 Found 48 hits with Last Name = 'sadoun' and Initial = 'f'
Found 48 hits with Last Name = 'sadoun' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
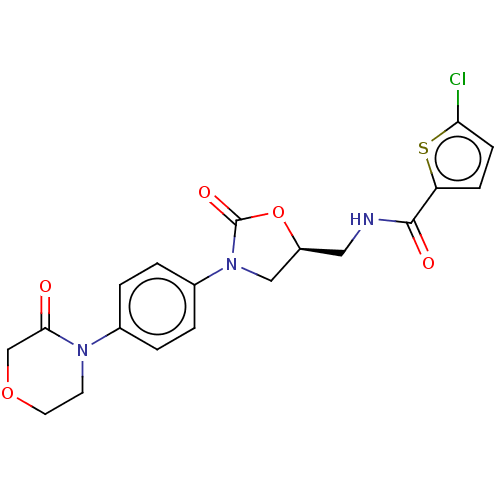
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of factor-10a (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443853
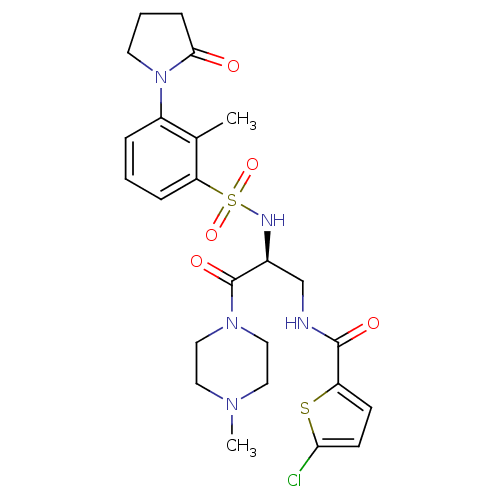
(CHEMBL3091519 | US20230391761, Reference 1)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O5S2/c1-16-18(30-10-4-7-22(30)31)5-3-6-20(16)37(34,35)27-17(24(33)29-13-11-28(2)12-14-29)15-26-23(32)19-8-9-21(25)36-19/h3,5-6,8-9,17,27H,4,7,10-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112086
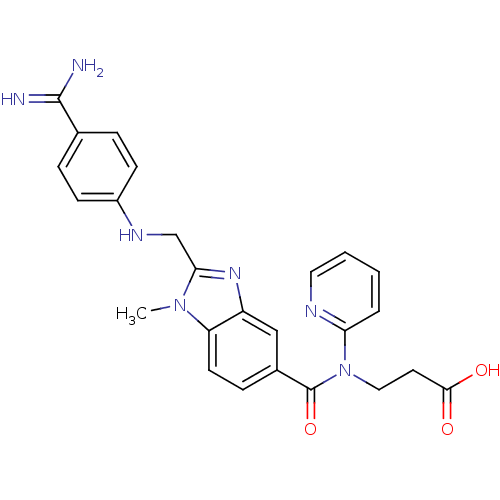
(3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C(=O)N(CCC(O)=O)c1ccccn1 Show InChI InChI=1S/C25H25N7O3/c1-31-20-10-7-17(25(35)32(13-11-23(33)34)21-4-2-3-12-28-21)14-19(20)30-22(31)15-29-18-8-5-16(6-9-18)24(26)27/h2-10,12,14,29H,11,13,15H2,1H3,(H3,26,27)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of factor-10a (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50443853

(CHEMBL3091519 | US20230391761, Reference 1)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O5S2/c1-16-18(30-10-4-7-22(30)31)5-3-6-20(16)37(34,35)27-17(24(33)29-13-11-28(2)12-14-29)15-26-23(32)19-8-9-21(25)36-19/h3,5-6,8-9,17,27H,4,7,10-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of t-PA (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443855

(CHEMBL3091517)Show SMILES [#6]-[#6](=O)-[#7]-c1c(cccc1S(=O)(=O)[#7]-[#6@@H](-[#6]-[#6]-[#6]-c1ccc(-[#7])cn1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](/F)F)-c1ccccc1 |r| Show InChI InChI=1S/C30H33F2N5O4S/c1-20(38)35-28-25(21-7-3-2-4-8-21)10-6-12-27(28)42(40,41)36-26(11-5-9-24-14-13-23(33)19-34-24)30(39)37-17-15-22(16-18-37)29(31)32/h2-4,6-8,10,12-14,19,26,36H,5,9,11,15-18,33H2,1H3,(H,35,38)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of factor-10a (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50443853

(CHEMBL3091519 | US20230391761, Reference 1)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O5S2/c1-16-18(30-10-4-7-22(30)31)5-3-6-20(16)37(34,35)27-17(24(33)29-13-11-28(2)12-14-29)15-26-23(32)19-8-9-21(25)36-19/h3,5-6,8-9,17,27H,4,7,10-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50323009

