 Found 708 hits with Last Name = 'roy' and Initial = 'c'
Found 708 hits with Last Name = 'roy' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120056
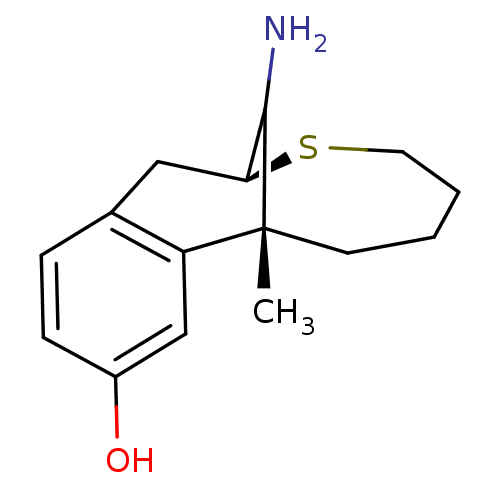
(15-Amino-1-methyl-10-thia-tricyclo[7.5.1.0*2,7*]pe...)Show InChI InChI=1S/C15H21NOS/c1-15-6-2-3-7-18-13(14(15)16)8-10-4-5-11(17)9-12(10)15/h4-5,9,13-14,17H,2-3,6-8,16H2,1H3/t13-,14?,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120057
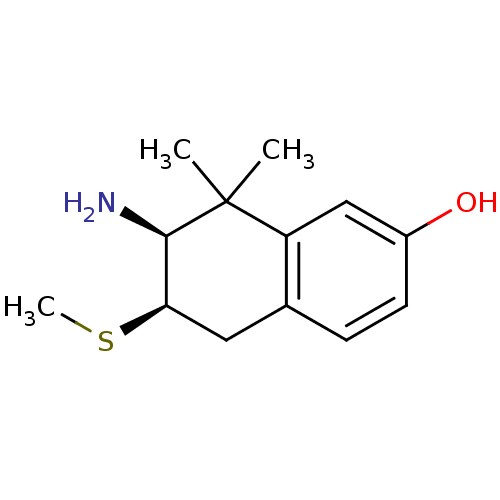
(7-Amino-8,8-dimethyl-6-methylsulfanyl-5,6,7,8-tetr...)Show InChI InChI=1S/C13H19NOS/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120058
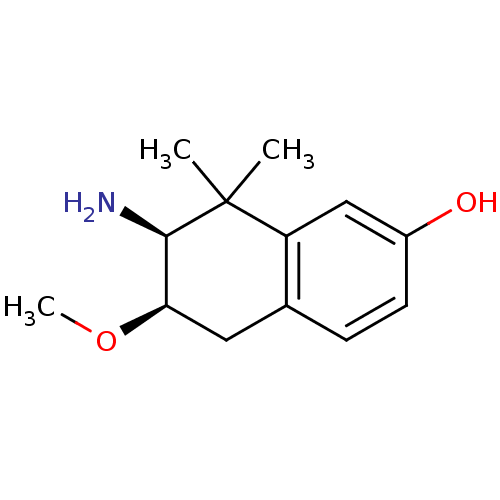
(7-Amino-6-methoxy-8,8-dimethyl-5,6,7,8-tetrahydro-...)Show InChI InChI=1S/C13H19NO2/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120053
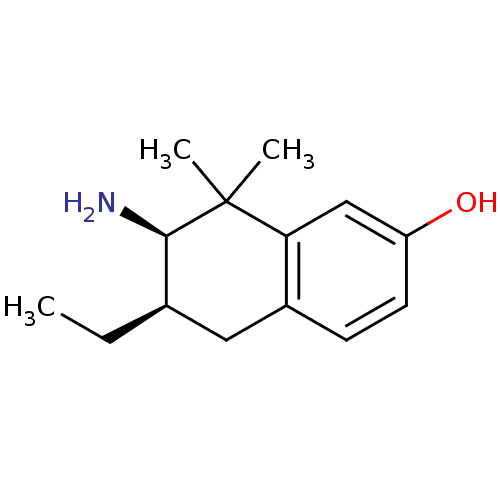
(7-Amino-6-ethyl-8,8-dimethyl-5,6,7,8-tetrahydro-na...)Show InChI InChI=1S/C14H21NO/c1-4-9-7-10-5-6-11(16)8-12(10)14(2,3)13(9)15/h5-6,8-9,13,16H,4,7,15H2,1-3H3/t9-,13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50120056

(15-Amino-1-methyl-10-thia-tricyclo[7.5.1.0*2,7*]pe...)Show InChI InChI=1S/C15H21NOS/c1-15-6-2-3-7-18-13(14(15)16)8-10-4-5-11(17)9-12(10)15/h4-5,9,13-14,17H,2-3,6-8,16H2,1H3/t13-,14?,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor kappa 1 was determined. |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor kappa 1 was determined. |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50120057

(7-Amino-8,8-dimethyl-6-methylsulfanyl-5,6,7,8-tetr...)Show InChI InChI=1S/C13H19NOS/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor kappa 1 was determined. |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor delta 1 was determined |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120061
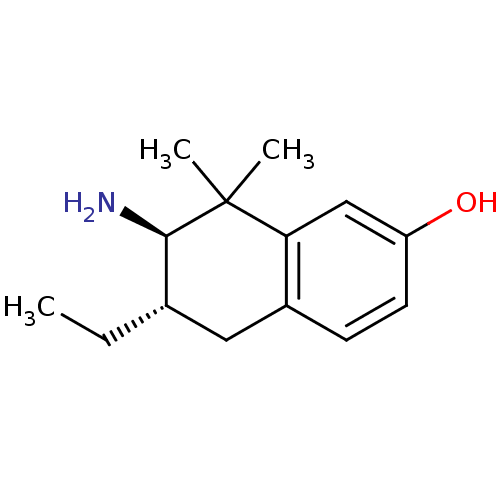
(7-Amino-6-ethyl-8,8-dimethyl-5,6,7,8-tetrahydro-na...)Show InChI InChI=1S/C14H21NO/c1-4-9-7-10-5-6-11(16)8-12(10)14(2,3)13(9)15/h5-6,8-9,13,16H,4,7,15H2,1-3H3/t9-,13+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120062

(7-Amino-6-mercapto-8,8-dimethyl-5,6,7,8-tetrahydro...)Show InChI InChI=1S/C12H17NOS/c1-12(2)9-6-8(14)4-3-7(9)5-10(15)11(12)13/h3-4,6,10-11,14-15H,5,13H2,1-2H3/t10-,11-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50120058

