 Found 3169 hits with Last Name = 'rudolph' and Initial = 'm'
Found 3169 hits with Last Name = 'rudolph' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197090
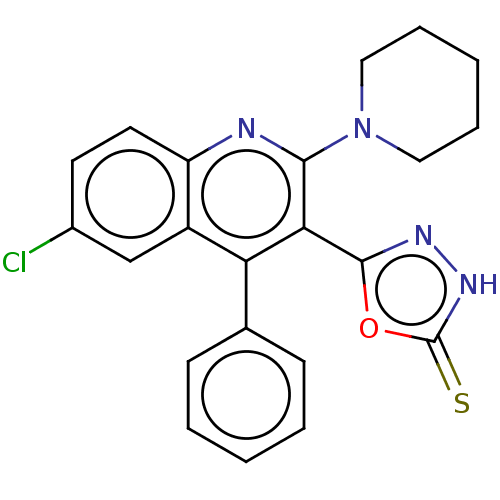
(CHEMBL3970105)Show SMILES Clc1ccc2nc(N3CCCCC3)c(-c3n[nH]c(=S)o3)c(-c3ccccc3)c2c1 Show InChI InChI=1S/C22H19ClN4OS/c23-15-9-10-17-16(13-15)18(14-7-3-1-4-8-14)19(21-25-26-22(29)28-21)20(24-17)27-11-5-2-6-12-27/h1,3-4,7-10,13H,2,5-6,11-12H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from recombinant human His6-tagged FABP4 expressed in Escherichia coli after 30 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197093
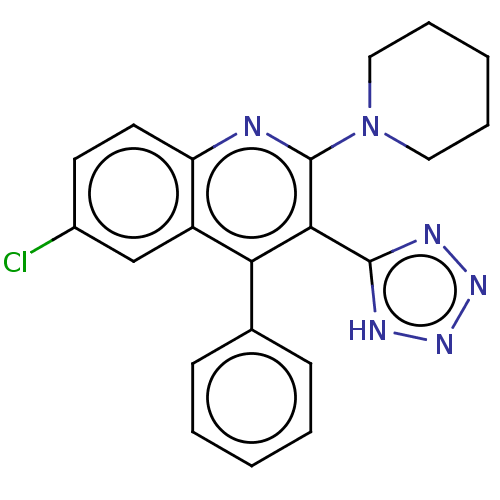
(CHEMBL3959018)Show SMILES Clc1ccc2nc(N3CCCCC3)c(-c3nnn[nH]3)c(-c3ccccc3)c2c1 Show InChI InChI=1S/C21H19ClN6/c22-15-9-10-17-16(13-15)18(14-7-3-1-4-8-14)19(20-24-26-27-25-20)21(23-17)28-11-5-2-6-12-28/h1,3-4,7-10,13H,2,5-6,11-12H2,(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from recombinant human His6-tagged FABP4 expressed in Escherichia coli after 30 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197092
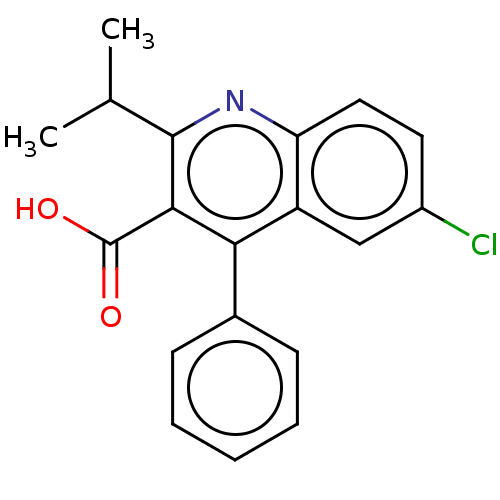
(CHEMBL3947458)Show InChI InChI=1S/C19H16ClNO2/c1-11(2)18-17(19(22)23)16(12-6-4-3-5-7-12)14-10-13(20)8-9-15(14)21-18/h3-11H,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197092