(1'-(5-(4-chlorophenyl)-4-(2,4-dichlorophenyl)-1-me...)Show SMILES Cn1c(cc(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)N1CCC(CC1)(N1CCCCC1)C(N)=O Show InChI InChI=1S/C29H31Cl3N4O2/c1-34-25(27(37)35-15-11-29(12-16-35,28(33)38)36-13-3-2-4-14-36)18-23(22-10-9-21(31)17-24(22)32)26(34)19-5-7-20(30)8-6-19/h5-10,17-18H,2-4,11-16H2,1H3,(H2,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor in rat brain synaptosomal membranes |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443857
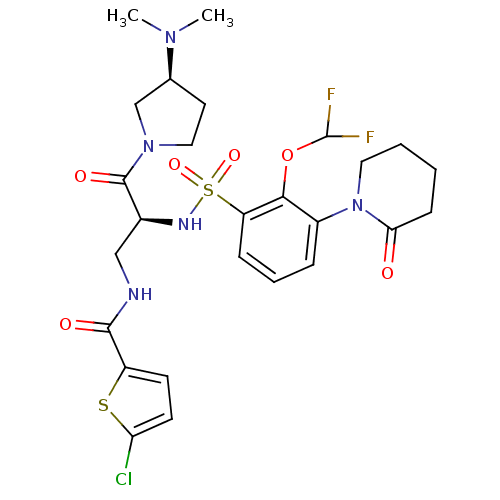
(CHEMBL3091501)Show SMILES CN(C)[C@H]1CCN(C1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCCC2=O)c1OC(F)F |r| Show InChI InChI=1S/C26H32ClF2N5O6S2/c1-32(2)16-11-13-33(15-16)25(37)17(14-30-24(36)19-9-10-21(27)41-19)31-42(38,39)20-7-5-6-18(23(20)40-26(28)29)34-12-4-3-8-22(34)35/h5-7,9-10,16-17,26,31H,3-4,8,11-15H2,1-2H3,(H,30,36)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50323003
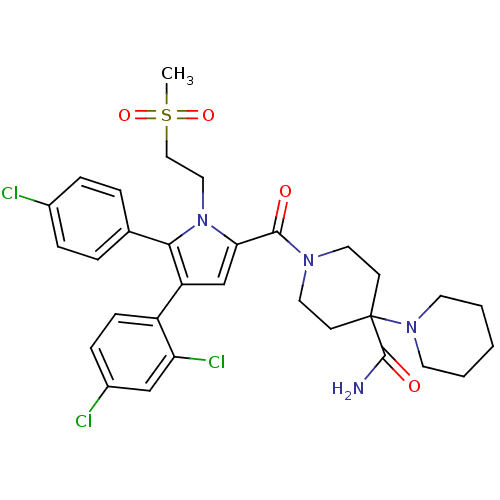
(1'-(5-(4-chlorophenyl)-4-(2,4-dichlorophenyl)-1-(2...)Show SMILES CS(=O)(=O)CCn1c(cc(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)N1CCC(CC1)(N1CCCCC1)C(N)=O Show InChI InChI=1S/C31H35Cl3N4O4S/c1-43(41,42)18-17-38-27(29(39)36-15-11-31(12-16-36,30(35)40)37-13-3-2-4-14-37)20-25(24-10-9-23(33)19-26(24)34)28(38)21-5-7-22(32)8-6-21/h5-10,19-20H,2-4,11-18H2,1H3,(H2,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor in rat brain synaptosomal membranes |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443858
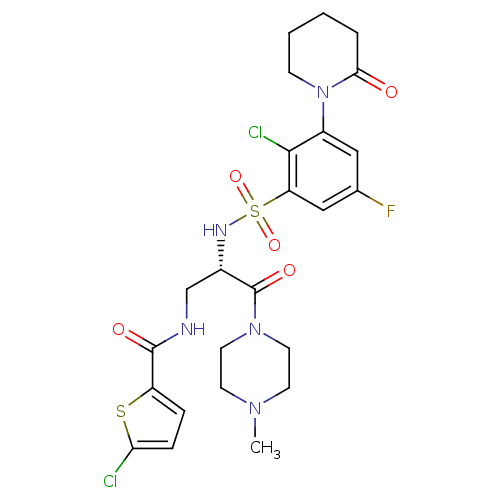
(CHEMBL3091502)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cc(F)cc(N2CCCCC2=O)c1Cl |r| Show InChI InChI=1S/C24H28Cl2FN5O5S2/c1-30-8-10-31(11-9-30)24(35)16(14-28-23(34)18-5-6-20(25)38-18)29-39(36,37)19-13-15(27)12-17(22(19)26)32-7-3-2-4-21(32)33/h5-6,12-13,16,29H,2-4,7-11,14H2,1H3,(H,28,34)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443846
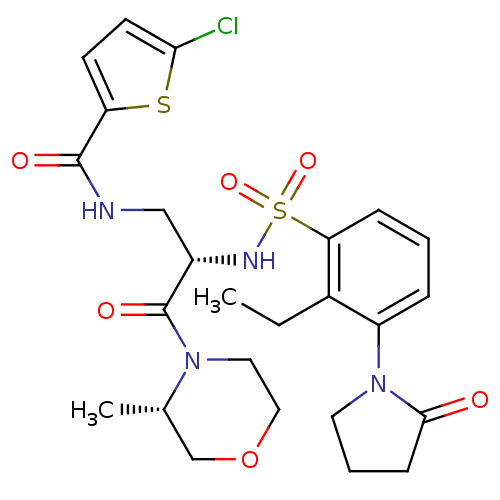
(CHEMBL3091527)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCOC[C@@H]1C)N1CCCC1=O |r| Show InChI InChI=1S/C25H31ClN4O6S2/c1-3-17-19(30-11-5-8-23(30)31)6-4-7-21(17)38(34,35)28-18(25(33)29-12-13-36-15-16(29)2)14-27-24(32)20-9-10-22(26)37-20/h4,6-7,9-10,16,18,28H,3,5,8,11-15H2,1-2H3,(H,27,32)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443847
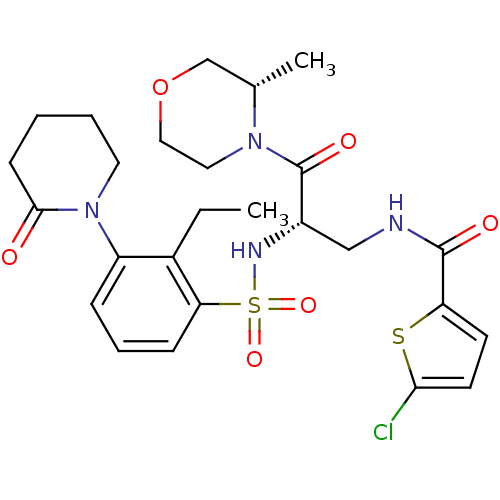