(7-Amino-6-methoxy-8,8-dimethyl-5,6,7,8-tetrahydro-...)Show InChI InChI=1S/C13H19NO2/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor kappa 1 was determined. |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120054
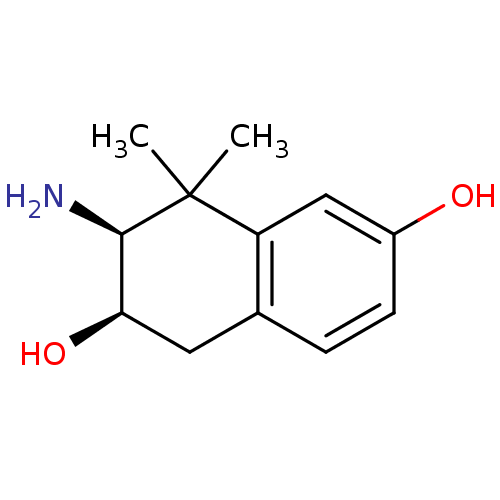
(3-Amino-4,4-dimethyl-1,2,3,4-tetrahydro-naphthalen...)Show InChI InChI=1S/C12H17NO2/c1-12(2)9-6-8(14)4-3-7(9)5-10(15)11(12)13/h3-4,6,10-11,14-15H,5,13H2,1-2H3/t10-,11-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120059
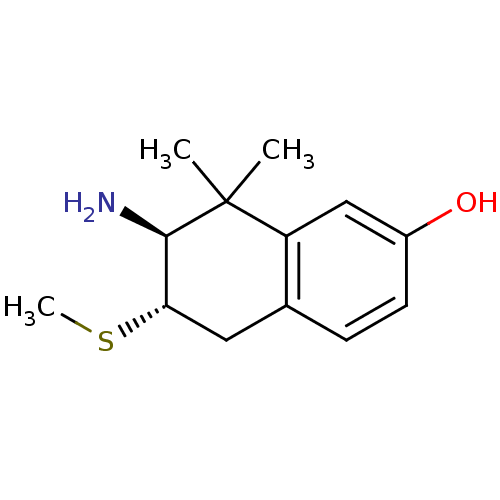
(7-Amino-8,8-dimethyl-6-methylsulfanyl-5,6,7,8-tetr...)Show InChI InChI=1S/C13H19NOS/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50120053

(7-Amino-6-ethyl-8,8-dimethyl-5,6,7,8-tetrahydro-na...)Show InChI InChI=1S/C14H21NO/c1-4-9-7-10-5-6-11(16)8-12(10)14(2,3)13(9)15/h5-6,8-9,13,16H,4,7,15H2,1-3H3/t9-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 345 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor kappa 1 was determined. |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50120057

(7-Amino-8,8-dimethyl-6-methylsulfanyl-5,6,7,8-tetr...)Show InChI InChI=1S/C13H19NOS/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 349 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor delta 1 was determined |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50120056

(15-Amino-1-methyl-10-thia-tricyclo[7.5.1.0*2,7*]pe...)Show InChI InChI=1S/C15H21NOS/c1-15-6-2-3-7-18-13(14(15)16)8-10-4-5-11(17)9-12(10)15/h4-5,9,13-14,17H,2-3,6-8,16H2,1H3/t13-,14?,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor delta 1 was determined |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120055
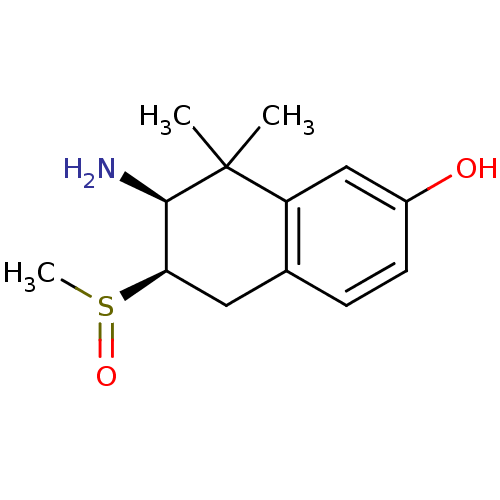
(7-Amino-6-methanesulfinyl-8,8-dimethyl-5,6,7,8-tet...)Show InChI InChI=1S/C13H19NO2S/c1-13(2)10-7-9(15)5-4-8(10)6-11(12(13)14)17(3)16/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-,17?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120055

(7-Amino-6-methanesulfinyl-8,8-dimethyl-5,6,7,8-tet...)Show InChI InChI=1S/C13H19NO2S/c1-13(2)10-7-9(15)5-4-8(10)6-11(12(13)14)17(3)16/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-,17?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120060

(7-Amino-6-methanesulfonyl-8,8-dimethyl-5,6,7,8-tet...)Show SMILES CC1(C)[C@H](N)[C@@H](Cc2ccc(O)cc12)S(C)(=O)=O Show InChI InChI=1S/C13H19NO3S/c1-13(2)10-7-9(15)5-4-8(10)6-11(12(13)14)18(3,16)17/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50120053