(CHEMBL3947458)Show InChI InChI=1S/C19H16ClNO2/c1-11(2)18-17(19(22)23)16(12-6-4-3-5-7-12)14-10-13(20)8-9-15(14)21-18/h3-11H,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from recombinant human His6-tagged FABP4 expressed in Escherichia coli after 30 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197087
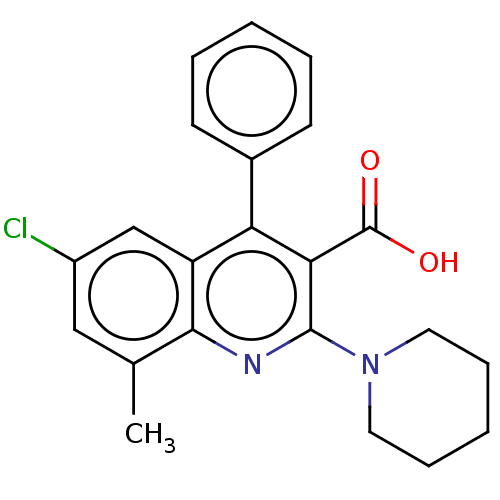
(CHEMBL3950316)Show SMILES Cc1cc(Cl)cc2c(-c3ccccc3)c(C(O)=O)c(nc12)N1CCCCC1 Show InChI InChI=1S/C22H21ClN2O2/c1-14-12-16(23)13-17-18(15-8-4-2-5-9-15)19(22(26)27)21(24-20(14)17)25-10-6-3-7-11-25/h2,4-5,8-9,12-13H,3,6-7,10-11H2,1H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from recombinant human His6-tagged FABP4 expressed in Escherichia coli after 30 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197088
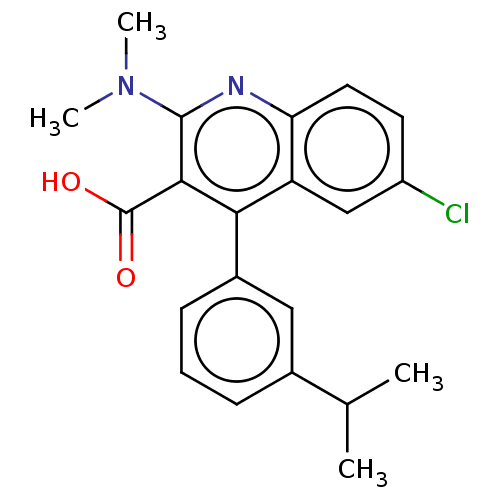
(CHEMBL3979347)Show SMILES CC(C)c1cccc(c1)-c1c(C(O)=O)c(nc2ccc(Cl)cc12)N(C)C Show InChI InChI=1S/C21H21ClN2O2/c1-12(2)13-6-5-7-14(10-13)18-16-11-15(22)8-9-17(16)23-20(24(3)4)19(18)21(25)26/h5-12H,1-4H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from recombinant human His6-tagged FABP4 expressed in Escherichia coli after 30 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197086
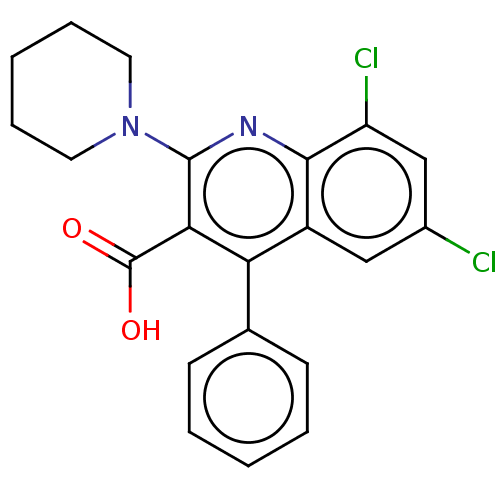
(CHEMBL3971182)Show SMILES OC(=O)c1c(nc2c(Cl)cc(Cl)cc2c1-c1ccccc1)N1CCCCC1 Show InChI InChI=1S/C21H18Cl2N2O2/c22-14-11-15-17(13-7-3-1-4-8-13)18(21(26)27)20(24-19(15)16(23)12-14)25-9-5-2-6-10-25/h1,3-4,7-8,11-12H,2,5-6,9-10H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from recombinant human His6-tagged FABP4 expressed in Escherichia coli after 30 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197089
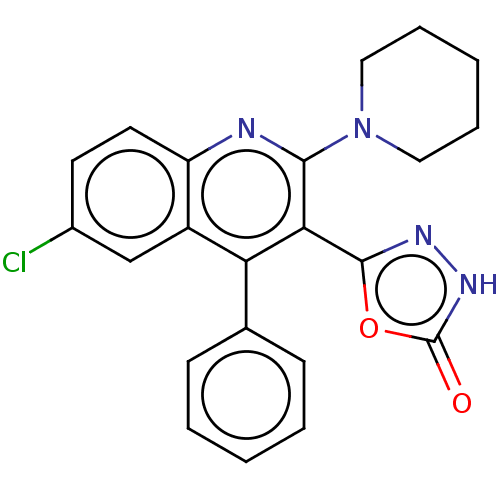
(CHEMBL3889982)Show SMILES Clc1ccc2nc(N3CCCCC3)c(-c3n[nH]c(=O)o3)c(-c3ccccc3)c2c1 Show InChI InChI=1S/C22H19ClN4O2/c23-15-9-10-17-16(13-15)18(14-7-3-1-4-8-14)19(21-25-26-22(28)29-21)20(24-17)27-11-5-2-6-12-27/h1,3-4,7-10,13H,2,5-6,11-12H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from recombinant human His6-tagged FABP4 expressed in Escherichia coli after 30 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197094
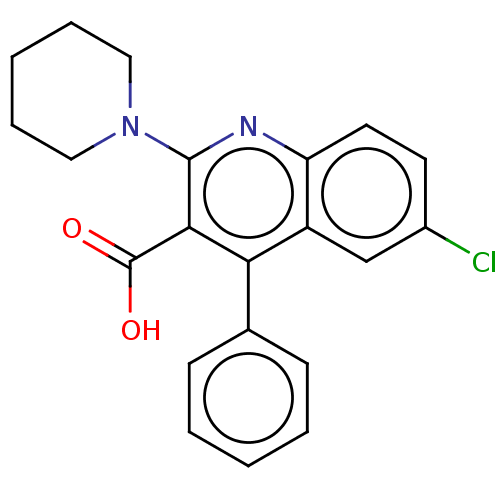
(CHEMBL3941588)Show SMILES OC(=O)c1c(nc2ccc(Cl)cc2c1-c1ccccc1)N1CCCCC1 Show InChI InChI=1S/C21H19ClN2O2/c22-15-9-10-17-16(13-15)18(14-7-3-1-4-8-14)19(21(25)26)20(23-17)24-11-5-2-6-12-24/h1,3-4,7-10,13H,2,5-6,11-12H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from recombinant human His6-tagged FABP4 expressed in Escherichia coli after 30 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197094