(CHEMBL3091526)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCOC[C@@H]1C)N1CCCCC1=O |r| Show InChI InChI=1S/C26H33ClN4O6S2/c1-3-18-20(31-12-5-4-9-24(31)32)7-6-8-22(18)39(35,36)29-19(26(34)30-13-14-37-16-17(30)2)15-28-25(33)21-10-11-23(27)38-21/h6-8,10-11,17,19,29H,3-5,9,12-16H2,1-2H3,(H,28,33)/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50323007
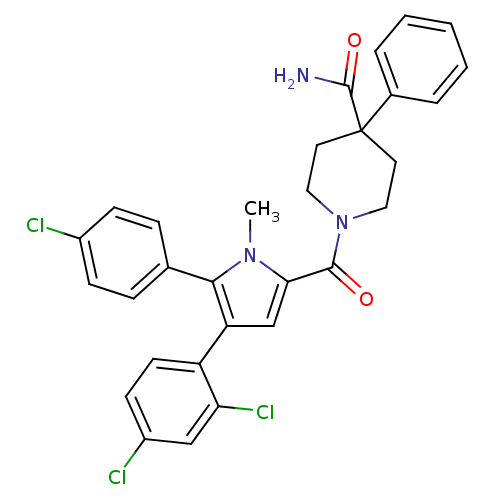
(1-(5-(4-chlorophenyl)-4-(2,4-dichlorophenyl)-1-met...)Show SMILES Cn1c(cc(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)N1CCC(CC1)(C(N)=O)c1ccccc1 Show InChI InChI=1S/C30H26Cl3N3O2/c1-35-26(28(37)36-15-13-30(14-16-36,29(34)38)20-5-3-2-4-6-20)18-24(23-12-11-22(32)17-25(23)33)27(35)19-7-9-21(31)10-8-19/h2-12,17-18H,13-16H2,1H3,(H2,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor in rat brain synaptosomal membranes |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443861
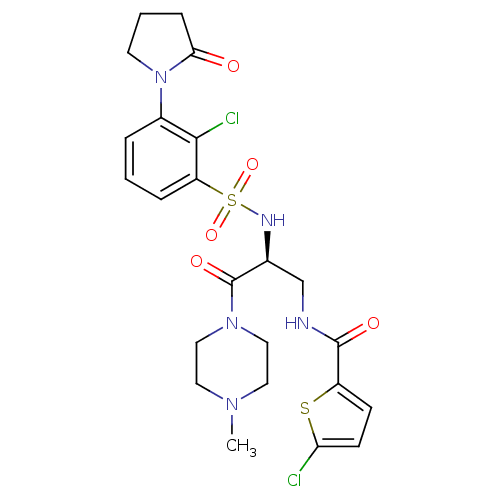
(CHEMBL3091515)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1Cl |r| Show InChI InChI=1S/C23H27Cl2N5O5S2/c1-28-10-12-29(13-11-28)23(33)15(14-26-22(32)17-7-8-19(24)36-17)27-37(34,35)18-5-2-4-16(21(18)25)30-9-3-6-20(30)31/h2,4-5,7-8,15,27H,3,6,9-14H2,1H3,(H,26,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443844

(CHEMBL3091506)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCOCC1)N1CCOCC1=O |r| Show InChI InChI=1S/C24H29ClN4O7S2/c1-2-16-18(29-10-13-36-15-22(29)30)4-3-5-20(16)38(33,34)27-17(24(32)28-8-11-35-12-9-28)14-26-23(31)19-6-7-21(25)37-19/h3-7,17,27H,2,8-15H2,1H3,(H,26,31)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443853

(CHEMBL3091519 | US20230391761, Reference 1)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O5S2/c1-16-18(30-10-4-7-22(30)31)5-3-6-20(16)37(34,35)27-17(24(33)29-13-11-28(2)12-14-29)15-26-23(32)19-8-9-21(25)36-19/h3,5-6,8-9,17,27H,4,7,10-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50323006
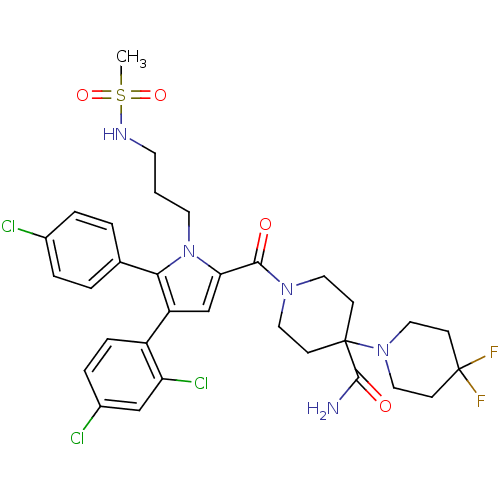
(1'-(5-(4-chlorophenyl)-4-(2,4-dichlorophenyl)-1-(3...)Show SMILES CS(=O)(=O)NCCCn1c(cc(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)N1CCC(CC1)(N1CCC(F)(F)CC1)C(N)=O Show InChI InChI=1S/C32H36Cl3F2N5O4S/c1-47(45,46)39-13-2-14-42-27(20-25(24-8-7-23(34)19-26(24)35)28(42)21-3-5-22(33)6-4-21)29(43)40-15-9-31(10-16-40,30(38)44)41-17-11-32(36,37)12-18-41/h3-8,19-20,39H,2,9-18H2,1H3,(H2,38,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor in rat brain synaptosomal membranes |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443848

