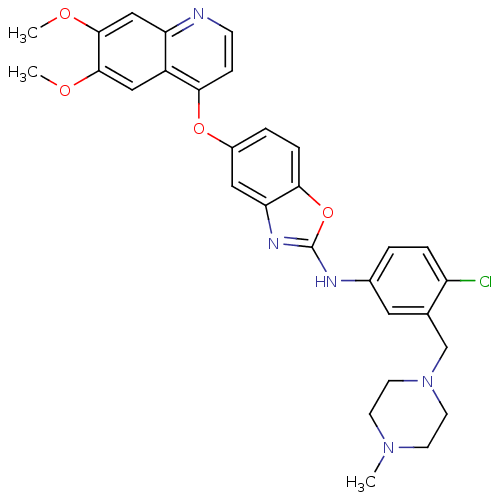 (2-aminobenzoxazole, 30 | N-{4-chloro-3-[(4-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17704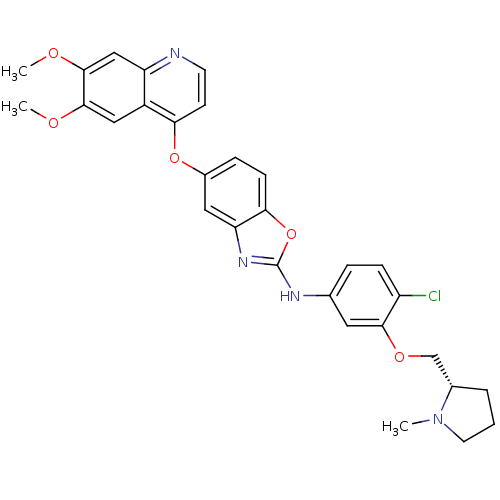 (2-aminobenzoxazole, 29 | N-(4-chloro-3-{[(2S)-1-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM82279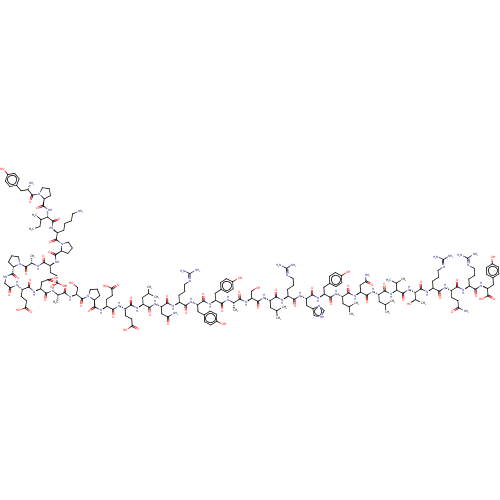 (CAS_118997-30-1 | PYY, human) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Curated by PDSP Ki Database | J Biol Chem 270: 22661-4 (1995) Article DOI: 10.1074/jbc.270.39.22661 BindingDB Entry DOI: 10.7270/Q2TT4PGD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50258515 (CHEMBL4083754) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM82277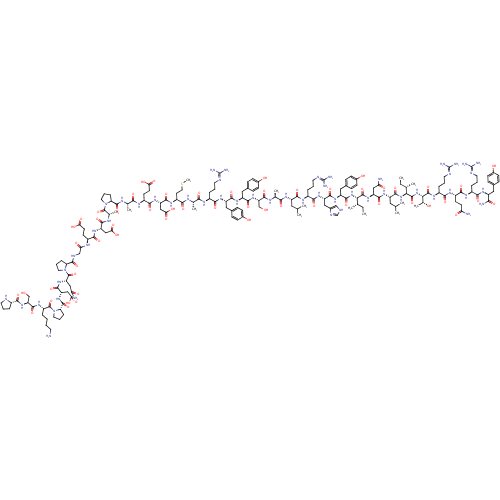 (NPY2-36, human | NPY2-36, rat, human) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Curated by PDSP Ki Database | J Biol Chem 270: 22661-4 (1995) Article DOI: 10.1074/jbc.270.39.22661 BindingDB Entry DOI: 10.7270/Q2TT4PGD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258544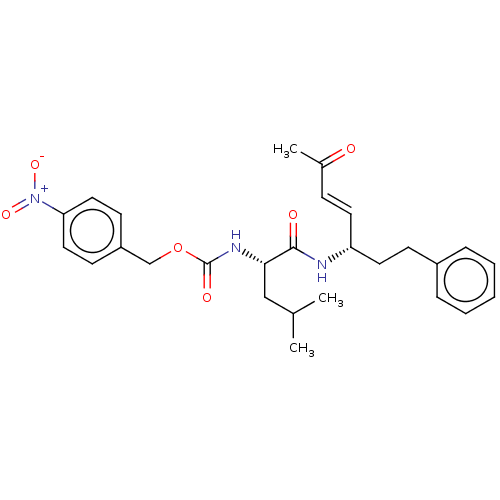 (CHEMBL4096388) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM82279 (CAS_118997-30-1 | PYY, human) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Curated by PDSP Ki Database | J Biol Chem 270: 22661-4 (1995) Article DOI: 10.1074/jbc.270.39.22661 BindingDB Entry DOI: 10.7270/Q2TT4PGD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17687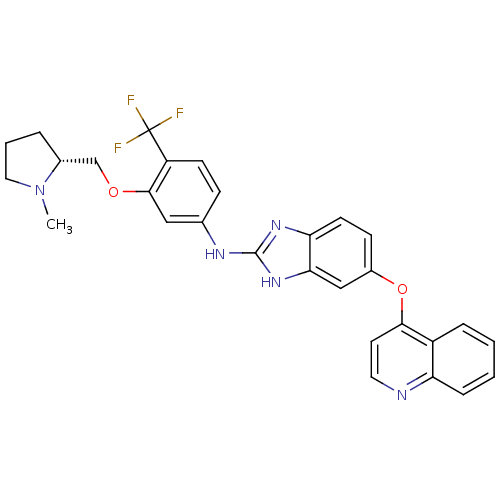 (2-aminobenzimidazole, 12 | N-(3-{[(2R)-1-methylpyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17709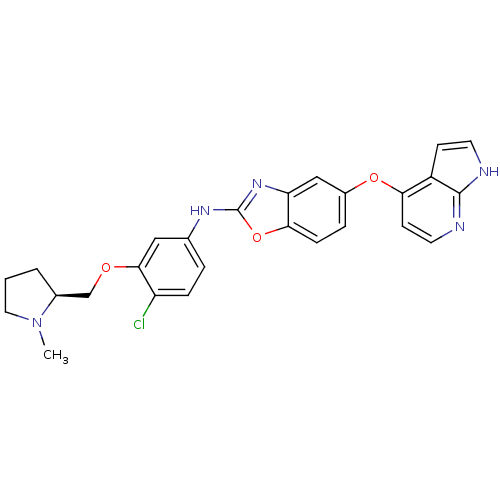 (2-aminobenzoxazole, 34 | N-(4-chloro-3-{[(2S)-1-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17684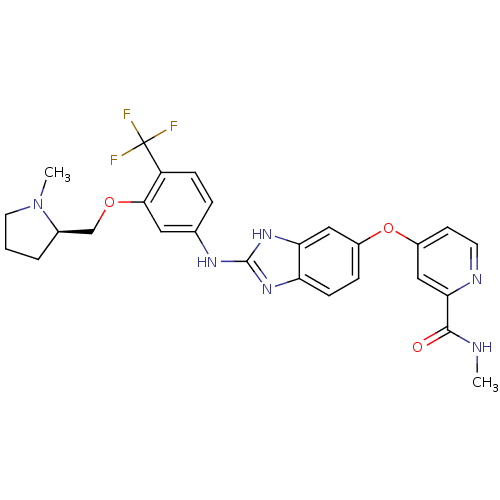 (2-aminobenzimidazole, 9 | N-methyl-4-({2-[(3-{[(2R...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17710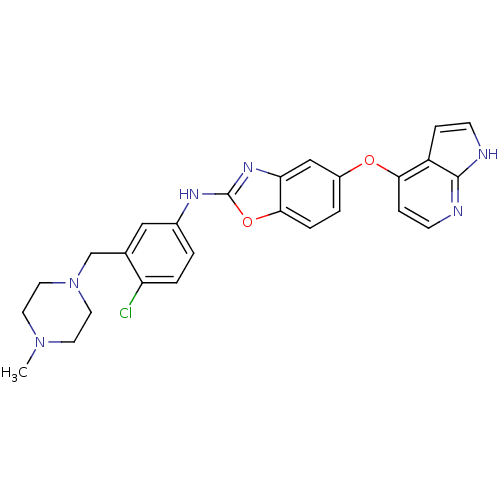 (2-aminobenzoxazole, 35 | N-{4-chloro-3-[(4-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258542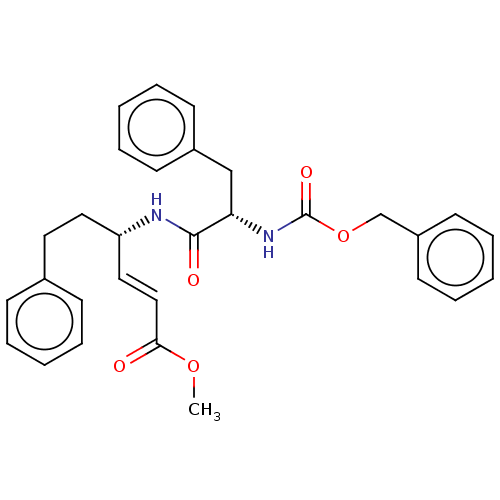 (CHEMBL4082758) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17713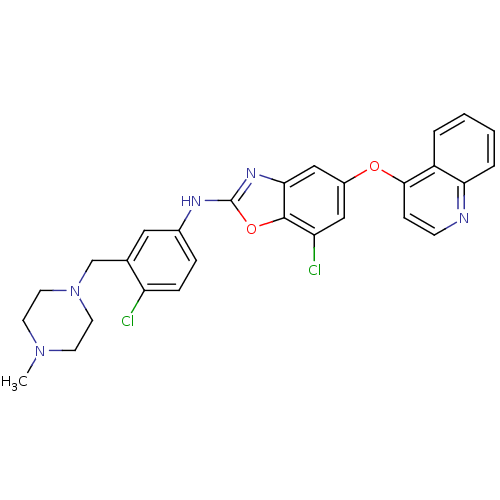 (2-aminobenzoxazole, 38 | 7-chloro-N-{4-chloro-3-[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17688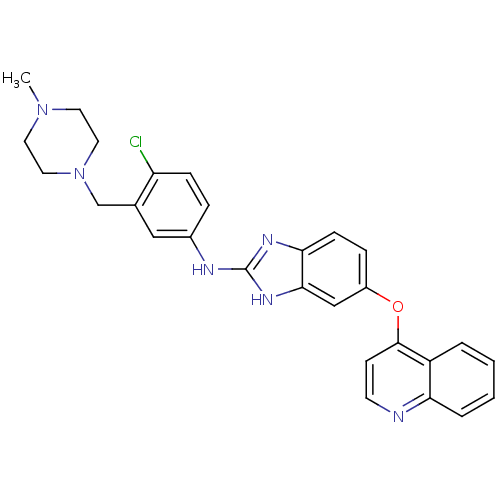 (2-aminobenzimidazole, 13 | N-{4-chloro-3-[(4-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50258514 (CHEMBL4062015) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50015490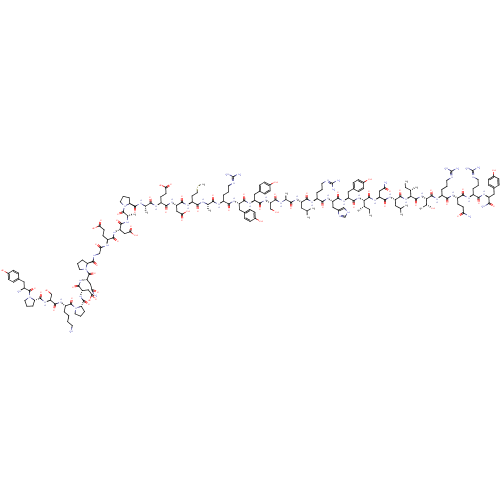 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Curated by PDSP Ki Database | J Biol Chem 270: 22661-4 (1995) Article DOI: 10.1074/jbc.270.39.22661 BindingDB Entry DOI: 10.7270/Q2TT4PGD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17697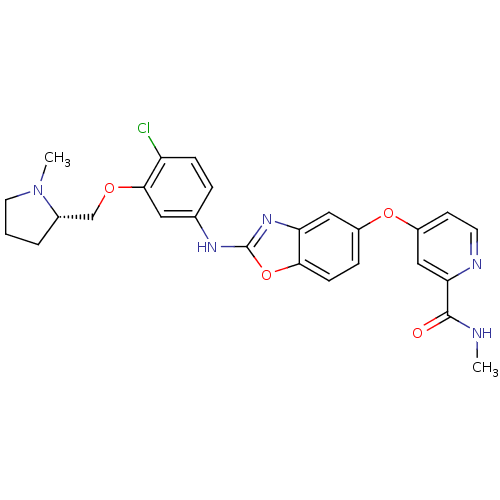 (2-aminobenzoxazole, 22 | 4-({2-[(4-chloro-3-{[(2S)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.80 | -48.3 | 4.60 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17702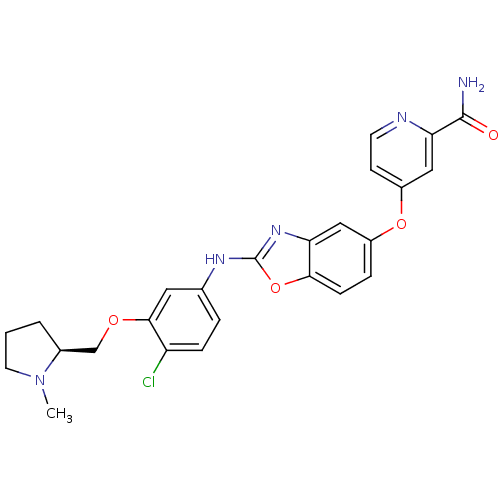 (2-aminobenzoxazole, 27 | 4-({2-[(4-chloro-3-{[(2S)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17711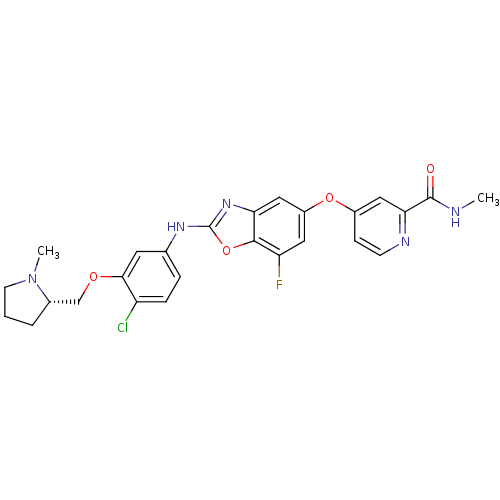 (2-aminobenzoxazole, 36 | 4-({2-[(4-chloro-3-{[(2S)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17683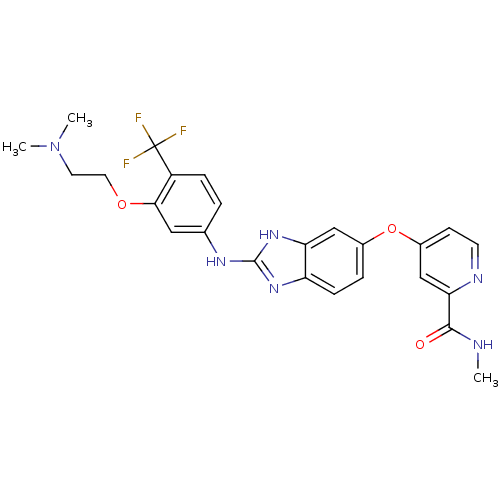 (2-aminobenzimidazole, 8 | 4-{[2-({3-[2-(dimethylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17701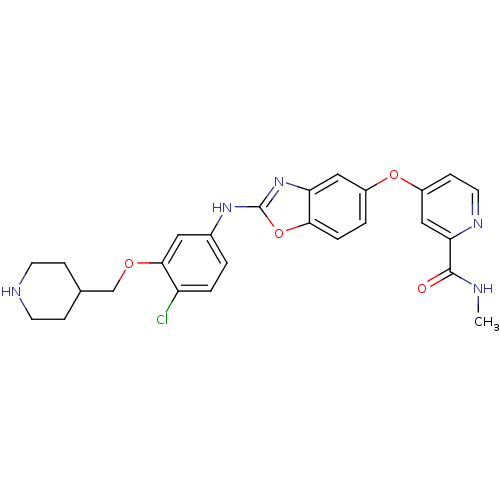 (2-aminobenzoxazole, 26 | 4-[(2-{[4-chloro-3-(piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17691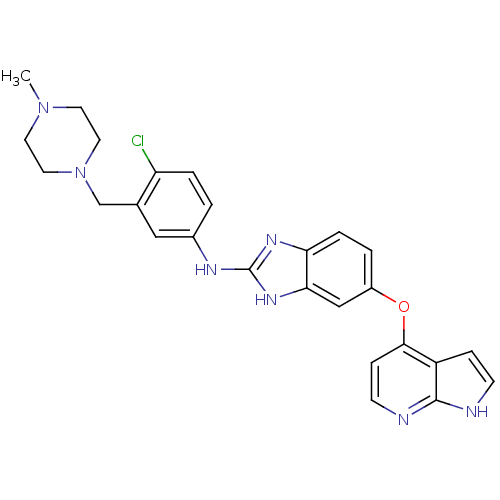 (2-aminobenzimidazole, 16 | N-{4-chloro-3-[(4-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM82277 (NPY2-36, human | NPY2-36, rat, human) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Curated by PDSP Ki Database | J Biol Chem 270: 22661-4 (1995) Article DOI: 10.1074/jbc.270.39.22661 BindingDB Entry DOI: 10.7270/Q2TT4PGD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50015490 