(7-Amino-6-ethyl-8,8-dimethyl-5,6,7,8-tetrahydro-na...)Show InChI InChI=1S/C14H21NO/c1-4-9-7-10-5-6-11(16)8-12(10)14(2,3)13(9)15/h5-6,8-9,13,16H,4,7,15H2,1-3H3/t9-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor delta 1 was determined |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50120058

(7-Amino-6-methoxy-8,8-dimethyl-5,6,7,8-tetrahydro-...)Show InChI InChI=1S/C13H19NO2/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor delta 1 was determined |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50254293
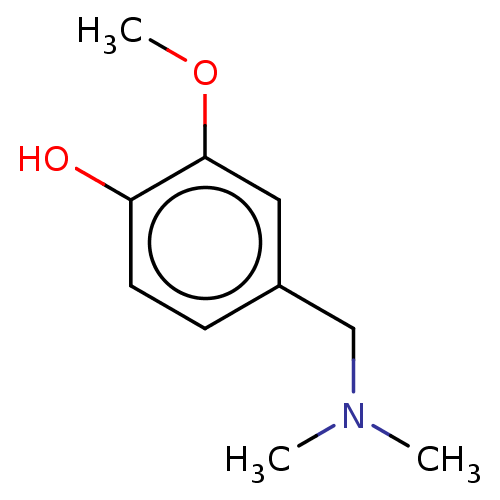
(CHEMBL4070283)Show InChI InChI=1S/C10H15NO2/c1-11(2)7-8-4-5-9(12)10(6-8)13-3/h4-6,12H,7H2,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States.
Curated by ChEMBL
| Assay Description
Mixed type inhibition of Electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate after 7 min by Ellman's method |
ACS Med Chem Lett 8: 622-627 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00037
BindingDB Entry DOI: 10.7270/Q20V8G76 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50254289

(CHEMBL4091215)Show InChI InChI=1S/C12H17NO2/c1-15-12-8-10(4-5-11(12)14)9-13-6-2-3-7-13/h4-5,8,14H,2-3,6-7,9H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States.
Curated by ChEMBL
| Assay Description
Mixed type inhibition of Electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate after 7 min by Ellman's method |
ACS Med Chem Lett 8: 622-627 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00037
BindingDB Entry DOI: 10.7270/Q20V8G76 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50254288

(CHEMBL4074057)Show InChI InChI=1S/C13H19NO2/c1-16-13-9-11(5-6-12(13)15)10-14-7-3-2-4-8-14/h5-6,9,15H,2-4,7-8,10H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States.
Curated by ChEMBL
| Assay Description
Mixed type inhibition of Electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate after 7 min by Ellman's method |
ACS Med Chem Lett 8: 622-627 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00037
BindingDB Entry DOI: 10.7270/Q20V8G76 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50254287
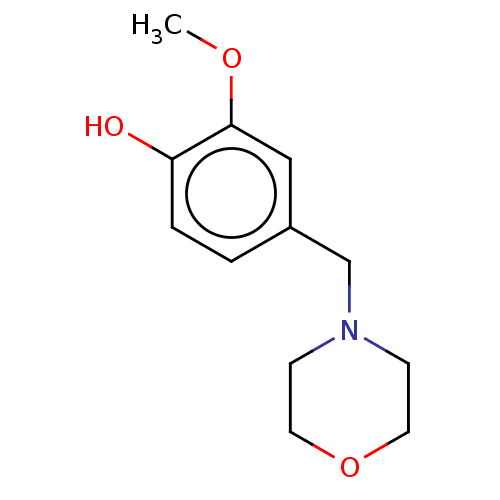
(CHEMBL4094588)Show InChI InChI=1S/C12H17NO3/c1-15-12-8-10(2-3-11(12)14)9-13-4-6-16-7-5-13/h2-3,8,14H,4-7,9H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.57E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States.