(CHEMBL3091525)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCOC[C@@H]1C)-c1ccccn1 |r| Show InChI InChI=1S/C26H29ClN4O5S2/c1-3-18-19(20-8-4-5-12-28-20)7-6-9-23(18)38(34,35)30-21(26(33)31-13-14-36-16-17(31)2)15-29-25(32)22-10-11-24(27)37-22/h4-12,17,21,30H,3,13-16H2,1-2H3,(H,29,32)/t17-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443869
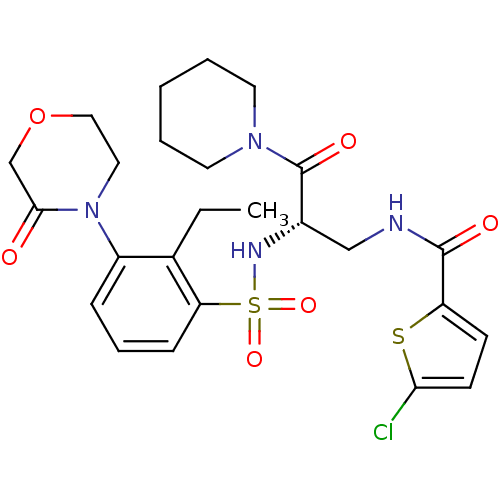
(CHEMBL3091507)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCCCC1)N1CCOCC1=O |r| Show InChI InChI=1S/C25H31ClN4O6S2/c1-2-17-19(30-13-14-36-16-23(30)31)7-6-8-21(17)38(34,35)28-18(25(33)29-11-4-3-5-12-29)15-27-24(32)20-9-10-22(26)37-20/h6-10,18,28H,2-5,11-16H2,1H3,(H,27,32)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443862
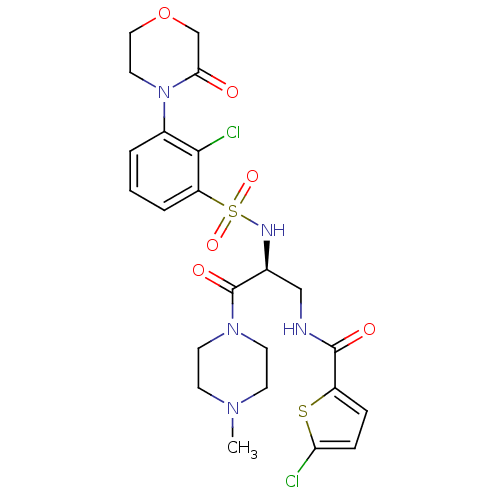
(CHEMBL3091514)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCOCC2=O)c1Cl |r| Show InChI InChI=1S/C23H27Cl2N5O6S2/c1-28-7-9-29(10-8-28)23(33)15(13-26-22(32)17-5-6-19(24)37-17)27-38(34,35)18-4-2-3-16(21(18)25)30-11-12-36-14-20(30)31/h2-6,15,27H,7-14H2,1H3,(H,26,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443867
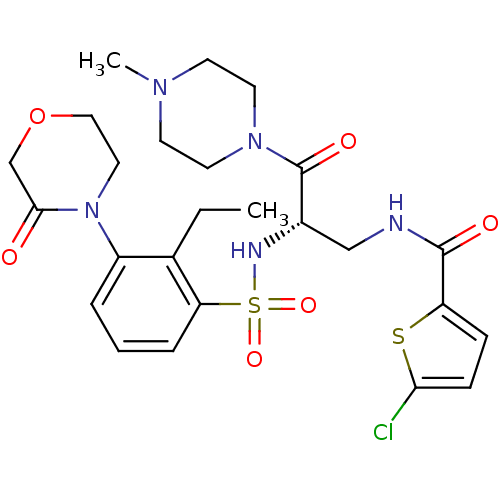
(CHEMBL3091509)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCN(C)CC1)N1CCOCC1=O |r| Show InChI InChI=1S/C25H32ClN5O6S2/c1-3-17-19(31-13-14-37-16-23(31)32)5-4-6-21(17)39(35,36)28-18(25(34)30-11-9-29(2)10-12-30)15-27-24(33)20-7-8-22(26)38-20/h4-8,18,28H,3,9-16H2,1-2H3,(H,27,33)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50323004

(1'-(5-(4-chlorophenyl)-4-(2,4-dichlorophenyl)-1-(3...)Show SMILES CS(=O)(=O)NCCCn1c(cc(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)N1CCC(CC1)(N1CCCCC1)C(N)=O Show InChI InChI=1S/C32H38Cl3N5O4S/c1-45(43,44)37-14-5-17-40-28(30(41)38-18-12-32(13-19-38,31(36)42)39-15-3-2-4-16-39)21-26(25-11-10-24(34)20-27(25)35)29(40)22-6-8-23(33)9-7-22/h6-11,20-21,37H,2-5,12-19H2,1H3,(H2,36,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor in rat brain synaptosomal membranes |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443865
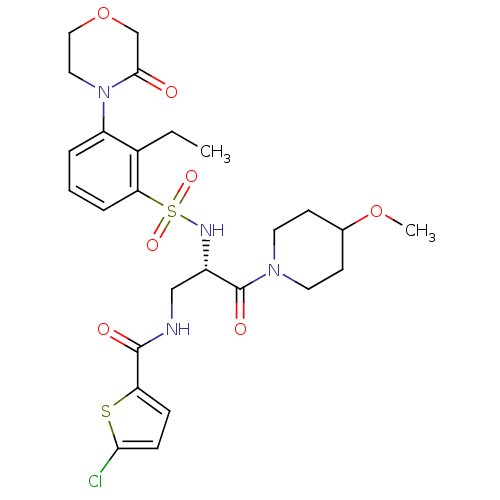
(CHEMBL3091511)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCC(CC1)OC)N1CCOCC1=O |r| Show InChI InChI=1S/C26H33ClN4O7S2/c1-3-18-20(31-13-14-38-16-24(31)32)5-4-6-22(18)40(35,36)29-19(15-28-25(33)21-7-8-23(27)39-21)26(34)30-11-9-17(37-2)10-12-30/h4-8,17,19,29H,3,9-16H2,1-2H3,(H,28,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50323006