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Curated by PDSP Ki Database | J Biol Chem 270: 22661-4 (1995) Article DOI: 10.1074/jbc.270.39.22661 BindingDB Entry DOI: 10.7270/Q2TT4PGD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50258544 (CHEMBL4096388) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17706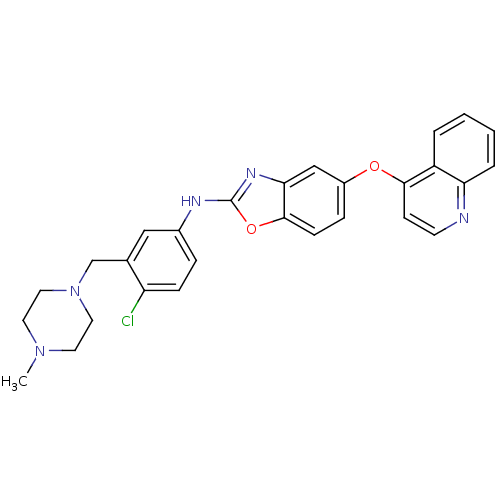 (2-aminobenzoxazole, 31 | N-{4-chloro-3-[(4-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258500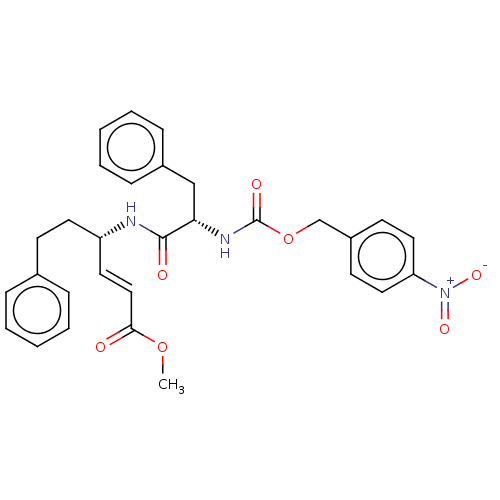 (CHEMBL4093034) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50304793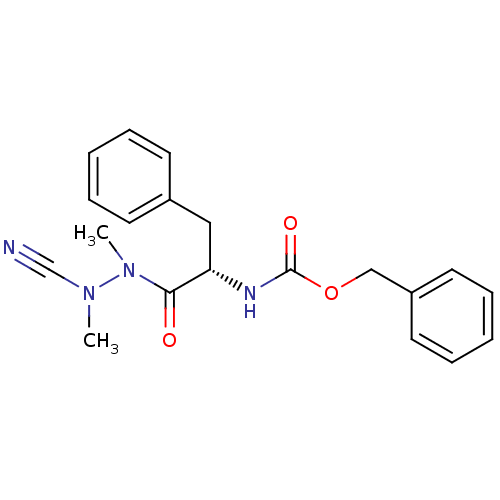 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by microtiter plate spectrofluorimetry | Bioorg Med Chem Lett 20: 252-5 (2010) Article DOI: 10.1016/j.bmcl.2009.10.122 BindingDB Entry DOI: 10.7270/Q24X57WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17682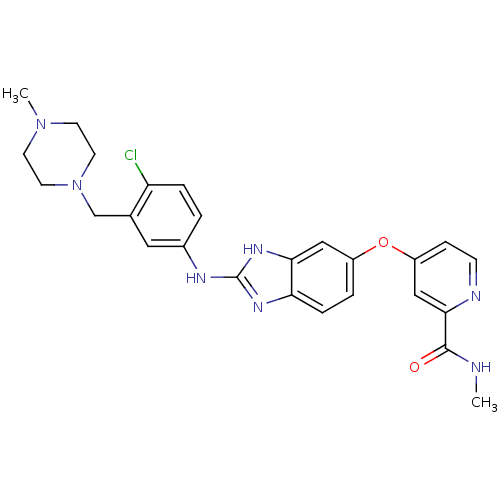 (2-aminobenzimidazole, 7 | 