(CHEMBL3941588)Show SMILES OC(=O)c1c(nc2ccc(Cl)cc2c1-c1ccccc1)N1CCCCC1 Show InChI InChI=1S/C21H19ClN2O2/c22-15-9-10-17-16(13-15)18(14-7-3-1-4-8-14)19(21(25)26)20(23-17)24-11-5-2-6-12-24/h1,3-4,7-10,13H,2,5-6,11-12H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50613583
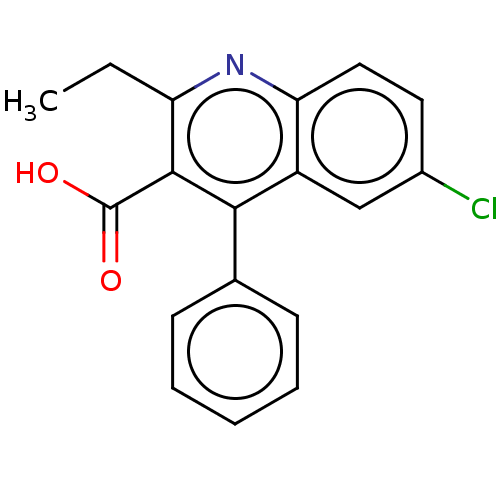
(CHEMBL5287034) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099928
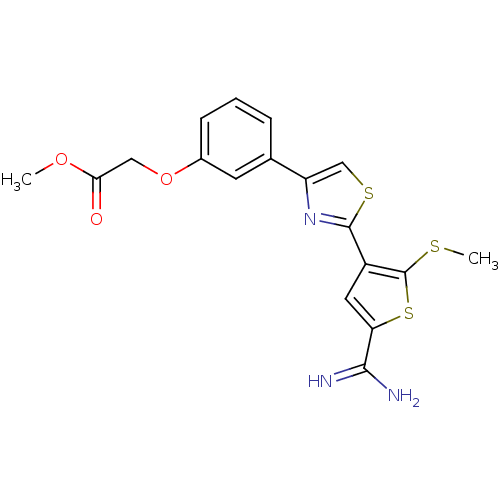
(CHEMBL28952 | {3-[2-(5-Carbamimidoyl-2-methylsulfa...)Show SMILES COC(=O)COc1cccc(c1)-c1csc(n1)-c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C18H17N3O3S3/c1-23-15(22)8-24-11-5-3-4-10(6-11)13-9-26-17(21-13)12-7-14(16(19)20)27-18(12)25-2/h3-7,9H,8H2,1-2H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099934
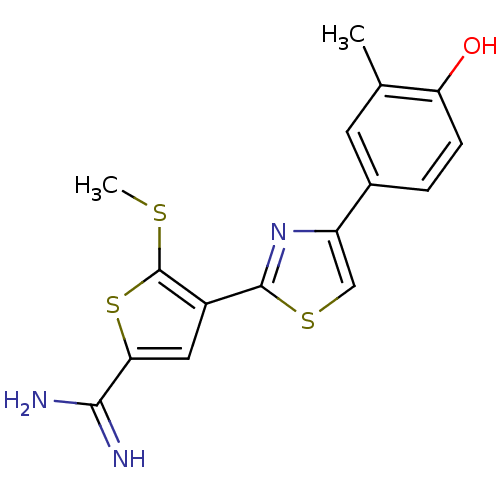
(4-[4-(4-Hydroxy-3-methyl-phenyl)-thiazol-2-yl]-5-m...)Show InChI InChI=1S/C16H15N3OS3/c1-8-5-9(3-4-12(8)20)11-7-22-15(19-11)10-6-13(14(17)18)23-16(10)21-2/h3-7,20H,1-2H3,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099923