(1'-(5-(4-chlorophenyl)-4-(2,4-dichlorophenyl)-1-(3...)Show SMILES CS(=O)(=O)NCCCn1c(cc(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)N1CCC(CC1)(N1CCC(F)(F)CC1)C(N)=O Show InChI InChI=1S/C32H36Cl3F2N5O4S/c1-47(45,46)39-13-2-14-42-27(20-25(24-8-7-23(34)19-26(24)35)28(42)21-3-5-22(33)6-4-21)29(43)40-15-9-31(10-16-40,30(38)44)41-17-11-32(36,37)12-18-41/h3-8,19-20,39H,2,9-18H2,1H3,(H2,38,44) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443864
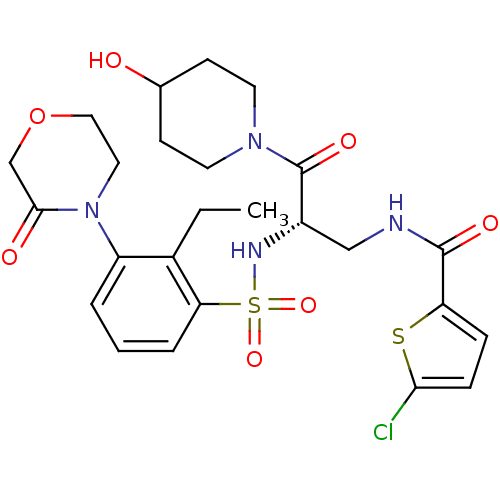
(CHEMBL3091512)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCC(O)CC1)N1CCOCC1=O |r| Show InChI InChI=1S/C25H31ClN4O7S2/c1-2-17-19(30-12-13-37-15-23(30)32)4-3-5-21(17)39(35,36)28-18(25(34)29-10-8-16(31)9-11-29)14-27-24(33)20-6-7-22(26)38-20/h3-7,16,18,28,31H,2,8-15H2,1H3,(H,27,33)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443845
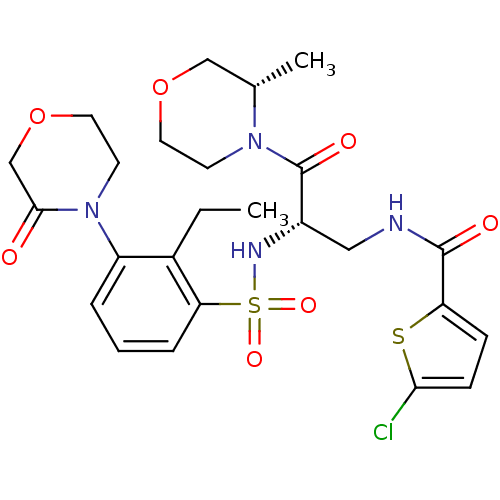
(CHEMBL3091528)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCOC[C@@H]1C)N1CCOCC1=O |r| Show InChI InChI=1S/C25H31ClN4O7S2/c1-3-17-19(30-10-12-37-15-23(30)31)5-4-6-21(17)39(34,35)28-18(25(33)29-9-11-36-14-16(29)2)13-27-24(32)20-7-8-22(26)38-20/h4-8,16,18,28H,3,9-15H2,1-2H3,(H,27,32)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443856
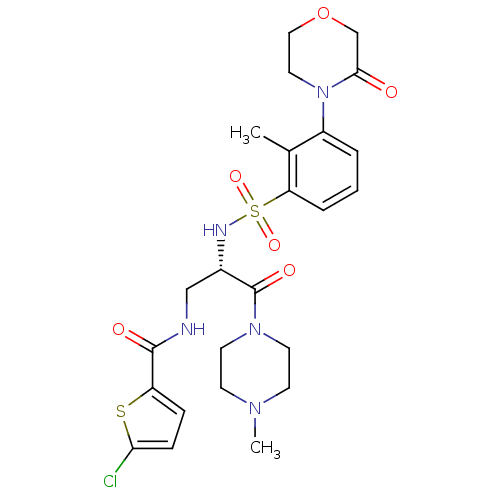
(CHEMBL3091503)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCOCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O6S2/c1-16-18(30-12-13-36-15-22(30)31)4-3-5-20(16)38(34,35)27-17(24(33)29-10-8-28(2)9-11-29)14-26-23(32)19-6-7-21(25)37-19/h3-7,17,27H,8-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443866
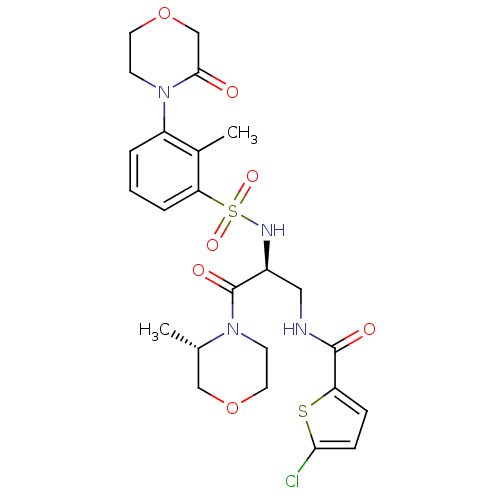
(CHEMBL3091510)Show SMILES C[C@H]1COCCN1C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCOCC2=O)c1C |r| Show InChI InChI=1S/C24H29ClN4O7S2/c1-15-13-35-10-8-28(15)24(32)17(12-26-23(31)19-6-7-21(25)37-19)27-38(33,34)20-5-3-4-18(16(20)2)29-9-11-36-14-22(29)30/h3-7,15,17,27H,8-14H2,1-2H3,(H,26,31)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor in rat brain synaptosomal membranes |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443860
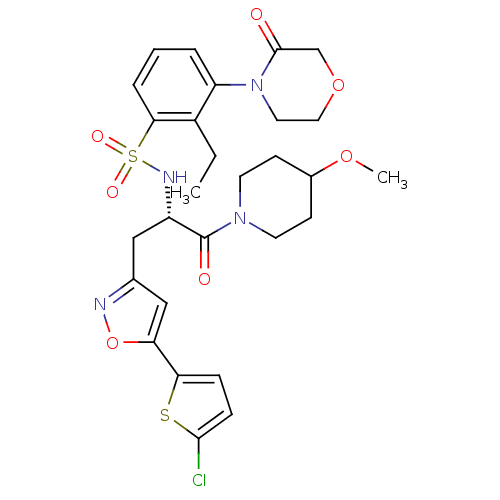
(CHEMBL3091505)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](Cc1cc(on1)-c1ccc(Cl)s1)C(=O)N1CCC(CC1)OC)N1CCOCC1=O |r| Show InChI InChI=1S/C28H33ClN4O7S2/c1-3-20-22(33-13-14-39-17-27(33)34)5-4-6-25(20)42(36,37)31-21(28(35)32-11-9-19(38-2)10-12-32)15-18-16-23(40-30-18)24-7-8-26(29)41-24/h4-8,16,19,21,31H,3,9-15,17H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328705