4-{[2-({4-chloro-3-[(4-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17698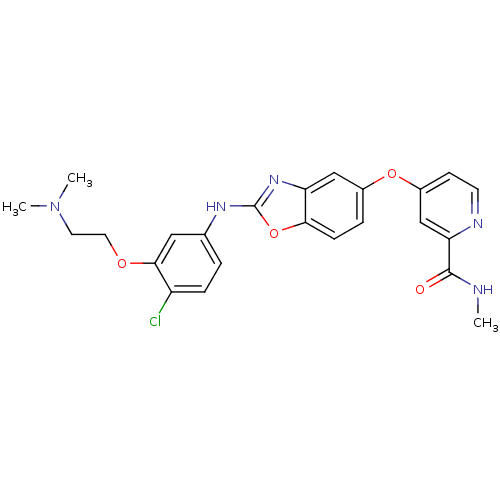 (2-aminobenzoxazole, 23 | 4-{[2-({4-chloro-3-[2-(di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17695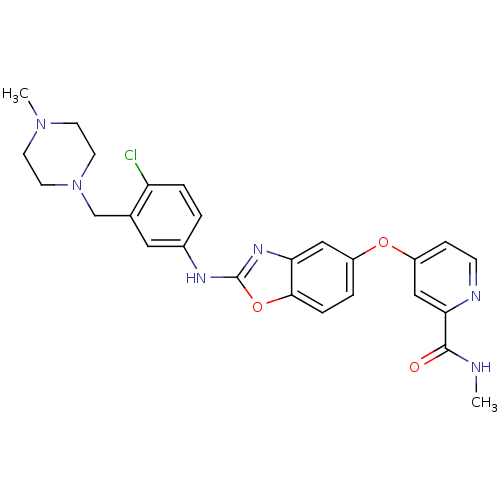 (2-aminobenzoxazole, 20 | 4-{[2-({4-chloro-3-[(4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17700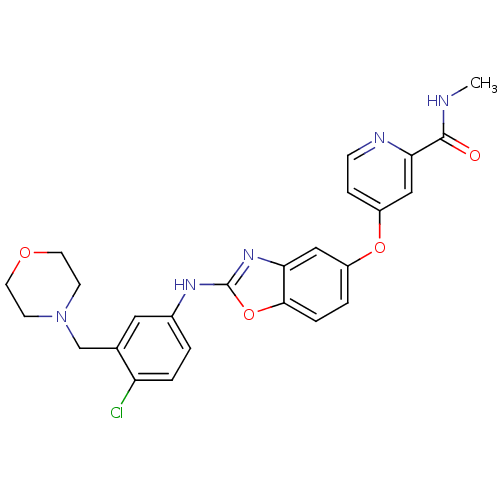 (2-aminobenzoxazole, 25 | 4-[(2-{[4-chloro-3-(morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17699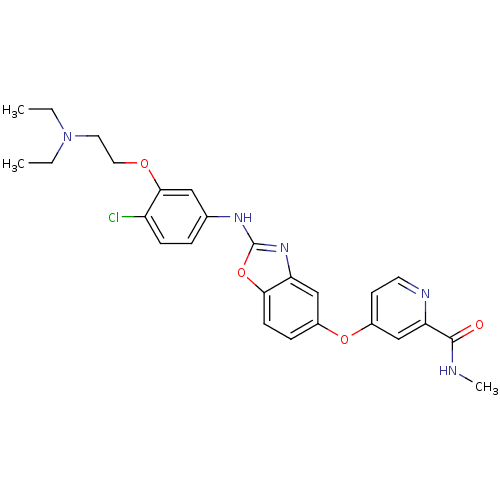 (2-aminobenzoxazole, 24 | 4-{[2-({4-chloro-3-[2-(di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258523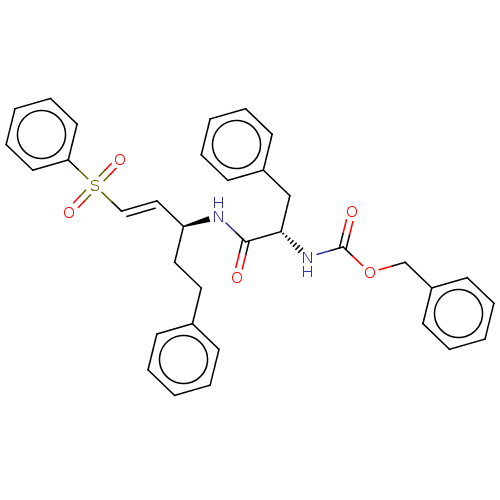 (CHEMBL2402204) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM82278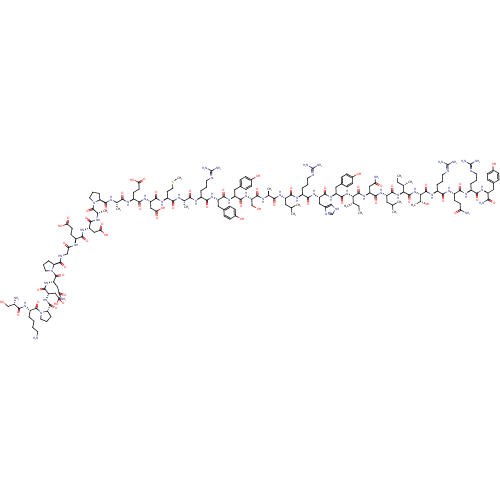 (NPY3-36) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Curated by PDSP Ki Database | J Biol Chem 270: 22661-4 (1995) Article DOI: 10.1074/jbc.270.39.22661 BindingDB Entry DOI: 