(CHEMBL29037 | N-{3-[2-(5-Carbamimidoyl-2-methylsul...)Show SMILES CSc1sc(cc1-c1nc(cs1)-c1cccc(NC(=O)c2ccc(F)cc2)c1)C(N)=N Show InChI InChI=1S/C22H17FN4OS3/c1-29-22-16(10-18(31-22)19(24)25)21-27-17(11-30-21)13-3-2-4-15(9-13)26-20(28)12-5-7-14(23)8-6-12/h2-11H,1H3,(H3,24,25)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50109377
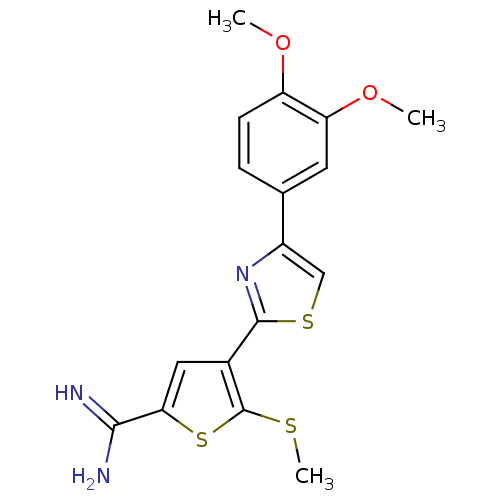
(4-[4-(3,4-Dimethoxy-phenyl)-thiazol-2-yl]-5-methyl...)Show InChI InChI=1S/C17H17N3O2S3/c1-21-12-5-4-9(6-13(12)22-2)11-8-24-16(20-11)10-7-14(15(18)19)25-17(10)23-3/h4-8H,1-3H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human kidney cell urokinase |
Bioorg Med Chem Lett 12: 491-5 (2002)
BindingDB Entry DOI: 10.7270/Q2H994H5 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098163
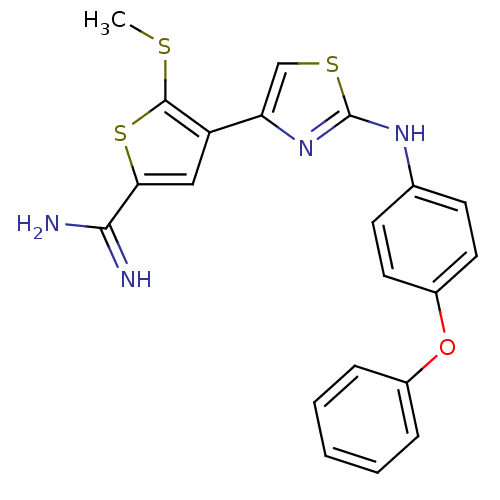
(5-Methylsulfanyl-4-[2-(4-phenoxy-phenylamino)-thia...)Show SMILES CSc1sc(cc1-c1csc(Nc2ccc(Oc3ccccc3)cc2)n1)C(N)=N Show InChI InChI=1S/C21H18N4OS3/c1-27-20-16(11-18(29-20)19(22)23)17-12-28-21(25-17)24-13-7-9-15(10-8-13)26-14-5-3-2-4-6-14/h2-12H,1H3,(H3,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 915-8 (2001)
BindingDB Entry DOI: 10.7270/Q2J67G5B |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50147047
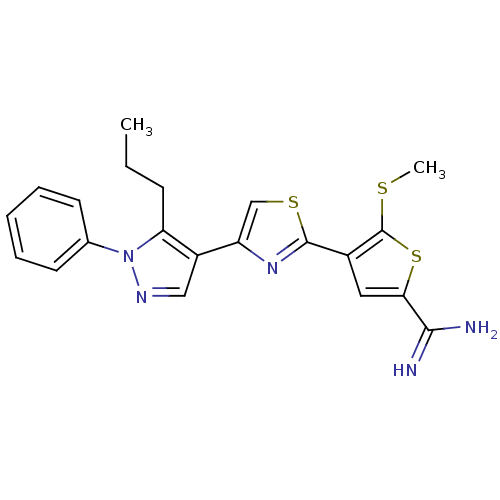
(5-Methylsulfanyl-4-[4-(1-phenyl-5-propyl-1H-pyrazo...)Show SMILES CCCc1c(cnn1-c1ccccc1)-c1csc(n1)-c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C21H21N5S3/c1-3-7-17-15(11-24-26(17)13-8-5-4-6-9-13)16-12-28-20(25-16)14-10-18(19(22)23)29-21(14)27-2/h4-6,8-12H,3,7H2,1-2H3,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Complement C1s subcomponent |
Bioorg Med Chem Lett 14: 3043-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.034
BindingDB Entry DOI: 10.7270/Q2K35T33 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50147046
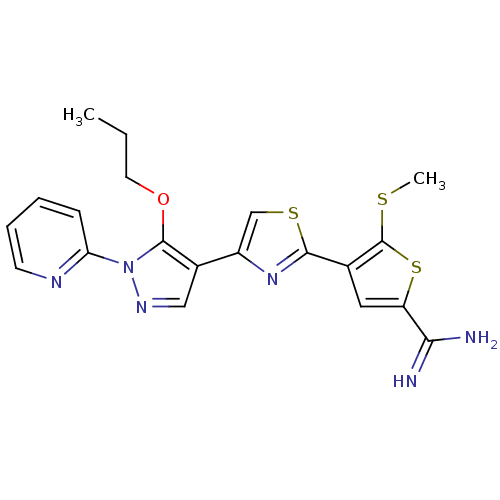
(5-Methylsulfanyl-4-[4-(5-propoxy-1-pyridin-2-yl-1H...)Show SMILES CCCOc1c(cnn1-c1ccccn1)-c1csc(n1)-c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C20H20N6OS3/c1-3-8-27-19-13(10-24-26(19)16-6-4-5-7-23-16)14-11-29-18(25-14)12-9-15(17(21)22)30-20(12)28-2/h4-7,9-11H,3,8H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Complement C1s subcomponent |
Bioorg Med Chem Lett 14: 3043-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.034
BindingDB Entry DOI: 10.7270/Q2K35T33 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098169
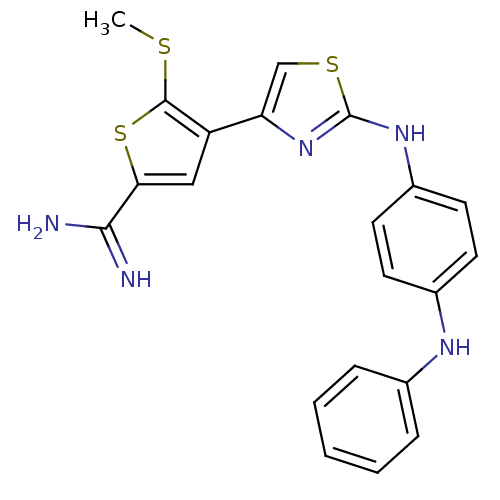
(5-Methylsulfanyl-4-[2-(4-phenylamino-phenylamino)-...)Show SMILES CSc1sc(cc1-c1csc(Nc2ccc(Nc3ccccc3)cc2)n1)C(N)=N Show InChI InChI=1S/C21H19N5S3/c1-27-20-16(11-18(29-20)19(22)23)17-12-28-21(26-17)25-15-9-7-14(8-10-15)24-13-5-3-2-4-6-13/h2-12,24H,1H3,(H3,22,23)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 915-8 (2001)
BindingDB Entry DOI: 10.7270/Q2J67G5B |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098144
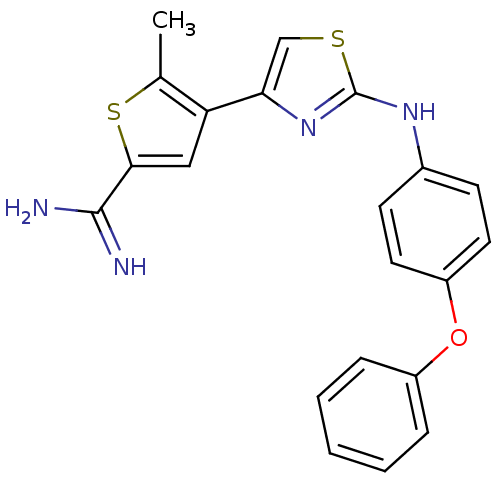
(5-Methyl-4-[2-(4-phenoxy-phenylamino)-thiazol-4-yl...)Show SMILES Cc1sc(cc1-c1csc(Nc2ccc(Oc3ccccc3)cc2)n1)C(N)=N Show InChI InChI=1S/C21H18N4OS2/c1-13-17(11-19(28-13)20(22)23)18-12-27-21(25-18)24-14-7-9-16(10-8-14)26-15-5-3-2-4-6-15/h2-12H,1H3,(H3,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 915-8 (2001)
BindingDB Entry DOI: 10.7270/Q2J67G5B |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein 5
(Homo sapiens (Human)) | BDBM50197093

