

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290221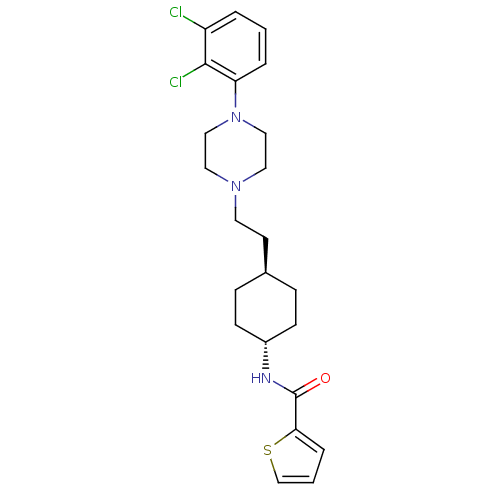 (CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063292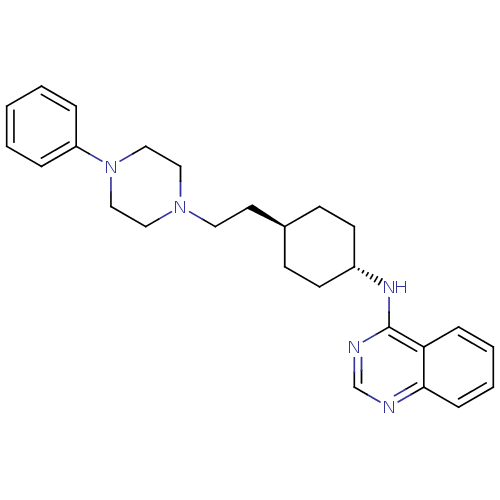 (CHEMBL349426 | {4-[2-(4-Phenyl-piperazin-1-yl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM430 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2208 ((6S)-3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2204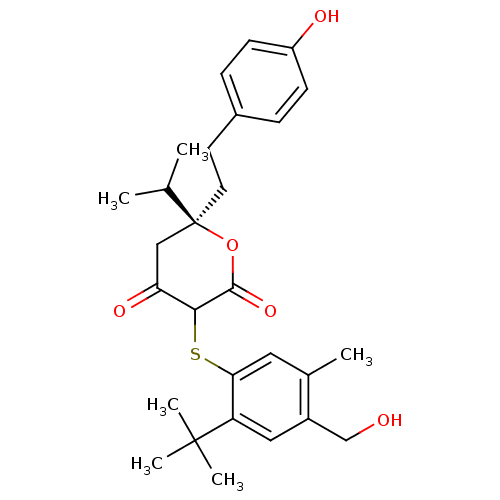 ((3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-phenylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072528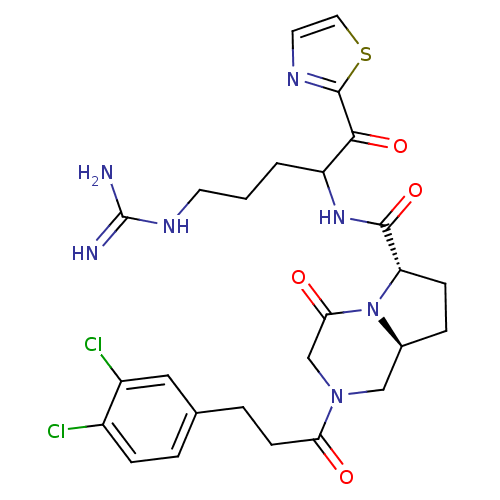 ((6S,8aS)-2-[3-(3,4-Dichloro-phenyl)-propionyl]-4-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2206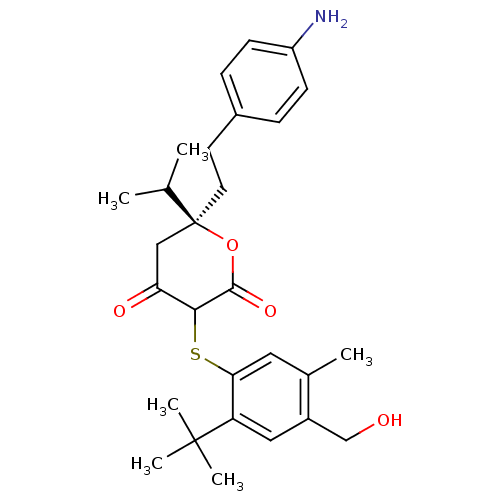 ((6S)-6-[2-(4-aminophenyl)ethyl]-3-{[2-tert-butyl-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063279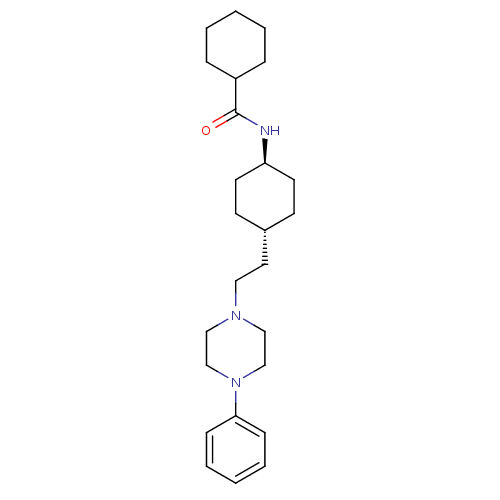 (CHEMBL309623 | Cyclohexanecarboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063279 (CHEMBL309623 | Cyclohexanecarboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2533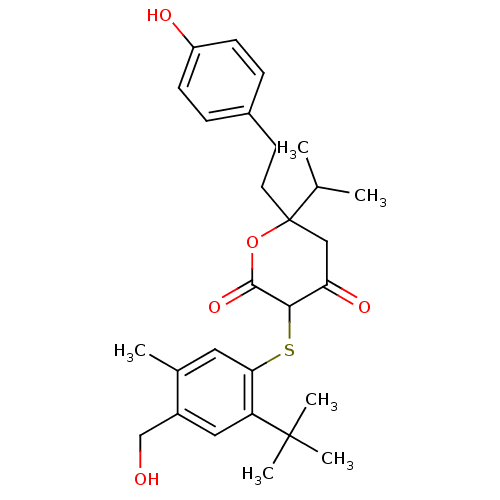 (3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylphenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.170 | -58.0 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072536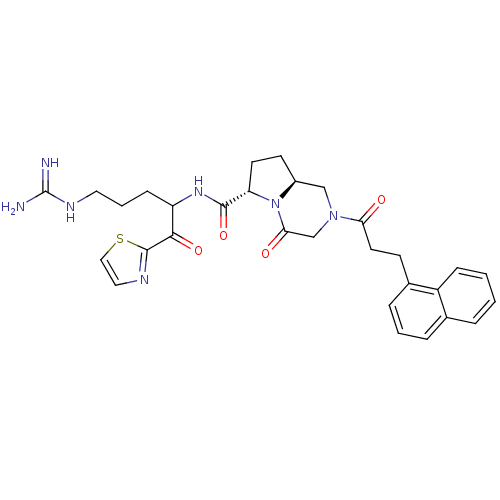 ((6S,8aS)-2-(3-Naphthalen-1-yl-propionyl)-4-oxo-oct...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2536 (6-Alkyl-6-phenethyldihydropyrone 13y | 6-[2-(4-ami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072527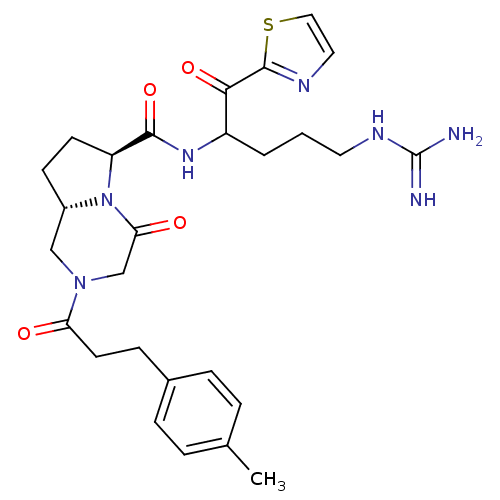 ((6S,8aS)-4-Oxo-2-(3-p-tolyl-propionyl)-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50054067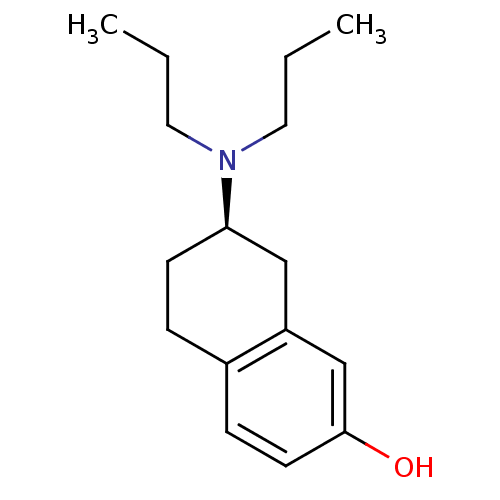 ((2R)-7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290206 (CHEMBL78800 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072534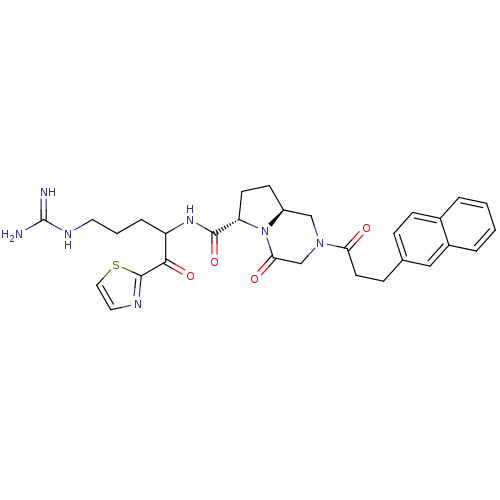 ((6S,8aS)-2-(3-Naphthalen-2-yl-propionyl)-4-oxo-oct...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50063281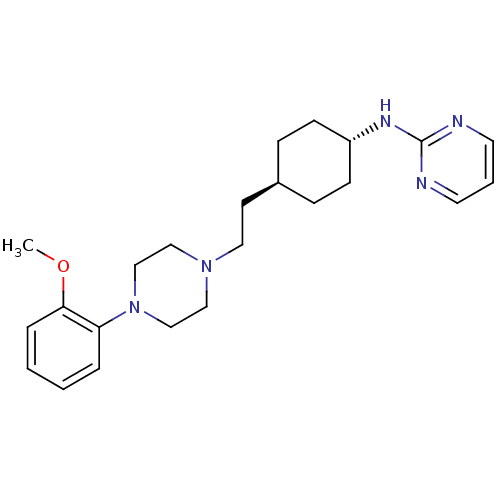 ((4-{2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-ethyl}...