

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027663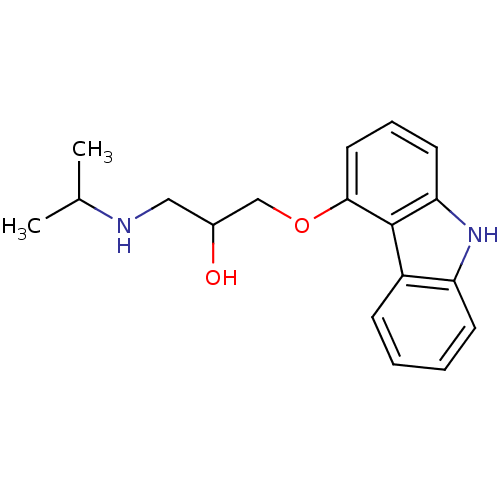 (1-(9H-Carbazol-4-yloxy)-3-isopropylamino-propan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DHA from human beta2 adrenoceptor by liquid scintillation counter | Bioorg Med Chem Lett 18: 5391-5 (2008) Article DOI: 10.1016/j.bmcl.2008.09.046 BindingDB Entry DOI: 10.7270/Q2FB52RP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50274012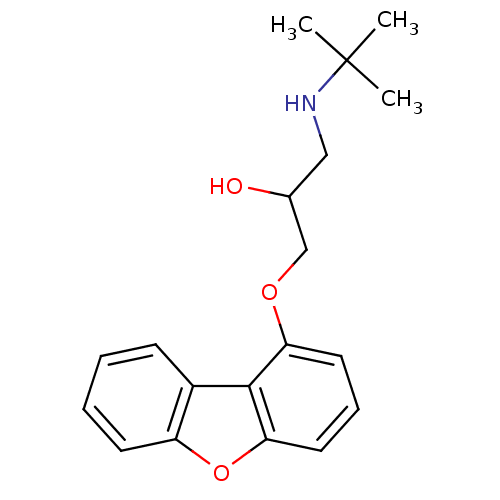 (1-tert-Butylamino-3-(dibenzofuran-1-yloxy)-propan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DHA from human beta2 adrenoceptor by liquid scintillation counter | Bioorg Med Chem Lett 18: 5391-5 (2008) Article DOI: 10.1016/j.bmcl.2008.09.046 BindingDB Entry DOI: 10.7270/Q2FB52RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50274013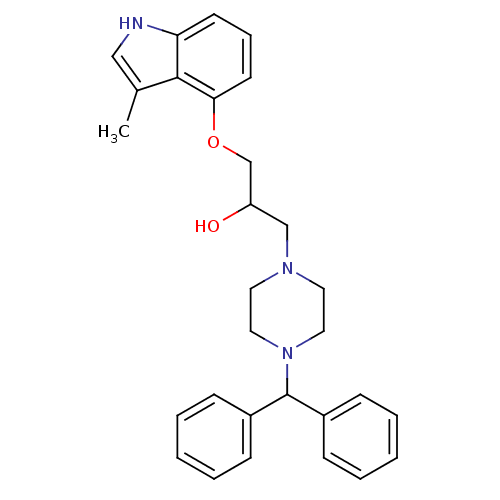 (1-(4-benzhydrylpiperazin-1-yl)-3-(3-methyl-1H-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DHA from human beta2 adrenoceptor by liquid scintillation counter | Bioorg Med Chem Lett 18: 5391-5 (2008) Article DOI: 10.1016/j.bmcl.2008.09.046 BindingDB Entry DOI: 10.7270/Q2FB52RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109106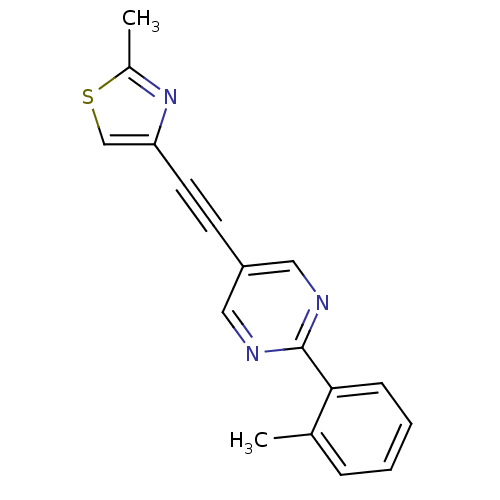 (US8609852, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine from mGlu5R in rat brain membranes | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109136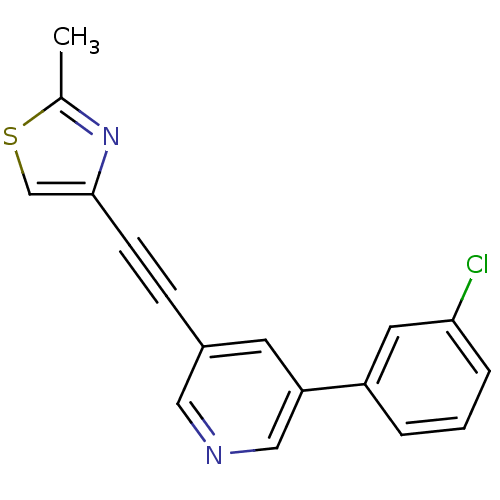 (US8609852, 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine from mGlu5R in rat brain membranes | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449332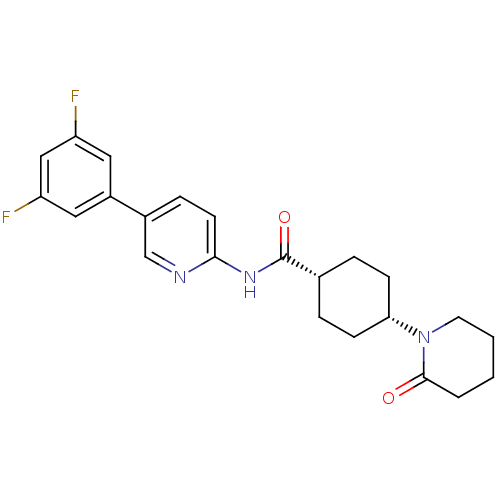 (CHEMBL3126041) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449331 (CHEMBL3126043) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50084137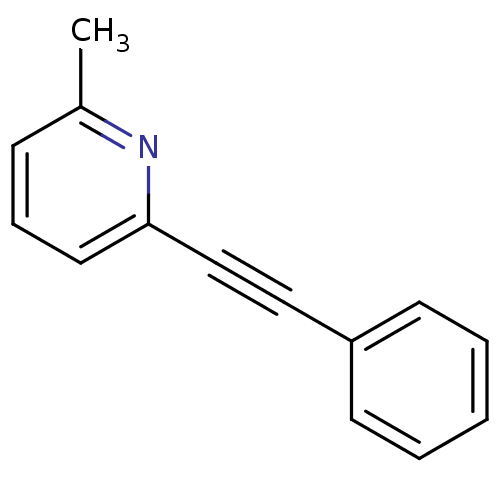 (2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]-M-MPEP from mGluR5 StaR domain (569 to 836 residues) (unknown origin) expressed in HEK293 cell membranes | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449330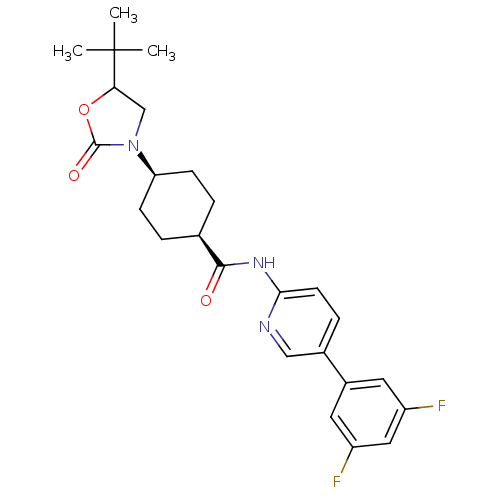 (CHEMBL3126045) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449327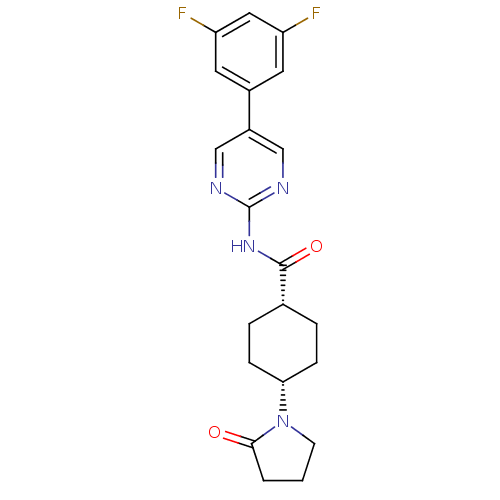 (CHEMBL3126039) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449328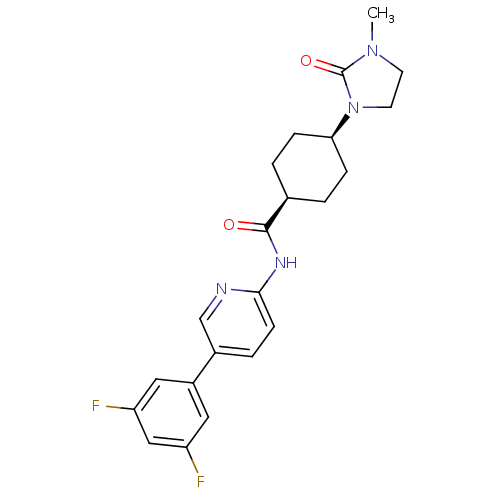 (CHEMBL3126047) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449329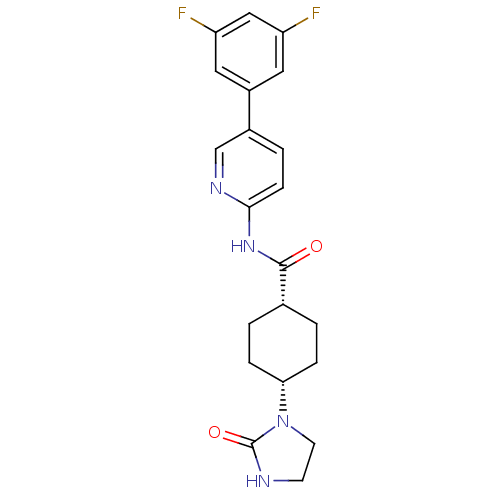 (CHEMBL3126046) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50443085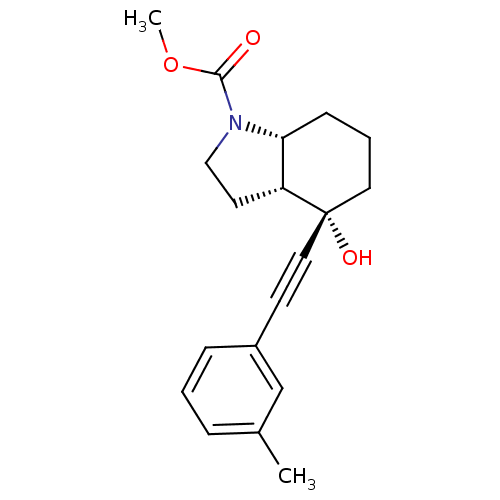 (AFQ056 | Mavoglurant) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]-M-MPEP from mGluR5 StaR domain (569 to 836 residues) (unknown origin) expressed in HEK293 cell membranes | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449326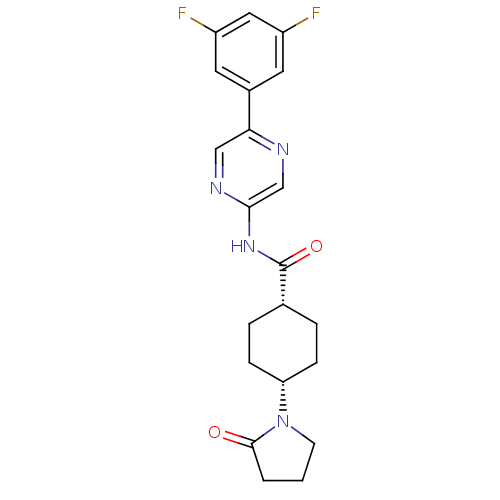 (CHEMBL3126040) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50143540 (CHEMBL3759750) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPgamma from mGluR5 in rat cerebral cortex membranes after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449325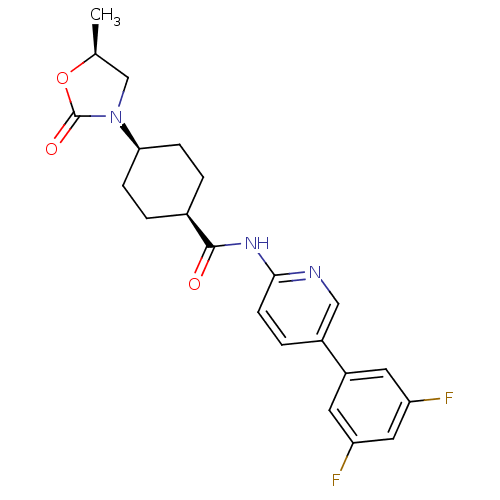 (CHEMBL3126044) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449323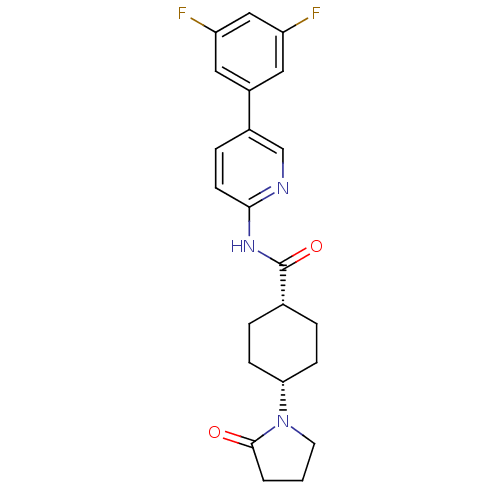 (CHEMBL3126053) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449324 (CHEMBL3126042) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449321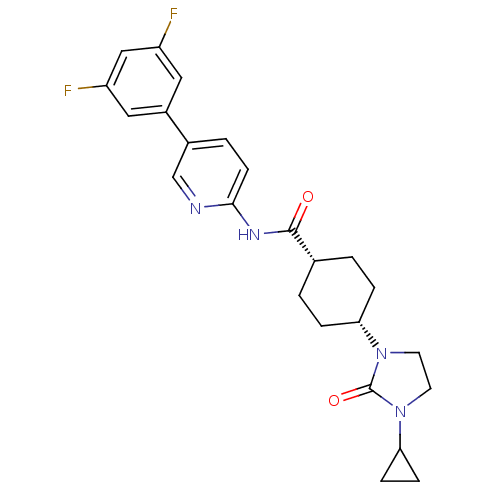 (CHEMBL3126052) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449322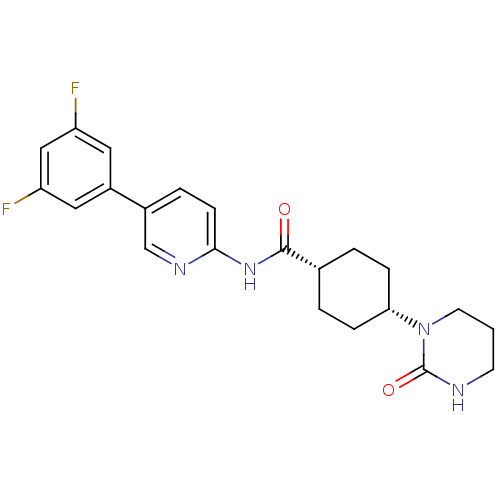 (CHEMBL3126048) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449320 (CHEMBL3126050) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50154977 (2-{2-[3-fluoro-5-(pyridin-3-yloxy)phenyl]-2H-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5R