(CHEMBL3959018)Show SMILES Clc1ccc2nc(N3CCCCC3)c(-c3nnn[nH]3)c(-c3ccccc3)c2c1 Show InChI InChI=1S/C21H19ClN6/c22-15-9-10-17-16(13-15)18(14-7-3-1-4-8-14)19(20-24-26-27-25-20)21(23-17)28-11-5-2-6-12-28/h1,3-4,7-10,13H,2,5-6,11-12H2,(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from human N-terminal His6-tagged FABP5 (127 to 132 residues) expressed in Escherichia coli after 30 mins b... |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099902
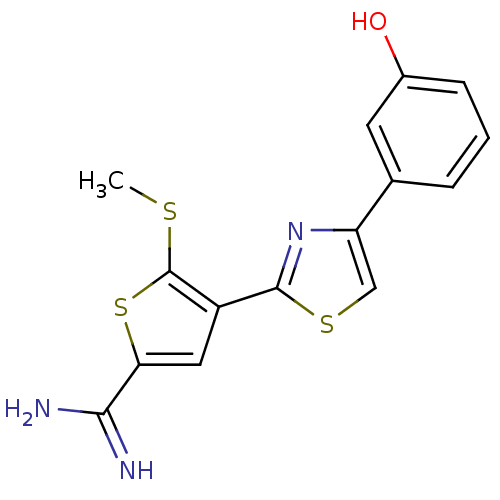
(4-[4-(3-Hydroxy-phenyl)-thiazol-2-yl]-5-methylsulf...)Show InChI InChI=1S/C15H13N3OS3/c1-20-15-10(6-12(22-15)13(16)17)14-18-11(7-21-14)8-3-2-4-9(19)5-8/h2-7,19H,1H3,(H3,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099933
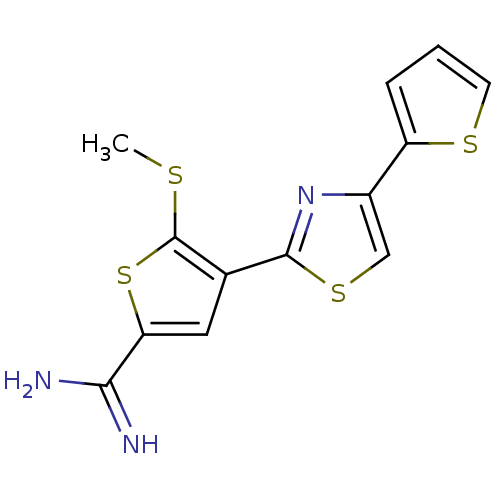
(5-Methylsulfanyl-4-(4-thiophen-2-yl-thiazol-2-yl)-...)Show InChI InChI=1S/C13H11N3S4/c1-17-13-7(5-10(20-13)11(14)15)12-16-8(6-19-12)9-3-2-4-18-9/h2-6H,1H3,(H3,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099921
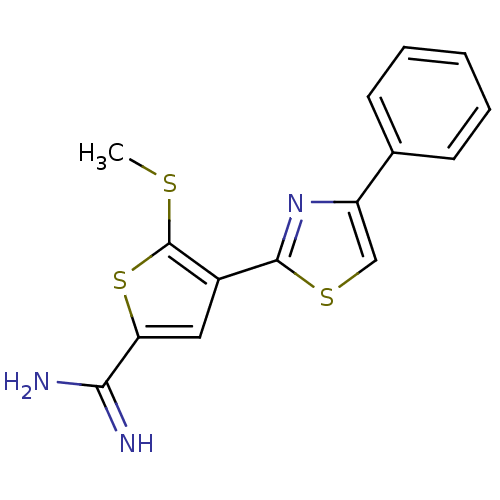
(5-Methylsulfanyl-4-(4-phenyl-thiazol-2-yl)-thiophe...)Show InChI InChI=1S/C15H13N3S3/c1-19-15-10(7-12(21-15)13(16)17)14-18-11(8-20-14)9-5-3-2-4-6-9/h2-8H,1H3,(H3,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50147059
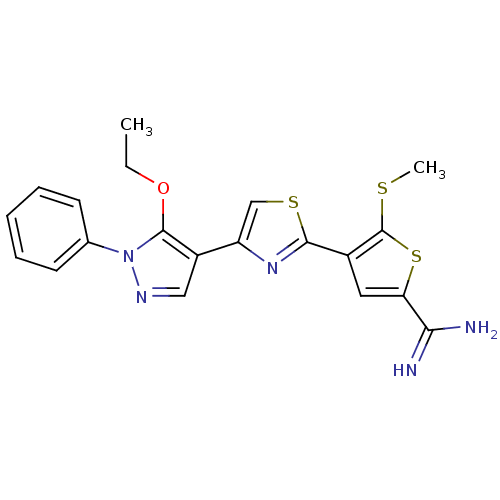
(4-[4-(5-Ethoxy-1-phenyl-1H-pyrazol-4-yl)-thiazol-2...)Show SMILES CCOc1c(cnn1-c1ccccc1)-c1csc(n1)-c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C20H19N5OS3/c1-3-26-19-14(10-23-25(19)12-7-5-4-6-8-12)15-11-28-18(24-15)13-9-16(17(21)22)29-20(13)27-2/h4-11H,3H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Complement C1s subcomponent |
Bioorg Med Chem Lett 14: 3043-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.034
BindingDB Entry DOI: 10.7270/Q2K35T33 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, heart
(Homo sapiens (Human)) | BDBM50197091
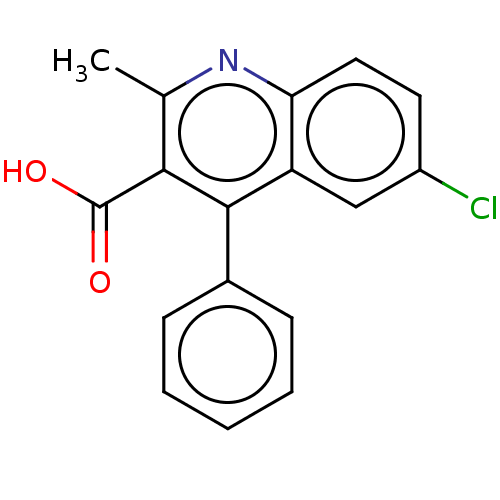
(CHEMBL1738980)Show InChI InChI=1S/C17H12ClNO2/c1-10-15(17(20)21)16(11-5-3-2-4-6-11)13-9-12(18)7-8-14(13)19-10/h2-9H,1H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099911

(4-(4-Benzo[1,3]dioxol-5-yl-thiazol-2-yl)-5-methyls...)Show InChI InChI=1S/C16H13N3O2S3/c1-22-16-9(5-13(24-16)14(17)18)15-19-10(6-23-15)8-2-3-11-12(4-8)21-7-20-11/h2-6H,7H2,1H3,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, heart
(Homo sapiens (Human)) | BDBM50197091