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The binding affinity of the compound was determined by measuring its ability to displace [3H]-8-OH-DPAT radioligand in 5-hydroxytryptamine 1A recepto... | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072537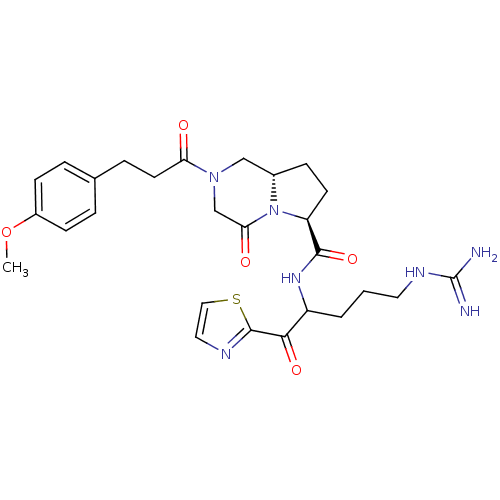 ((6S,8aS)-2-[3-(4-Methoxy-phenyl)-propionyl]-4-oxo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072524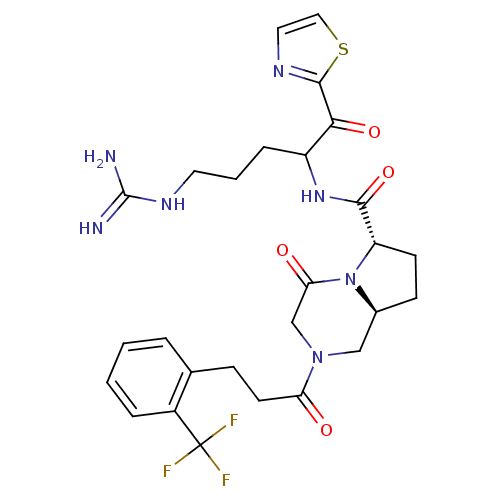 ((6S,8aS)-4-Oxo-2-[3-(2-trifluoromethyl-phenyl)-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072532 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Rattus norvegicus) | BDBM50072740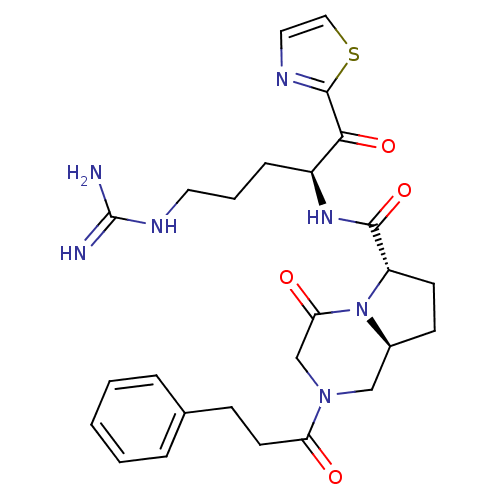 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the amidolytic activity of thrombin | Bioorg Med Chem Lett 11: 3161-4 (2001) BindingDB Entry DOI: 10.7270/Q2JM28XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072740 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound towards Thrombin | J Med Chem 