Curated by ChEMBL
| Assay Description
Mixed type inhibition of Electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate after 7 min by Ellman's method |
ACS Med Chem Lett 8: 622-627 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00037
BindingDB Entry DOI: 10.7270/Q20V8G76 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495264
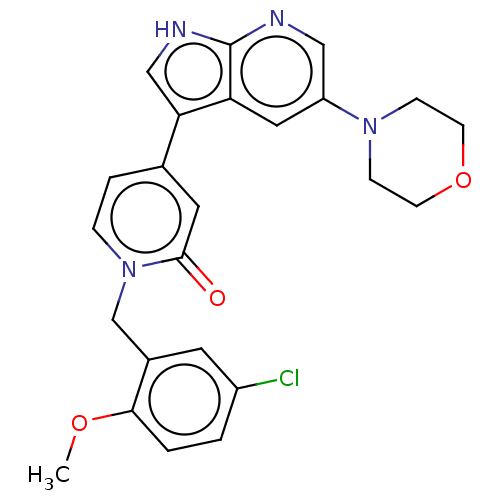
(1-(5-Chloro-2-methoxybenzyl)-4-(5-morpholino-1H-py...)Show SMILES COc1ccc(Cl)cc1Cn1ccc(cc1=O)-c1c[nH]c2ncc(cc12)N1CCOCC1 Show InChI InChI=1S/C24H23ClN4O3/c1-31-22-3-2-18(25)10-17(22)15-29-5-4-16(11-23(29)30)21-14-27-24-20(21)12-19(13-26-24)28-6-8-32-9-7-28/h2-5,10-14H,6-9,15H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495265
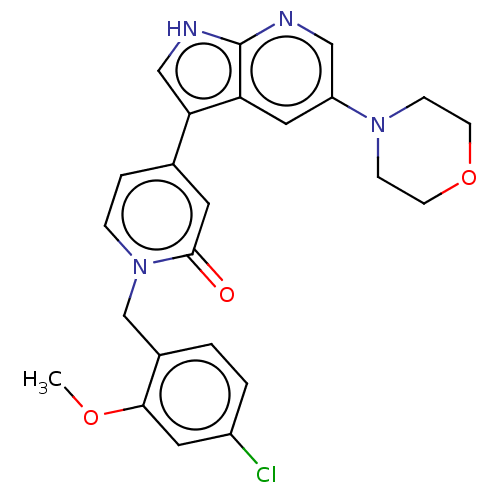
(1-(4-Chloro-2-methoxybenzyl)-4-(5-morpholino-1H-py...)Show SMILES COc1cc(Cl)ccc1Cn1ccc(cc1=O)-c1c[nH]c2ncc(cc12)N1CCOCC1 Show InChI InChI=1S/C24H23ClN4O3/c1-31-22-11-18(25)3-2-17(22)15-29-5-4-16(10-23(29)30)21-14-27-24-20(21)12-19(13-26-24)28-6-8-32-9-7-28/h2-5,10-14H,6-9,15H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495266
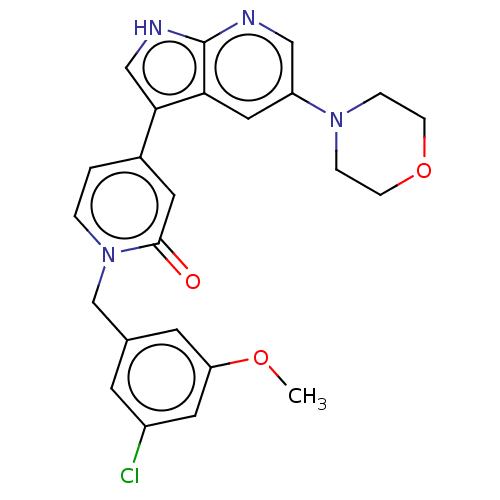
(1-(3-Chloro-5-methoxybenzyl)-4-(5-morpholino-1H-py...)Show SMILES COc1cc(Cl)cc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)c1 Show InChI InChI=1S/C24H23ClN4O3/c1-31-20-9-16(8-18(25)11-20)15-29-3-2-17(10-23(29)30)22-14-27-24-21(22)12-19(13-26-24)28-4-6-32-7-5-28/h2-3,8-14H,4-7,15H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495248
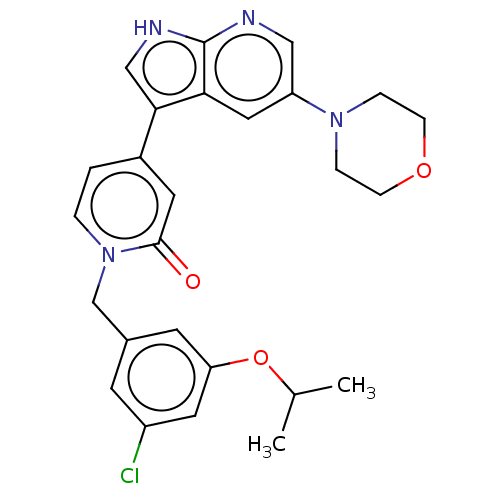
(1-(3-Chloro-5-isopropoxybenzyl)-4-(5-morpholino-1H...)Show SMILES CC(C)Oc1cc(Cl)cc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)c1 Show InChI InChI=1S/C26H27ClN4O3/c1-17(2)34-22-10-18(9-20(27)12-22)16-31-4-3-19(11-25(31)32)24-15-29-26-23(24)13-21(14-28-26)30-5-7-33-8-6-30/h3-4,9-15,17H,5-8,16H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495250