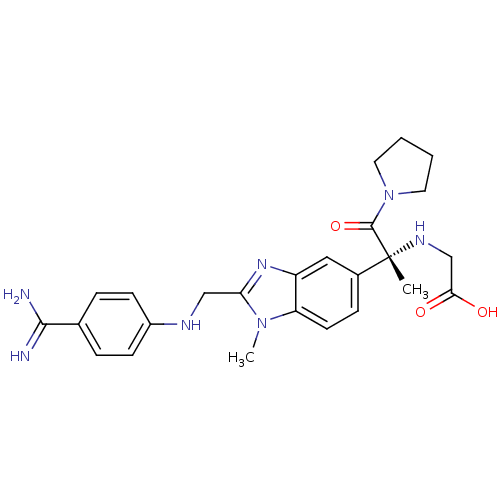
(CHEMBL1270156 | Tanogitran)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)[C@@](C)(NCC(O)=O)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C25H31N7O3/c1-25(29-15-22(33)34,24(35)32-11-3-4-12-32)17-7-10-20-19(13-17)30-21(31(20)2)14-28-18-8-5-16(6-9-18)23(26)27/h5-10,13,28-29H,3-4,11-12,14-15H2,1-2H3,(H3,26,27)(H,33,34)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of factor-10a (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443863
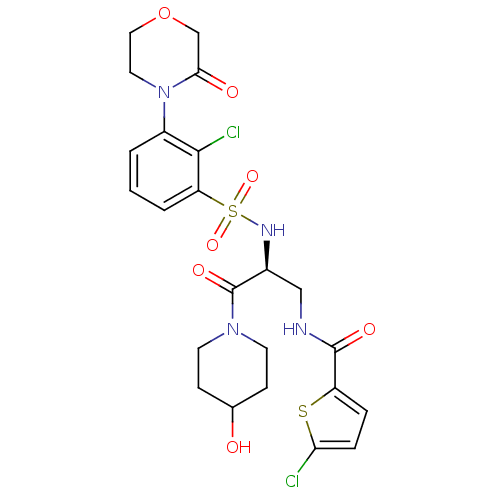
(CHEMBL3091513)Show SMILES OC1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCOCC2=O)c1Cl |r| Show InChI InChI=1S/C23H26Cl2N4O7S2/c24-19-5-4-17(37-19)22(32)26-12-15(23(33)28-8-6-14(30)7-9-28)27-38(34,35)18-3-1-2-16(21(18)25)29-10-11-36-13-20(29)31/h1-5,14-15,27,30H,6-13H2,(H,26,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443868

(CHEMBL3091508)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCC(C)CC1)N1CCOCC1=O |r| Show InChI InChI=1S/C26H33ClN4O6S2/c1-3-18-20(31-13-14-37-16-24(31)32)5-4-6-22(18)39(35,36)29-19(26(34)30-11-9-17(2)10-12-30)15-28-25(33)21-7-8-23(27)38-21/h4-8,17,19,29H,3,9-16H2,1-2H3,(H,28,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443859

(CHEMBL3091504)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](Cc1cc(on1)-c1ccc(Cl)s1)C(=O)N1CCC(O)CC1)N1CCOCC1=O |r| Show InChI InChI=1S/C27H31ClN4O7S2/c1-2-19-21(32-12-13-38-16-26(32)34)4-3-5-24(19)41(36,37)30-20(27(35)31-10-8-18(33)9-11-31)14-17-15-22(39-29-17)23-6-7-25(28)40-23/h3-7,15,18,20,30,33H,2,8-14,16H2,1H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50323005

(1'-(5-(4-chlorophenyl)-4-(2,4-dichlorophenyl)-1-(3...)Show SMILES CC1(C)CCN(CC1)C1(CCN(CC1)C(=O)c1cc(c(-c2ccc(Cl)cc2)n1CCCNS(C)(=O)=O)-c1ccc(Cl)cc1Cl)C(N)=O Show InChI InChI=1S/C34H42Cl3N5O4S/c1-33(2)11-19-41(20-12-33)34(32(38)44)13-17-40(18-14-34)31(43)29-22-27(26-10-9-25(36)21-28(26)37)30(23-5-7-24(35)8-6-23)42(29)16-4-15-39-47(3,45)46/h5-10,21-22,39H,4,11-20H2,1-3H3,(H2,38,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor in rat brain synaptosomal membranes |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50323010
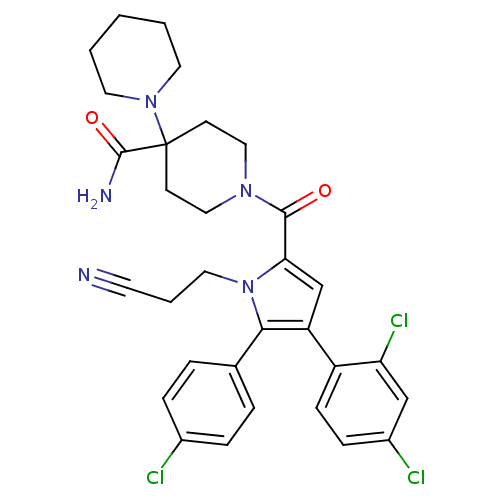