43: 361-8 (2000) BindingDB Entry DOI: 10.7270/Q2P26XCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50290221 (CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054067 ((2R)-7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290225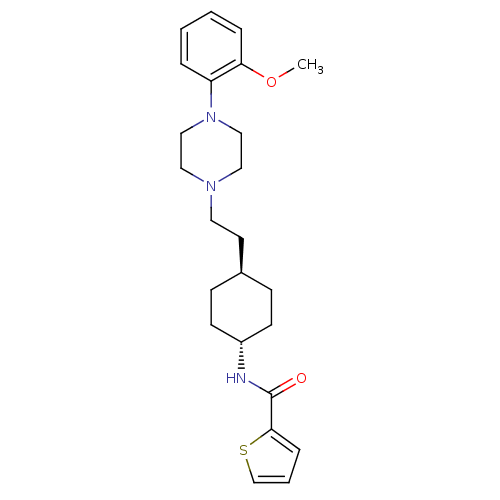 (CHEMBL78950 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063291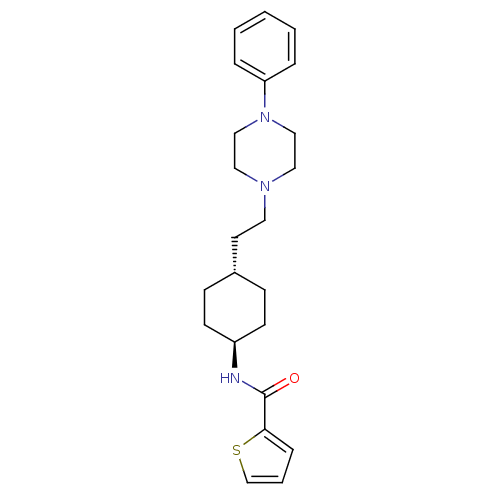 (CHEMBL78791 | Thiophene-2-carboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063291 (CHEMBL78791 | Thiophene-2-carboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM85093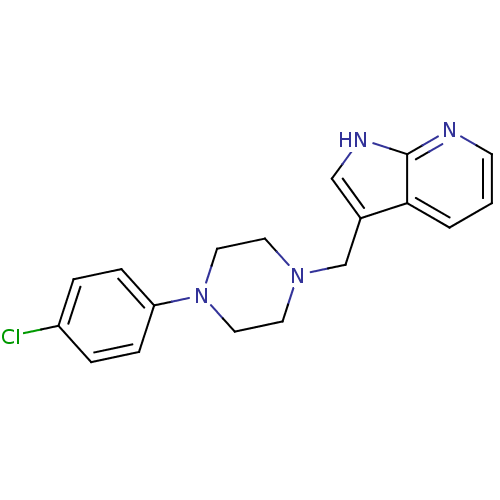 (CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity at human dopamine D4 receptor expressed in CHO cells by [3H]spiperone displacement. | Bioorg Med Chem Lett 8: 1499-502 (1999) BindingDB Entry DOI: 10.7270/Q2WH2P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072520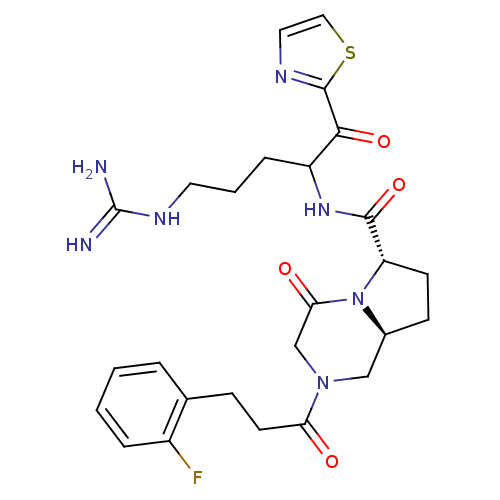 ((6S,8aS)-2-[3-(2-Fluoro-phenyl)-propionyl]-4-oxo-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50290206 (CHEMBL78800 