10.7270/Q2TT4PGD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50304796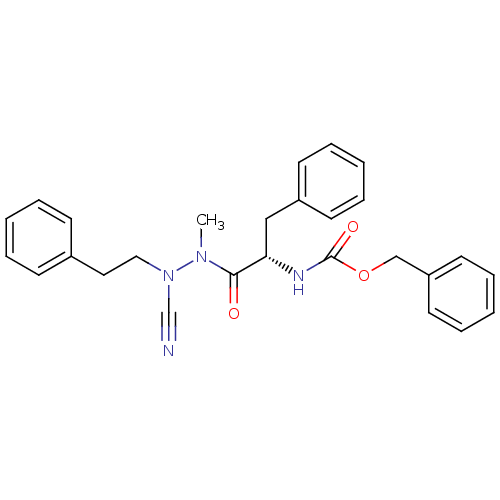 ((S)-benzyl 1-(2-cyano-1-methyl-2-phenethylhydrazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by microtiter plate spectrofluorimetry | Bioorg Med Chem Lett 20: 252-5 (2010) Article DOI: 10.1016/j.bmcl.2009.10.122 BindingDB Entry DOI: 10.7270/Q24X57WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258524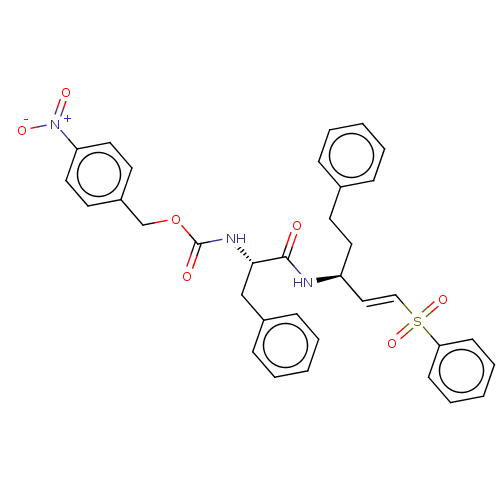 (CHEMBL4081250) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258498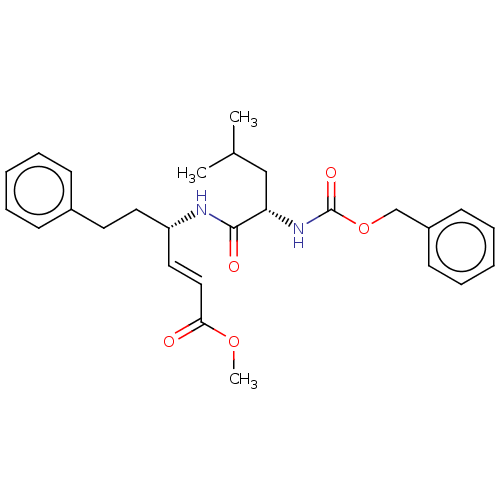 (CHEMBL4101714) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17680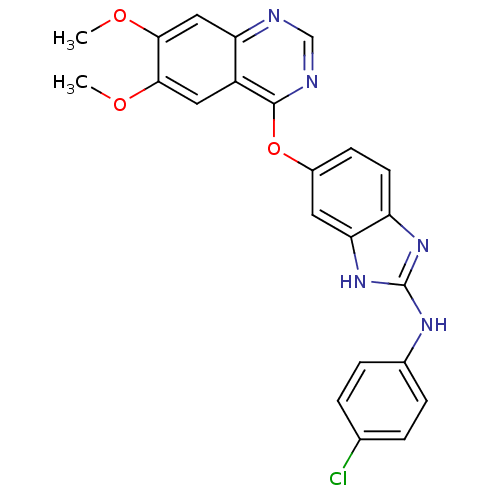 (2-aminobenzimidazole, 5 | N-(4-chlorophenyl)-5-[(6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50304794 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-4-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by microtiter plate spectrofluorimetry | Bioorg Med Chem Lett 20: 252-5 (2010) Article DOI: 10.1016/j.bmcl.2009.10.122 