(CHEMBL1738980)Show InChI InChI=1S/C17H12ClNO2/c1-10-15(17(20)21)16(11-5-3-2-4-6-11)13-9-12(18)7-8-14(13)19-10/h2-9H,1H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from human FABP3 after 30 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099897
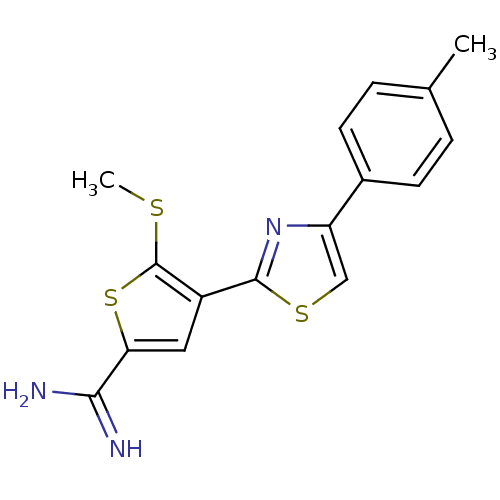
(5-Methylsulfanyl-4-(4-p-tolyl-thiazol-2-yl)-thioph...)Show InChI InChI=1S/C16H15N3S3/c1-9-3-5-10(6-4-9)12-8-21-15(19-12)11-7-13(14(17)18)22-16(11)20-2/h3-8H,1-2H3,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099901
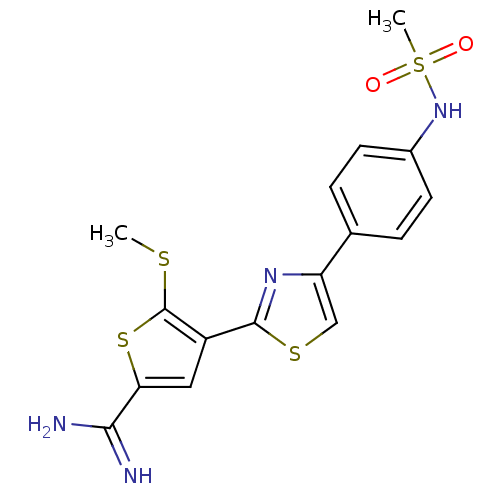
(4-[4-(4-Methanesulfonylamino-phenyl)-thiazol-2-yl]...)Show SMILES CSc1sc(cc1-c1nc(cs1)-c1ccc(NS(C)(=O)=O)cc1)C(N)=N Show InChI InChI=1S/C16H16N4O2S4/c1-23-16-11(7-13(25-16)14(17)18)15-19-12(8-24-15)9-3-5-10(6-4-9)20-26(2,21)22/h3-8,20H,1-2H3,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099900
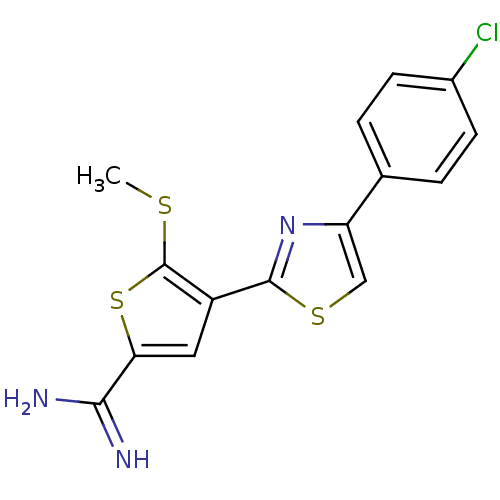
(4-[4-(4-Chloro-phenyl)-thiazol-2-yl]-5-methylsulfa...)Show InChI InChI=1S/C15H12ClN3S3/c1-20-15-10(6-12(22-15)13(17)18)14-19-11(7-21-14)8-2-4-9(16)5-3-8/h2-7H,1H3,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197091

(CHEMBL1738980)Show InChI InChI=1S/C17H12ClNO2/c1-10-15(17(20)21)16(11-5-3-2-4-6-11)13-9-12(18)7-8-14(13)19-10/h2-9H,1H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty acid-binding protein, heart
(Homo sapiens (Human)) | BDBM50197087

(CHEMBL3950316)Show SMILES Cc1cc(Cl)cc2c(-c3ccccc3)c(C(O)=O)c(nc12)N1CCCCC1 Show InChI InChI=1S/C22H21ClN2O2/c1-14-12-16(23)13-17-18(15-8-4-2-5-9-15)19(22(26)27)21(24-20(14)17)25-10-6-3-7-11-25/h2,4-5,8-9,12-13H,3,6-7,10-11H2,1H3,(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from human FABP3 after 30 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein 5
(Homo sapiens (Human)) | BDBM50197087

(CHEMBL3950316)Show SMILES Cc1cc(Cl)cc2c(-c3ccccc3)c(C(O)=O)c(nc12)N1CCCCC1 Show InChI InChI=1S/C22H21ClN2O2/c1-14-12-16(23)13-17-18(15-8-4-2-5-9-15)19(22(26)27)21(24-20(14)17)25-10-6-3-7-11-25/h2,4-5,8-9,12-13H,3,6-7,10-11H2,1H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from human N-terminal His6-tagged FABP5 (127 to 132 residues) expressed in Escherichia coli after 30 mins b... |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099915
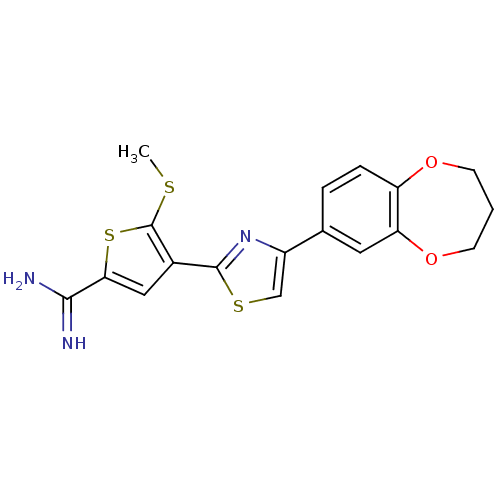
(4-[4-(3,4-Dihydro-2H-benzo[b][1,4]dioxepin-7-yl)-t...)Show SMILES CSc1sc(cc1-c1nc(cs1)-c1ccc2OCCCOc2c1)C(N)=N Show InChI InChI=1S/C18H17N3O2S3/c1-24-18-11(8-15(26-18)16(19)20)17-21-12(9-25-17)10-3-4-13-14(7-10)23-6-2-5-22-13/h3-4,7-9H,2,5-6H2,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099921