in rat cortical membranes by liquid scintillation spectrometric analysis | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449319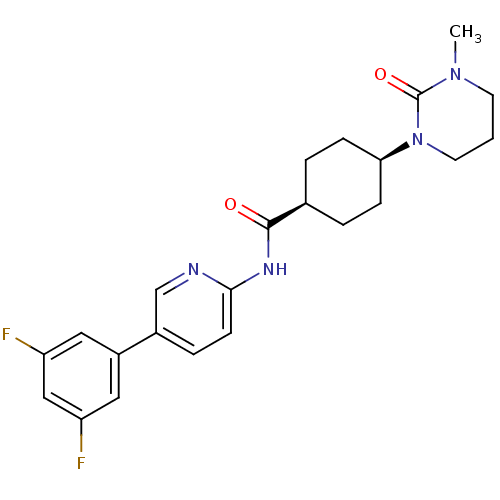 (CHEMBL3126049) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50122758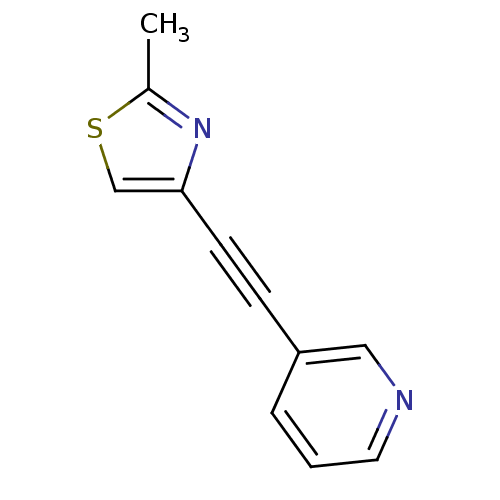 (2-methyl-4-(pyridin-3-ylethynyl)thiazole | 3-(2-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5R in rat cortical membranes by liquid scintillation spectrometric analysis | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50274014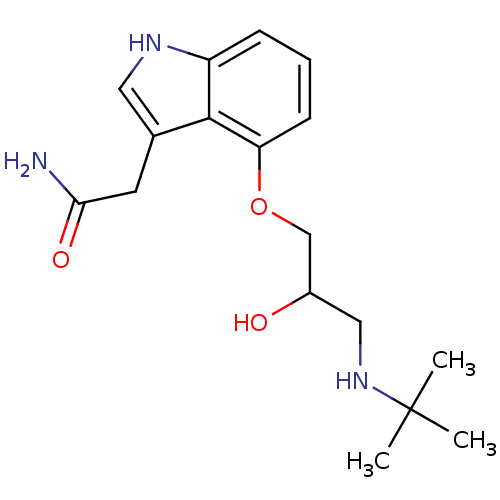 (2-(4-(3-(tert-butylamino)-2-hydroxypropoxy)-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DHA from human beta2 adrenoceptor by liquid scintillation counter | Bioorg Med Chem Lett 18: 5391-5 (2008) Article DOI: 10.1016/j.bmcl.2008.09.046 BindingDB Entry DOI: 10.7270/Q2FB52RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449318 (CHEMBL3126051) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50001689 (4-(3-(4-benzhydrylpiperazin-1-yl)-2-hydroxypropoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DHA from human beta2 adrenoceptor by liquid scintillation counter | Bioorg Med Chem Lett 18: 5391-5 (2008) Article DOI: 