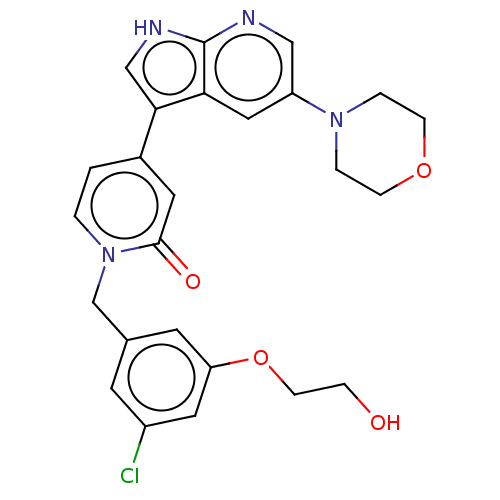
(1-(3-Chloro-5-(2-hydroxyethoxy)benzyl)-4-(5-morpho...)Show SMILES OCCOc1cc(Cl)cc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)c1 Show InChI InChI=1S/C25H25ClN4O4/c26-19-9-17(10-21(12-19)34-8-5-31)16-30-2-1-18(11-24(30)32)23-15-28-25-22(23)13-20(14-27-25)29-3-6-33-7-4-29/h1-2,9-15,31H,3-8,16H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495252
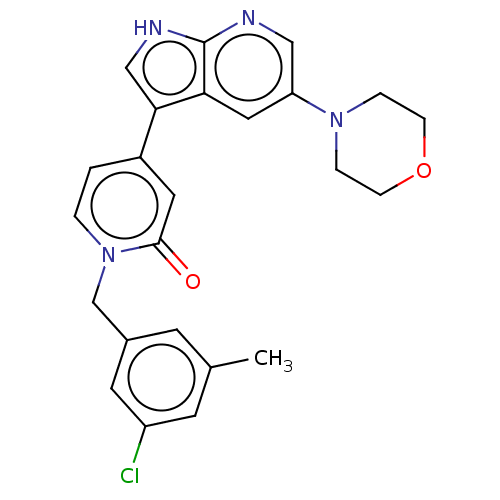
(1-(3-Chloro-5-methylbenzyl)-4-(5-morpholino-1H-pyr...)Show SMILES Cc1cc(Cl)cc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)c1 Show InChI InChI=1S/C24H23ClN4O2/c1-16-8-17(10-19(25)9-16)15-29-3-2-18(11-23(29)30)22-14-27-24-21(22)12-20(13-26-24)28-4-6-31-7-5-28/h2-3,8-14H,4-7,15H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495256
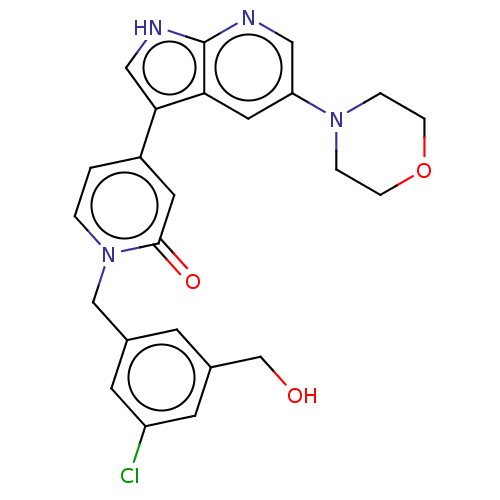
(1-(3-Chloro-5-(hydroxymethyl)benzyl)-4-(5-morpholi...)Show SMILES OCc1cc(Cl)cc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)c1 Show InChI InChI=1S/C24H23ClN4O3/c25-19-8-16(7-17(9-19)15-30)14-29-2-1-18(10-23(29)31)22-13-27-24-21(22)11-20(12-26-24)28-3-5-32-6-4-28/h1-2,7-13,30H,3-6,14-15H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495259
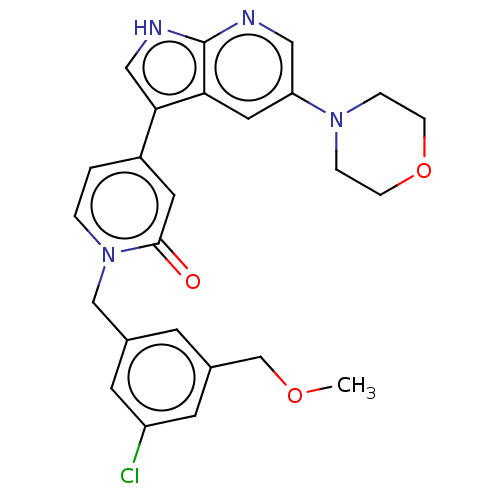
(1-(3-Chloro-5-(methoxymethyl)benzyl)-4-(5-morpholi...)Show SMILES COCc1cc(Cl)cc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)c1 Show InChI InChI=1S/C25H25ClN4O3/c1-32-16-18-8-17(9-20(26)10-18)15-30-3-2-19(11-24(30)31)23-14-28-25-22(23)12-21(13-27-25)29-4-6-33-7-5-29/h2-3,8-14H,4-7,15-16H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495261
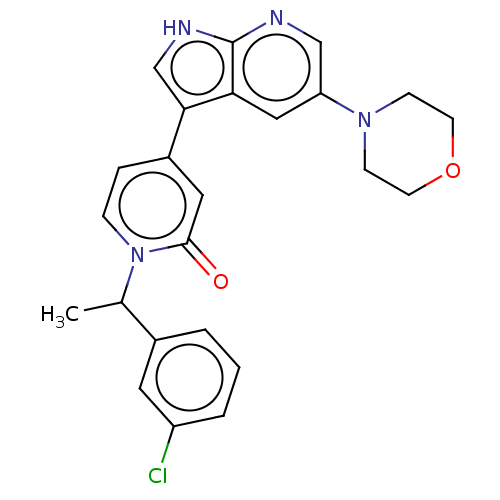
(1-(1-(3-Chlorophenyl)ethyl)-4-(5-morpholino-1H-pyr...)Show SMILES CC(c1cccc(Cl)c1)n1ccc(cc1=O)-c1c[nH]c2ncc(cc12)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495263
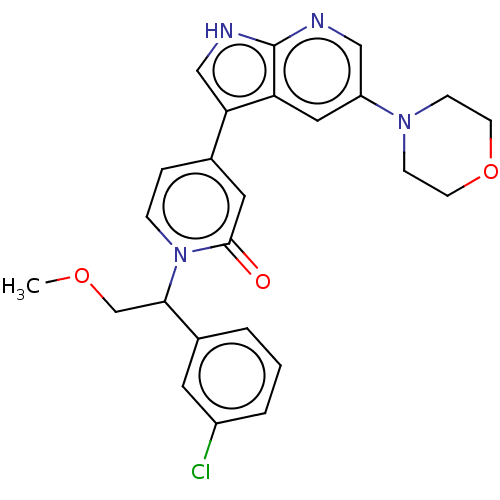