(5-Methylsulfanyl-4-(4-phenyl-thiazol-2-yl)-thiophe...)Show InChI InChI=1S/C15H13N3S3/c1-19-15-10(7-12(21-15)13(16)17)14-18-11(8-20-14)9-5-3-2-4-6-9/h2-8H,1H3,(H3,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human kidney cell urokinase |
Bioorg Med Chem Lett 12: 491-5 (2002)
BindingDB Entry DOI: 10.7270/Q2H994H5 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099900

(4-[4-(4-Chloro-phenyl)-thiazol-2-yl]-5-methylsulfa...)Show InChI InChI=1S/C15H12ClN3S3/c1-20-15-10(6-12(22-15)13(17)18)14-19-11(7-21-14)8-2-4-9(16)5-3-8/h2-7H,1H3,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human kidney cell urokinase |
Bioorg Med Chem Lett 12: 491-5 (2002)
BindingDB Entry DOI: 10.7270/Q2H994H5 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50109381

(5-Methyl-4-(4-phenyl-thiazol-2-yl)-thiophene-2-car...)Show InChI InChI=1S/C15H13N3S2/c1-9-11(7-13(20-9)14(16)17)15-18-12(8-19-15)10-5-3-2-4-6-10/h2-8H,1H3,(H3,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human kidney cell urokinase |
Bioorg Med Chem Lett 12: 491-5 (2002)
BindingDB Entry DOI: 10.7270/Q2H994H5 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, adipocyte
(Homo sapiens (Human)) | BDBM50197091

(CHEMBL1738980)Show InChI InChI=1S/C17H12ClNO2/c1-10-15(17(20)21)16(11-5-3-2-4-6-11)13-9-12(18)7-8-14(13)19-10/h2-9H,1H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from recombinant human His6-tagged FABP4 expressed in Escherichia coli after 30 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099896

(4-[4-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-thiazol-2...)Show InChI InChI=1S/C17H15N3O2S3/c1-23-17-10(7-14(25-17)15(18)19)16-20-11(8-24-16)9-2-3-12-13(6-9)22-5-4-21-12/h2-3,6-8H,4-5H2,1H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098141

(4-{2-[4-(4-Chloro-phenoxy)-phenylamino]-thiazol-4-...)Show SMILES CSc1sc(cc1-c1csc(Nc2ccc(Oc3ccc(Cl)cc3)cc2)n1)C(N)=N Show InChI InChI=1S/C21H17ClN4OS3/c1-28-20-16(10-18(30-20)19(23)24)17-11-29-21(26-17)25-13-4-8-15(9-5-13)27-14-6-2-12(22)3-7-14/h2-11H,1H3,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 915-8 (2001)
BindingDB Entry DOI: 10.7270/Q2J67G5B |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098127
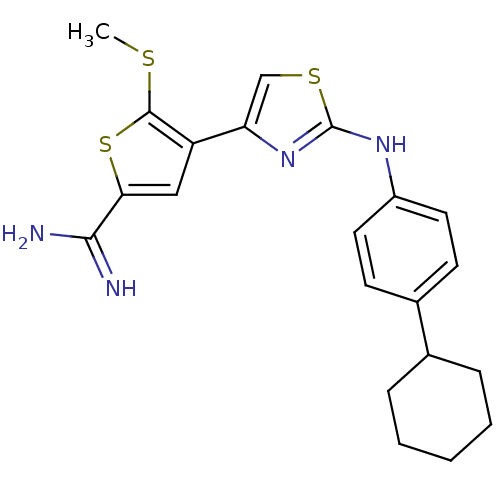
(4-[2-(4-Cyclohexyl-phenylamino)-thiazol-4-yl]-5-me...)Show SMILES CSc1sc(cc1-c1csc(Nc2ccc(cc2)C2CCCCC2)n1)C(N)=N Show InChI InChI=1S/C21H24N4S3/c1-26-20-16(11-18(28-20)19(22)23)17-12-27-21(25-17)24-15-9-7-14(8-10-15)13-5-3-2-4-6-13/h7-13H,2-6H2,1H3,(H3,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 915-8 (2001)
BindingDB Entry DOI: 10.7270/Q2J67G5B |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099903
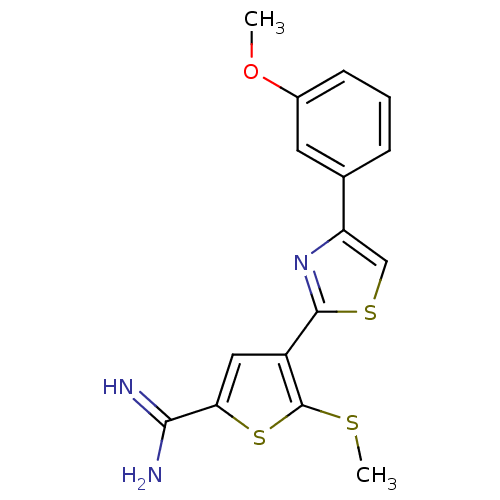
(4-[4-(3-Methoxy-phenyl)-thiazol-2-yl]-5-methylsulf...)Show InChI InChI=1S/C16H15N3OS3/c1-20-10-5-3-4-9(6-10)12-8-22-15(19-12)11-7-13(14(17)18)23-16(11)21-2/h3-8H,1-2H3,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein 5
(Homo sapiens (Human)) | BDBM50197086