(1'-(5-(4-chlorophenyl)-1-(2-cyanoethyl)-4-(2,4-dic...)Show SMILES NC(=O)C1(CCN(CC1)C(=O)c1cc(c(-c2ccc(Cl)cc2)n1CCC#N)-c1ccc(Cl)cc1Cl)N1CCCCC1 Show InChI InChI=1S/C31H32Cl3N5O2/c32-22-7-5-21(6-8-22)28-25(24-10-9-23(33)19-26(24)34)20-27(39(28)16-4-13-35)29(40)37-17-11-31(12-18-37,30(36)41)38-14-2-1-3-15-38/h5-10,19-20H,1-4,11-12,14-18H2,(H2,36,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor in rat brain synaptosomal membranes |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443849
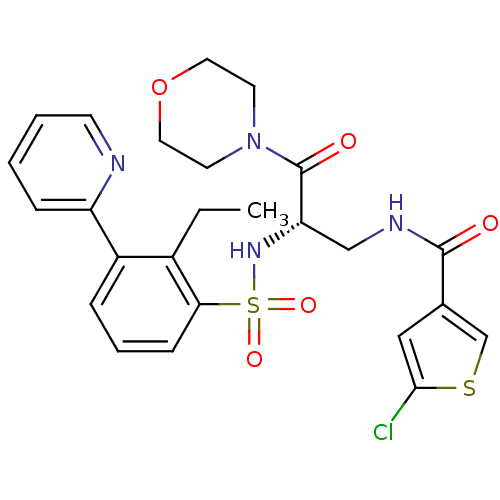
(CHEMBL3091524)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1csc(Cl)c1)C(=O)N1CCOCC1)-c1ccccn1 |r| Show InChI InChI=1S/C25H27ClN4O5S2/c1-2-18-19(20-7-3-4-9-27-20)6-5-8-22(18)37(33,34)29-21(25(32)30-10-12-35-13-11-30)15-28-24(31)17-14-23(26)36-16-17/h3-9,14,16,21,29H,2,10-13,15H2,1H3,(H,28,31)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17298
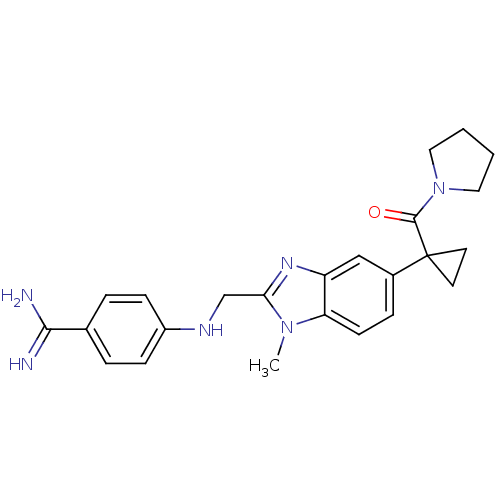
(4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C24H28N6O/c1-29-20-9-6-17(24(10-11-24)23(31)30-12-2-3-13-30)14-19(20)28-21(29)15-27-18-7-4-16(5-8-18)22(25)26/h4-9,14,27H,2-3,10-13,15H2,1H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of factor-10a (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50323008
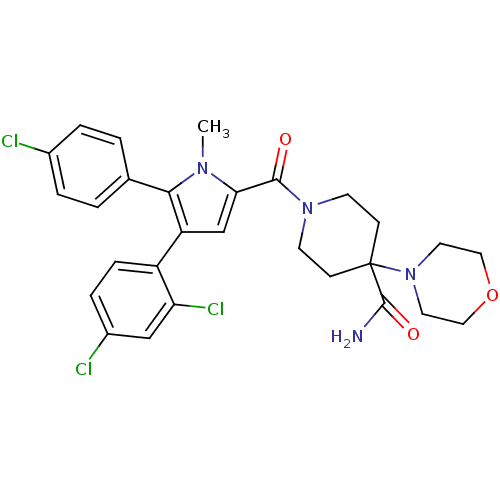
(1-(5-(4-chlorophenyl)-4-(2,4-dichlorophenyl)-1-met...)Show SMILES Cn1c(cc(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)N1CCC(CC1)(N1CCOCC1)C(N)=O Show InChI InChI=1S/C28H29Cl3N4O3/c1-33-24(26(36)34-10-8-28(9-11-34,27(32)37)35-12-14-38-15-13-35)17-22(21-7-6-20(30)16-23(21)31)25(33)18-2-4-19(29)5-3-18/h2-7,16-17H,8-15H2,1H3,(H2,32,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor in rat brain synaptosomal membranes |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443851
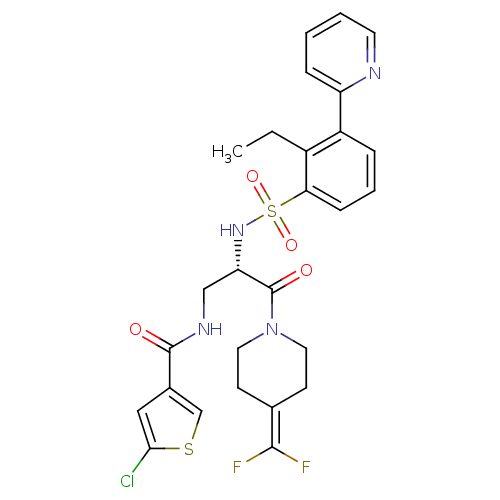
(CHEMBL3091521)Show SMILES [#6]-[#6]-c1c(cccc1S(=O)(=O)[#7]-[#6@@H](-[#6]-[#7]-[#6](=O)-c1csc(Cl)c1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](/F)F)-c1ccccn1 |r| Show InChI InChI=1S/C27H27ClF2N4O4S2/c1-2-19-20(21-7-3-4-11-31-21)6-5-8-23(19)40(37,38)33-22(15-32-26(35)18-14-24(28)39-16-18)27(36)34-12-9-17(10-13-34)25(29)30/h3-8,11,14,16,22,33H,2,9-10,12-13,15H2,1H3,(H,32,35)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50129976
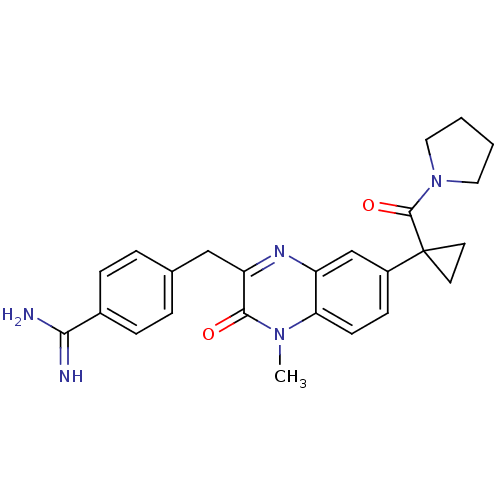