10.1016/j.bmcl.2008.09.046 BindingDB Entry DOI: 10.7270/Q2FB52RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449317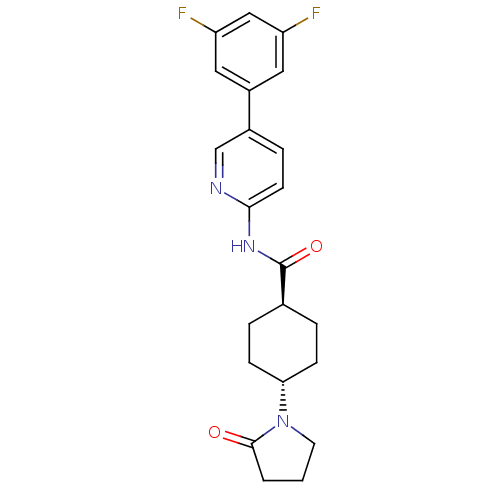 (CHEMBL3126054) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50449316 (CHEMBL3126038) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation counting | Bioorg Med Chem Lett 24: 1458-61 (2014) Article DOI: 10.1016/j.bmcl.2014.02.023 BindingDB Entry DOI: 10.7270/Q2S75HT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50143544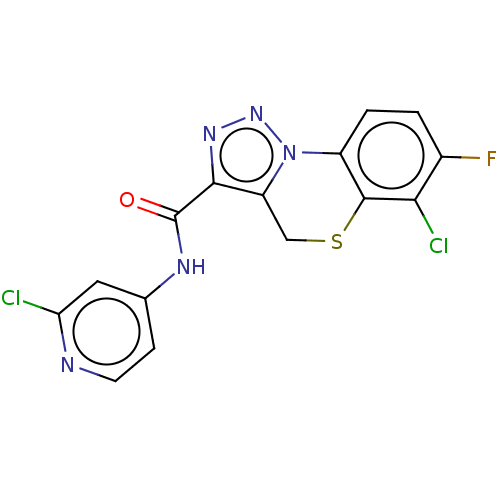 (CHEMBL3759766) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Antagonist activity at human mGluR5 expressed in CHO cells assessed as inhibition of glutamate-induced effect by aequorin bioluminescence assay | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106090 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((4-methoxy-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106091 ((S)-3-Benzo[1,3]dioxol-5-yl-3-((S)-4-methyl-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106083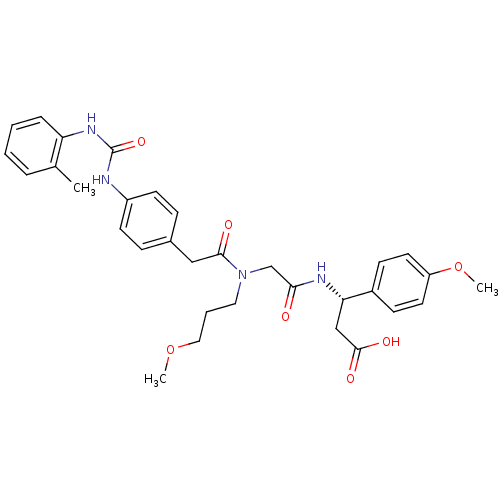 ((S)-3-(4-Methoxy-phenyl)-3-[2-((3-methoxy-propyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50143541 (CHEMBL3759977) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Antagonist