(CHEMBL3971182)Show SMILES OC(=O)c1c(nc2c(Cl)cc(Cl)cc2c1-c1ccccc1)N1CCCCC1 Show InChI InChI=1S/C21H18Cl2N2O2/c22-14-11-15-17(13-7-3-1-4-8-13)18(21(26)27)20(24-19(15)16(23)12-14)25-9-5-2-6-10-25/h1,3-4,7-8,11-12H,2,5-6,9-10H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from human N-terminal His6-tagged FABP5 (127 to 132 residues) expressed in Escherichia coli after 30 mins b... |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099913
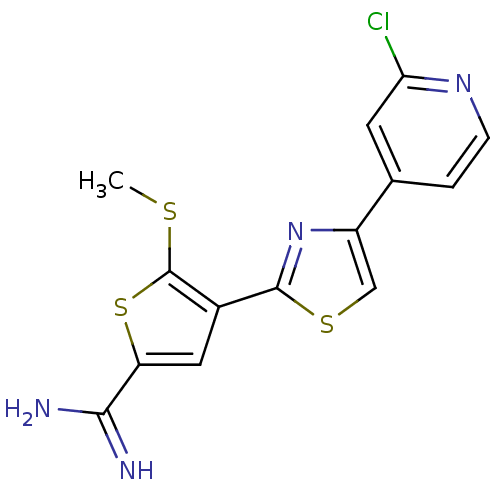
(4-[4-(2-Chloro-pyridin-4-yl)-thiazol-2-yl]-5-methy...)Show InChI InChI=1S/C14H11ClN4S3/c1-20-14-8(5-10(22-14)12(16)17)13-19-9(6-21-13)7-2-3-18-11(15)4-7/h2-6H,1H3,(H3,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099898
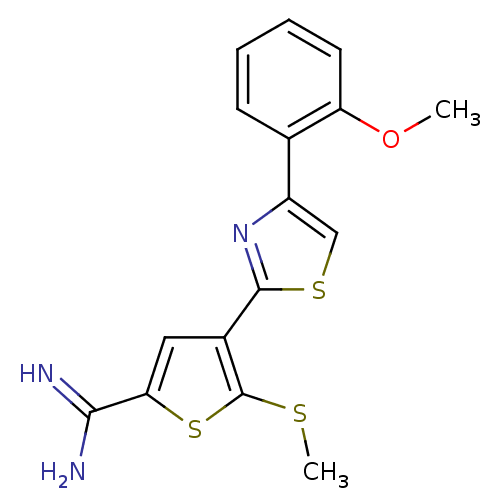
(4-[4-(2-Methoxy-phenyl)-thiazol-2-yl]-5-methylsulf...)Show InChI InChI=1S/C16H15N3OS3/c1-20-12-6-4-3-5-9(12)11-8-22-15(19-11)10-7-13(14(17)18)23-16(10)21-2/h3-8H,1-2H3,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein 5
(Homo sapiens (Human)) | BDBM50197090

(CHEMBL3970105)Show SMILES Clc1ccc2nc(N3CCCCC3)c(-c3n[nH]c(=S)o3)c(-c3ccccc3)c2c1 Show InChI InChI=1S/C22H19ClN4OS/c23-15-9-10-17-16(13-15)18(14-7-3-1-4-8-14)19(21-25-26-22(29)28-21)20(24-17)27-11-5-2-6-12-27/h1,3-4,7-10,13H,2,5-6,11-12H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labeled fatty acid from human N-terminal His6-tagged FABP5 (127 to 132 residues) expressed in Escherichia coli after 30 mins b... |
Bioorg Med Chem Lett 26: 5092-5097 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.071
BindingDB Entry DOI: 10.7270/Q25H7J69 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099936
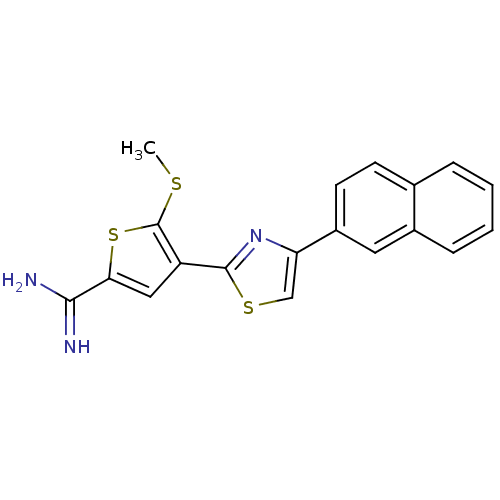
(5-Methylsulfanyl-4-(4-naphthalen-2-yl-thiazol-2-yl...)Show InChI InChI=1S/C19H15N3S3/c1-23-19-14(9-16(25-19)17(20)21)18-22-15(10-24-18)13-7-6-11-4-2-3-5-12(11)8-13/h2-10H,1H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50109378

(5-Ethyl-4-(4-phenyl-thiazol-2-yl)-thiophene-2-carb...)Show InChI InChI=1S/C16H15N3S2/c1-2-13-11(8-14(21-13)15(17)18)16-19-12(9-20-16)10-6-4-3-5-7-10/h3-9H,2H2,1H3,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human kidney cell urokinase |
Bioorg Med Chem Lett 12: 491-5 (2002)
BindingDB Entry DOI: 10.7270/Q2H994H5 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099935
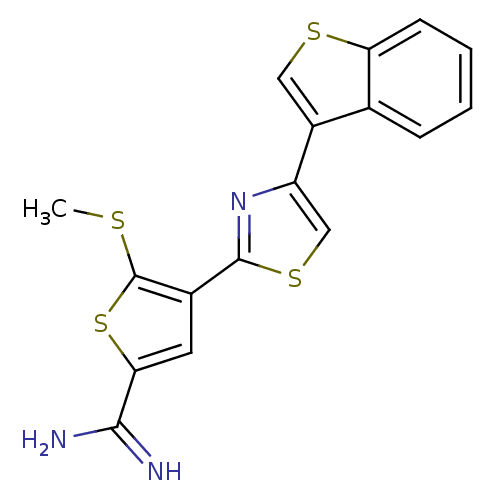
(4-(4-Benzo[b]thiophen-3-yl-thiazol-2-yl)-5-methyls...)Show InChI InChI=1S/C17H13N3S4/c1-21-17-10(6-14(24-17)15(18)19)16-20-12(8-23-16)11-7-22-13-5-3-2-4-9(11)13/h2-8H,1H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data