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM448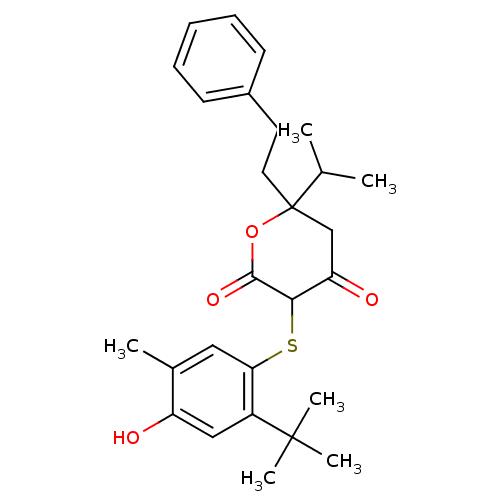 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080924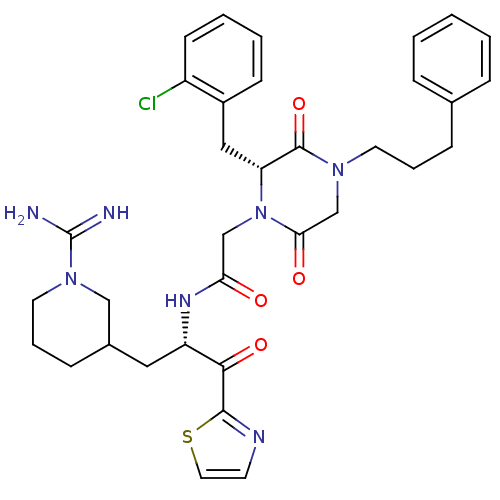 (CHEMBL83260 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 2503-8 (1999) BindingDB Entry DOI: 10.7270/Q2028QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072533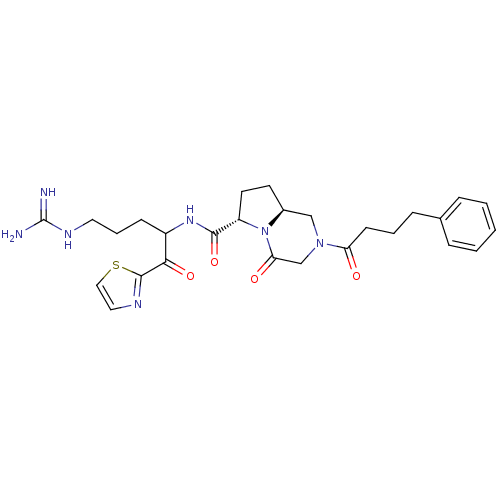 ((6S,8aS)-4-Oxo-2-(4-phenyl-butyryl)-octahydro-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290209 (CHEMBL310577 | Thiophene-2-carboxylic acid (4-{2-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50290225 (CHEMBL78950 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290214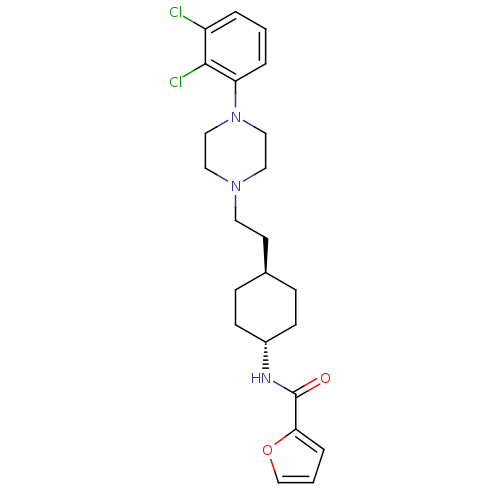 (CHEMBL312430 | Furan-2-carboxylic acid (4-{2-[4-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50290218 (CHEMBL78617 | Cyclopentanecarboxylic acid (4-{2-[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290221 (CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50455438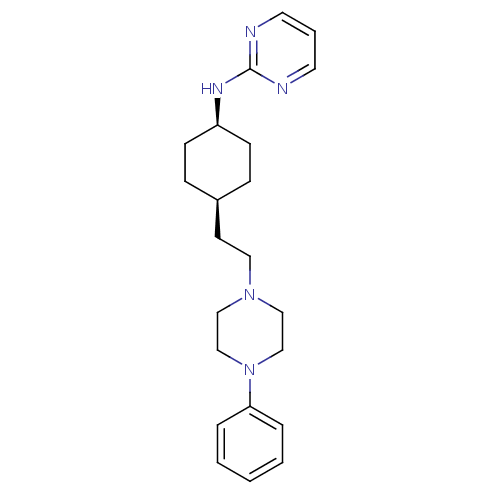 (CHEMBL3084971) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The binding affinity of the compound was determined by measuring its ability to displace [3H]-8-OH-DPAT radioligand in 5-hydroxytryptamine 1A recepto... | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290210 (CHEMBL78368 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063279 (CHEMBL309623 | Cyclohexanecarboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50063280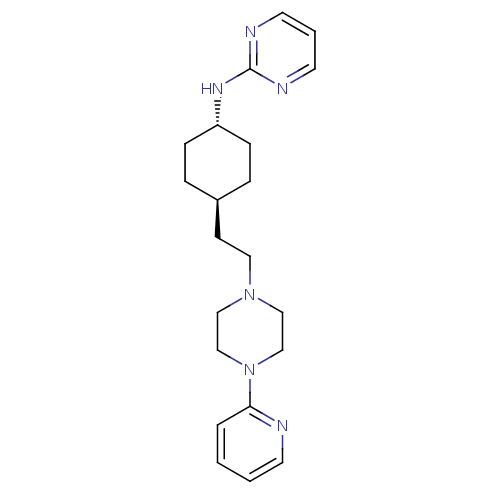 (CHEMBL355371 | {4-[2-(4-Pyridin-2-yl-piperazin-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The binding affinity of the compound was determined by measuring its ability to displace [3H]-8-OH-DPAT radioligand in 5-hydroxytryptamine 1A recepto... | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290213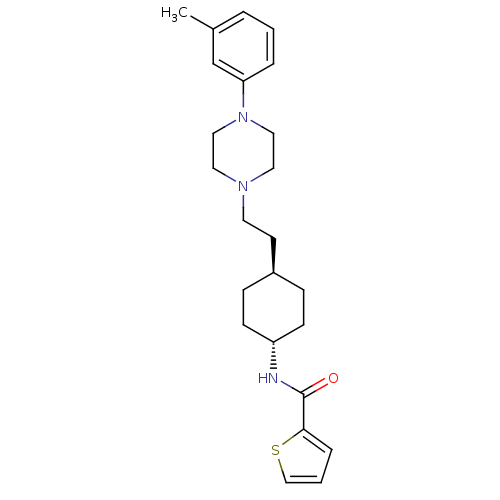 (CHEMBL80875 | Thiophene-2-carboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072530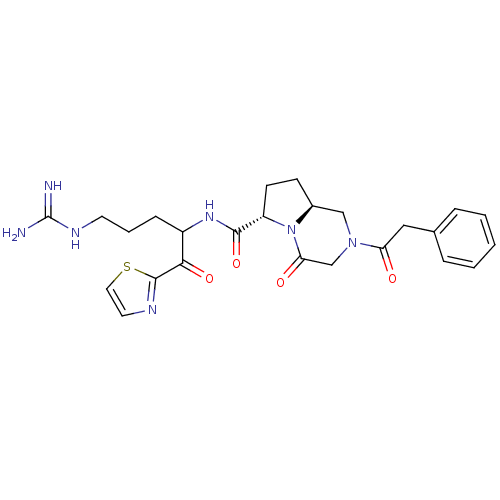 ((6S,8aS)-4-Oxo-2-phenylacetyl-octahydro-pyrrolo[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290217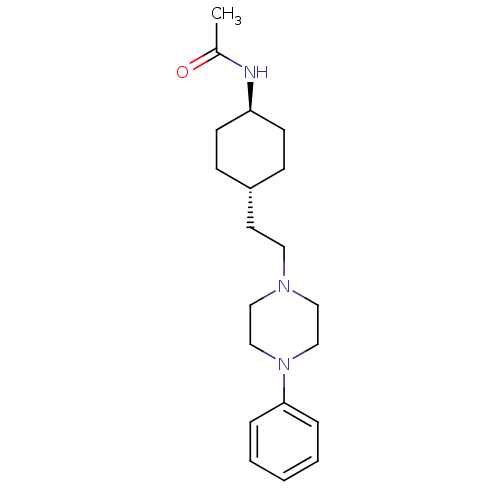 (CHEMBL78916 | N-{4-[2-(4-Phenyl-piperazin-1-yl)-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072541 ((6S,8aS)-4-Oxo-2-(3-pyridin-2-yl-propionyl)-octahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50063281 ((4-{2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-ethyl}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity determined by measuring its ability to displace [3H]-N-0437 radioligand in CHO-K1 cells on Cloned Human Dopamine receptor D2 | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50290214 (CHEMBL312430 | Furan-2-carboxylic acid (4-{2-[4-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072519 ((6S,8aS)-2-[(S)-2-Amino-3-(4-fluoro-phenyl)-propio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50071235 (CHEMBL65078 | Cyclopropylmethyl-[3-(2,4-dichloro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 8: 2067-70 (1999) BindingDB Entry DOI: 10.7270/Q2125RTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 825 total ) | Next | Last >> |