activity at human mGluR5d by fluo-3-based FLIPR assay | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085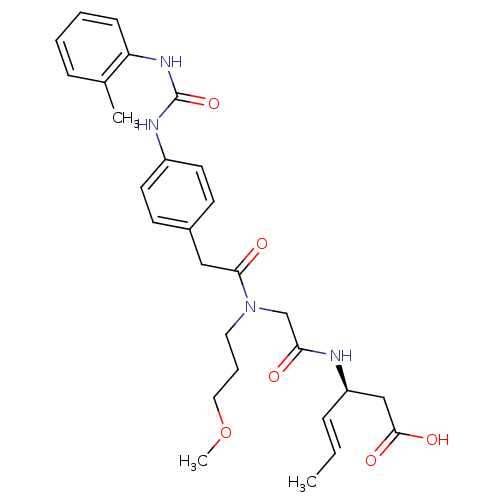 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by mouse Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to fibronectin by Very late antigen 4 (VLA-4) | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50143539 (CHEMBL3758266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Antagonist activity at mGluR5 (unknown origin) | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50143542 (CHEMBL3758851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]-MPEP (2-methyl-6-(phenylethynyl)pyridine) from mGlu5R in Sprague-Dawley rat cortical membranes after 60 mins by scintillation co... | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106082 ((S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to fibronectin by Very late antigen 5 (VLA-5) | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106082 ((S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to fibronectin by Very late antigen 4 (VLA-4) | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106098 ((R)-3-((S)-4-Methyl-2-{2-[4-(3-o-tolyl-ureido)-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106093 