(1-(1-(3-Chlorophenyl)-2-methoxyethyl)-4-(5-morphol...)Show SMILES COCC(c1cccc(Cl)c1)n1ccc(cc1=O)-c1c[nH]c2ncc(cc12)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495267
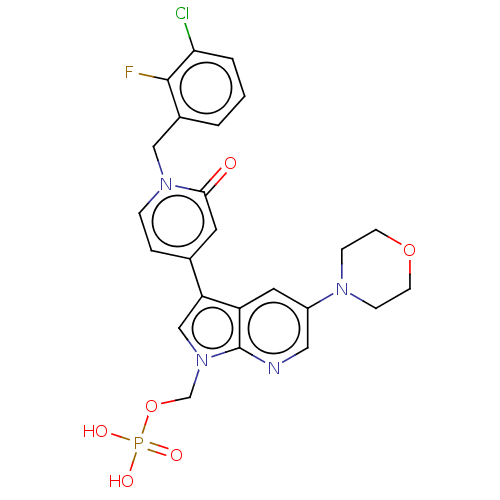
((3-(1-(3-Chloro-2-fluorobenzyl)-2-oxo-1,2-dihydrop...)Show SMILES OP(O)(=O)OCn1cc(-c2ccn(Cc3cccc(Cl)c3F)c(=O)c2)c2cc(cnc12)N1CCOCC1 Show InChI InChI=1S/C24H23ClFN4O6P/c25-21-3-1-2-17(23(21)26)13-29-5-4-16(10-22(29)31)20-14-30(15-36-37(32,33)34)24-19(20)11-18(12-27-24)28-6-8-35-9-7-28/h1-5,10-12,14H,6-9,13,15H2,(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495186
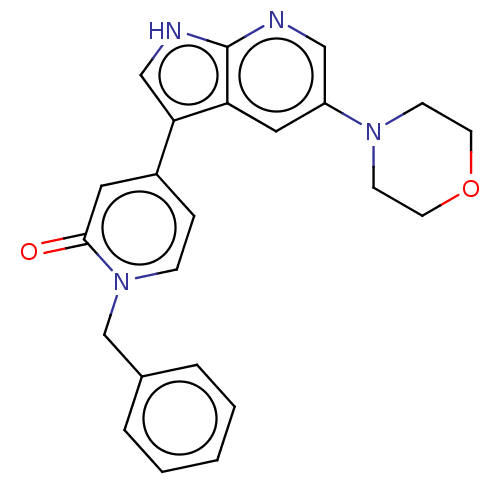
(1-Benzyl-4-(5-morpholino-1H-pyrrolo[2,3-b]pyridin-...)Show SMILES O=c1cc(ccn1Cc1ccccc1)-c1c[nH]c2ncc(cc12)N1CCOCC1 Show InChI InChI=1S/C23H22N4O2/c28-22-12-18(6-7-27(22)16-17-4-2-1-3-5-17)21-15-25-23-20(21)13-19(14-24-23)26-8-10-29-11-9-26/h1-7,12-15H,8-11,16H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495187
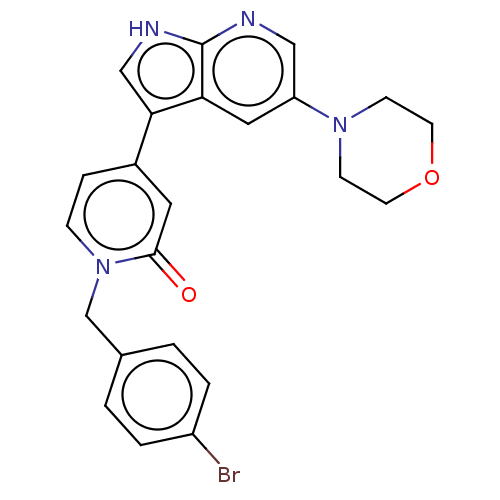
(1-(4-Bromobenzyl)-4-(5-morpholino-1H-pyrrolo[2,3-b...)Show SMILES Brc1ccc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)cc1 Show InChI InChI=1S/C23H21BrN4O2/c24-18-3-1-16(2-4-18)15-28-6-5-17(11-22(28)29)21-14-26-23-20(21)12-19(13-25-23)27-7-9-30-10-8-27/h1-6,11-14H,7-10,15H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495189

(1-(3-Iodobenzyl)-4-(5-morpholino-1H-pyrrolo[2,3-b]...)Show SMILES Ic1cccc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)c1 Show InChI InChI=1S/C23H21IN4O2/c24-18-3-1-2-16(10-18)15-28-5-4-17(11-22(28)29)21-14-26-23-20(21)12-19(13-25-23)27-6-8-30-9-7-27/h1-5,10-14H,6-9,15H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495192
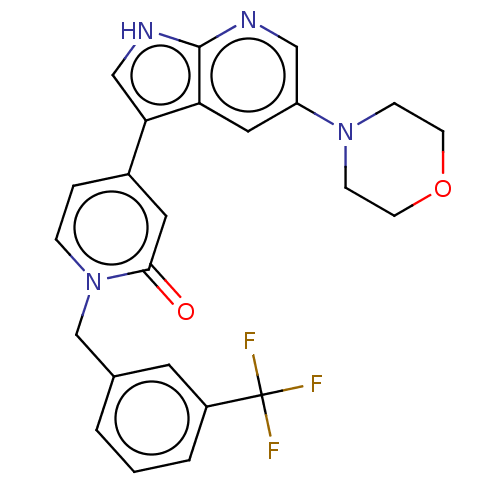