BindingDB Entry DOI: 10.7270/Q24X57WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17678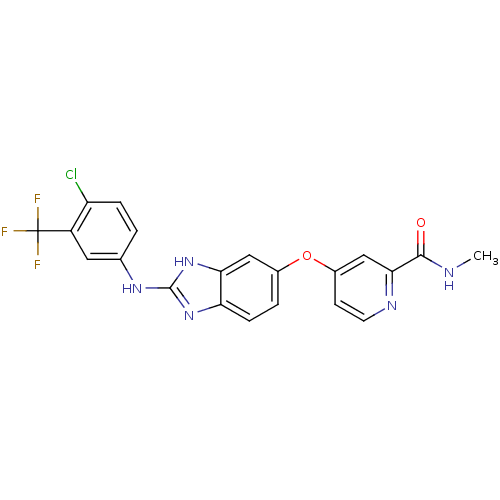 (2-aminobenzimidazole, 3 | 4-[(2-{[4-chloro-3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 9 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM17685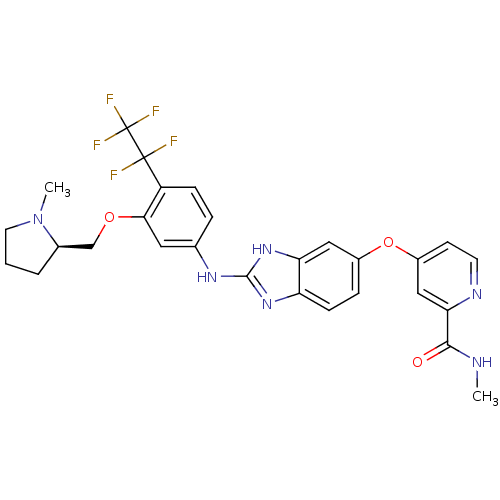 (2-aminobenzimidazole, 10 | N-methyl-4-({2-[(3-{[(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 50: 4351-4373 (2007) Article DOI: 10.1021/jm070034i BindingDB Entry DOI: 10.7270/Q21V5C74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258515 (CHEMBL4083754) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50304797 ((S)-benzyl 1-(2-cyano-1-methyl-2-pentylhydrazinyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by microtiter plate spectrofluorimetry | Bioorg Med Chem Lett 20: 252-5 (2010) Article DOI: 10.1016/j.bmcl.2009.10.122 BindingDB Entry DOI: 10.7270/Q24X57WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2179 total ) | Next | Last >> |