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methyl-butyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50143538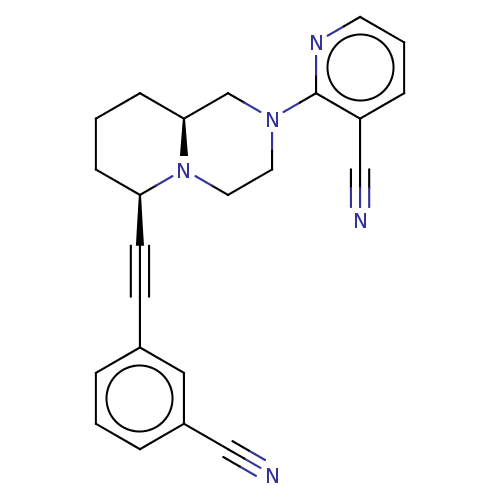 (CHEMBL3759888) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Antagonist activity at human mGluR5d by fluo-3-based FLIPR assay | Bioorg Med Chem Lett 26: 484-94 (2016) Article DOI: 10.1016/j.bmcl.2015.11.087 BindingDB Entry DOI: 10.7270/Q21C1ZQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by rat Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by rat Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106082 ((S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by mouse Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L/Intercellular adhesion molecule 1 (Homo sapiens (Human)) | BDBM50161080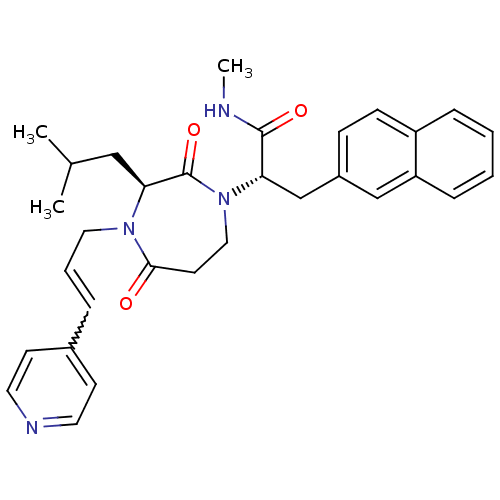 ((S)-2-[(S)-3-Isobutyl-2,5-dioxo-4-((E)-3-pyridin-4...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description In vitro inhibitory concentrationfor Lymphocyte function associated antigen 1/Intercellular adhesion molecule 1 (LFA-1/ICAM-1) | Bioorg Med Chem Lett 15: 1217-20 (2005) Article DOI: 10.1016/j.bmcl.2004.11.072 BindingDB Entry DOI: 10.7270/Q20Z72S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 315 total ) | Next | Last >> |