(4-(5-Morpholino-1H-pyrrolo[2,3-b]pyridin-3-yl)-1-(...)Show SMILES FC(F)(F)c1cccc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)c1 Show InChI InChI=1S/C24H21F3N4O2/c25-24(26,27)18-3-1-2-16(10-18)15-31-5-4-17(11-22(31)32)21-14-29-23-20(21)12-19(13-28-23)30-6-8-33-9-7-30/h1-5,10-14H,6-9,15H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495194
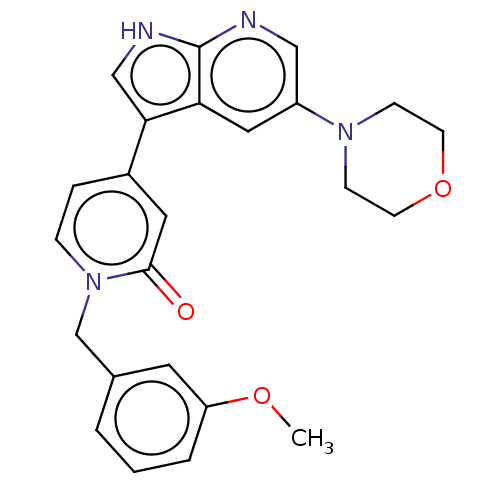
(1-(3-Methoxybenzyl)-4-(5-morpholino-1H-pyrrolo[2,3...)Show SMILES COc1cccc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)c1 Show InChI InChI=1S/C24H24N4O3/c1-30-20-4-2-3-17(11-20)16-28-6-5-18(12-23(28)29)22-15-26-24-21(22)13-19(14-25-24)27-7-9-31-10-8-27/h2-6,11-15H,7-10,16H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495196
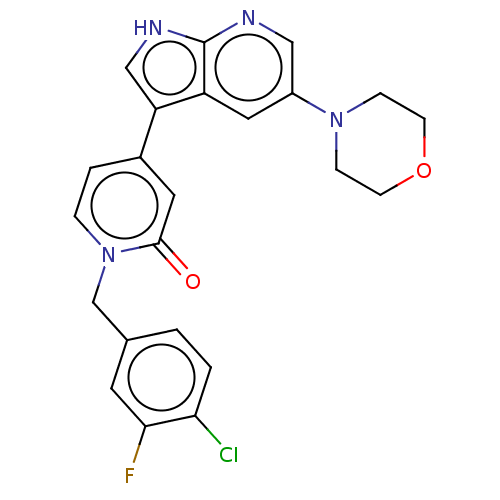
(1-(4-Chloro-3-fluorobenzyl)-4-(5-morpholino-1H-pyr...)Show SMILES Fc1cc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)ccc1Cl Show InChI InChI=1S/C23H20ClFN4O2/c24-20-2-1-15(9-21(20)25)14-29-4-3-16(10-22(29)30)19-13-27-23-18(19)11-17(12-26-23)28-5-7-31-8-6-28/h1-4,9-13H,5-8,14H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495179
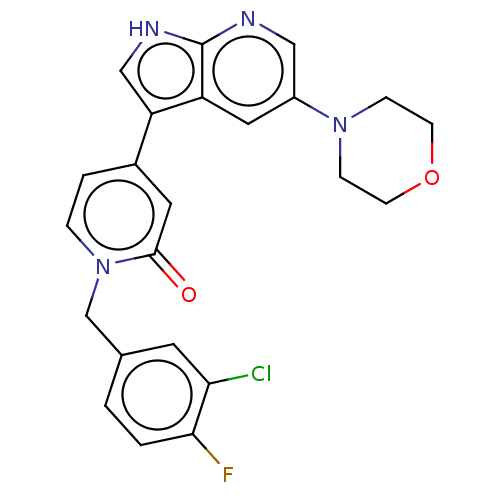
(1-(3-Chloro-4-fluorobenzyl)-4-(5-morpholino-1H-pyr...)Show SMILES Fc1ccc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)cc1Cl Show InChI InChI=1S/C23H20ClFN4O2/c24-20-9-15(1-2-21(20)25)14-29-4-3-16(10-22(29)30)19-13-27-23-18(19)11-17(12-26-23)28-5-7-31-8-6-28/h1-4,9-13H,5-8,14H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495181
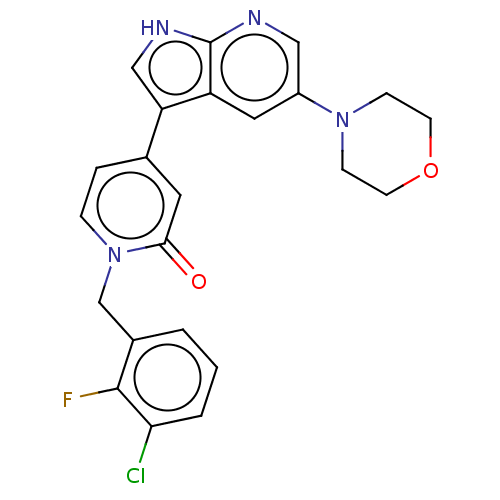
(1-(3-Chloro-2-fluorobenzyl)-4-(5-morpholino-1H-pyr...)Show SMILES Fc1c(Cl)cccc1Cn1ccc(cc1=O)-c1c[nH]c2ncc(cc12)N1CCOCC1 Show InChI InChI=1S/C23H20ClFN4O2/c24-20-3-1-2-16(22(20)25)14-29-5-4-15(10-21(29)30)19-13-27-23-18(19)11-17(12-26-23)28-6-8-31-9-7-28/h1-5,10-13H,6-9,14H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495183
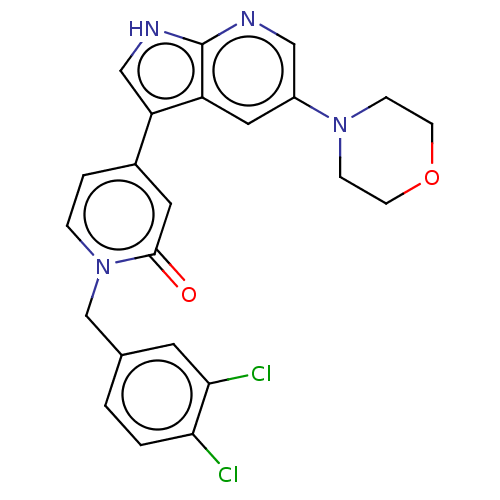
(1-(3,4-Dichlorobenzyl)-4-(5-morpholino-1H-pyrrolo[...)Show SMILES Clc1ccc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)cc1Cl Show InChI InChI=1S/C23H20Cl2N4O2/c24-20-2-1-15(9-21(20)25)14-29-4-3-16(10-22(29)30)19-13-27-23-18(19)11-17(12-26-23)28-5-7-31-8-6-28/h1-4,9-13H,5-8,14H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495185
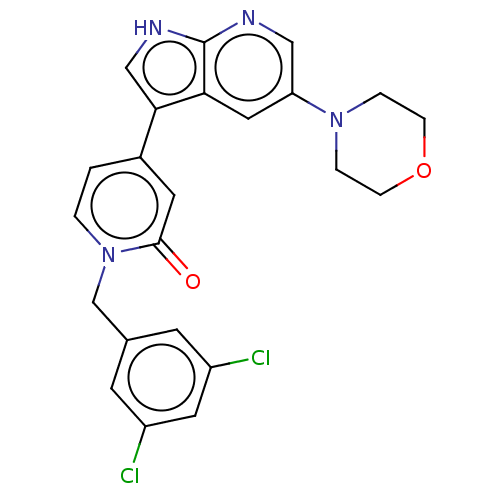
(1-(2,4-Dichlorobenzyl)-4-(5-morpholino-1H-pyrrolo[...)Show SMILES Clc1cc(Cl)cc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)c1 Show InChI InChI=1S/C23H20Cl2N4O2/c24-17-7-15(8-18(25)10-17)14-29-2-1-16(9-22(29)30)21-13-27-23-20(21)11-19(12-26-23)28-3-5-31-6-4-28/h1-2,7-13H,3-6,14H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495191
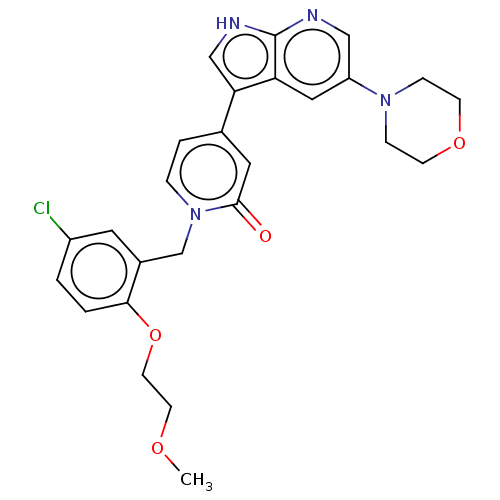
(1-(5-Chloro-2-(2-methoxyethoxy)benzyl)-4-(5-morpho...)Show SMILES COCCOc1ccc(Cl)cc1Cn1ccc(cc1=O)-c1c[nH]c2ncc(cc12)N1CCOCC1 Show InChI InChI=1S/C26H27ClN4O4/c1-33-10-11-35-24-3-2-20(27)12-19(24)17-31-5-4-18(13-25(31)32)23-16-29-26-22(23)14-21(15-28-26)30-6-8-34-9-7-30/h2-5,12-16H,6-11,17H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495193
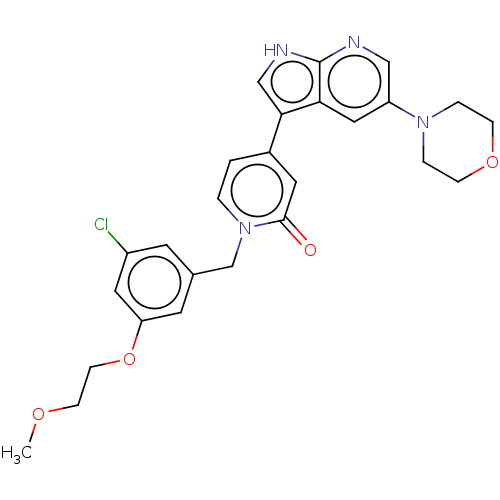
(1-(3-Chloro-5-(2-methoxyethoxy)benzyl)-4-(5-morpho...)Show SMILES COCCOc1cc(Cl)cc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)c1 Show InChI InChI=1S/C26H27ClN4O4/c1-33-8-9-35-22-11-18(10-20(27)13-22)17-31-3-2-19(12-25(31)32)24-16-29-26-23(24)14-21(15-28-26)30-4-6-34-7-5-30/h2-3,10-16H,4-9,17H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM495198
(1-((6-Chloropyridin-2-yl)methyl)-4-(5-morpholino-1...)Show SMILES Clc1cccc(Cn2ccc(cc2=O)-c2c[nH]c3ncc(cc23)N2CCOCC2)n1 Show InChI InChI=1S/C22H20ClN5O2/c23-20-3-1-2-16(26-20)14-28-5-4-15(10-21(28)29)19-13-25-22-18(19)11-17(12-24-22)27-6-8-30-9-7-27/h1-5,10-13H,6-9,14H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0DDB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data