(4-{4-Methyl-3-oxo-7-[1-(pyrrolidine-1-carbonyl)-cy...)Show SMILES Cn1c2ccc(cc2nc(Cc2ccc(cc2)C(N)=N)c1=O)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C25H27N5O2/c1-29-21-9-8-18(25(10-11-25)24(32)30-12-2-3-13-30)15-19(21)28-20(23(29)31)14-16-4-6-17(7-5-16)22(26)27/h4-9,15H,2-3,10-14H2,1H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of factor-10a (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443852

(CHEMBL3091520)Show SMILES [#6]-c1c(cccc1S(=O)(=O)[#7]-[#6@@H](-[#6]-[#7]-[#6](=O)-c1csc(Cl)c1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](/F)F)-c1ccccn1 |r| Show InChI InChI=1S/C26H25ClF2N4O4S2/c1-16-19(20-6-2-3-10-30-20)5-4-7-22(16)39(36,37)32-21(14-31-25(34)18-13-23(27)38-15-18)26(35)33-11-8-17(9-12-33)24(28)29/h2-7,10,13,15,21,32H,8-9,11-12,14H2,1H3,(H,31,34)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50323002

(5-(5-(4'-carbamoyl-1,4'-bipiperidine-1'-carbonyl)-...)Show SMILES CC(C)(CCCn1c(cc(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)N1CCC(CC1)(N1CCCCC1)C(N)=O)C(O)=O Show InChI InChI=1S/C35H41Cl3N4O4/c1-34(2,33(45)46)13-6-18-42-29(31(43)40-19-14-35(15-20-40,32(39)44)41-16-4-3-5-17-41)22-27(26-12-11-25(37)21-28(26)38)30(42)23-7-9-24(36)10-8-23/h7-12,21-22H,3-6,13-20H2,1-2H3,(H2,39,44)(H,45,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor in rat brain synaptosomal membranes |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50323001
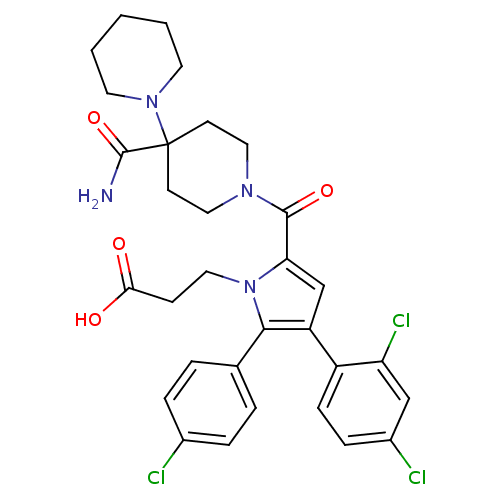
(3-(5-(4'-carbamoyl-1,4'-bipiperidine-1'-carbonyl)-...)Show SMILES NC(=O)C1(CCN(CC1)C(=O)c1cc(c(-c2ccc(Cl)cc2)n1CCC(O)=O)-c1ccc(Cl)cc1Cl)N1CCCCC1 Show InChI InChI=1S/C31H33Cl3N4O4/c32-21-6-4-20(5-7-21)28-24(23-9-8-22(33)18-25(23)34)19-26(38(28)15-10-27(39)40)29(41)36-16-11-31(12-17-36,30(35)42)37-13-2-1-3-14-37/h4-9,18-19H,1-3,10-17H2,(H2,35,42)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor in rat brain synaptosomal membranes |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377608
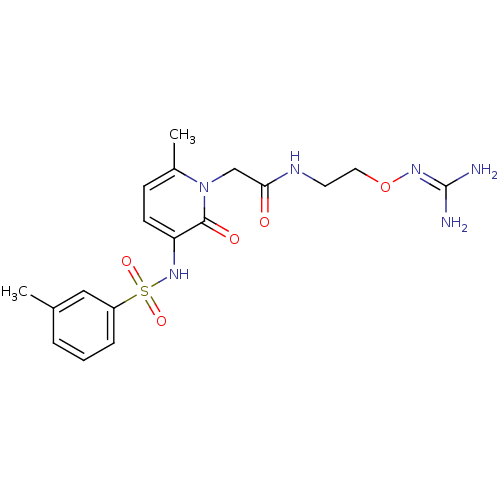
(CHEMBL256941)Show SMILES [#6]-c1cccc(c1)S(=O)(=O)[#7]-c1ccc(-[#6])n(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1=O Show InChI InChI=1S/C18H24N6O5S/c1-12-4-3-5-14(10-12)30(27,28)23-15-7-6-13(2)24(17(15)26)11-16(25)21-8-9-29-22-18(19)20/h3-7,10,23H,8-9,11H2,1-2H3,(H,21,25)(H4,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of factor-10a (unknown origin) |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443850
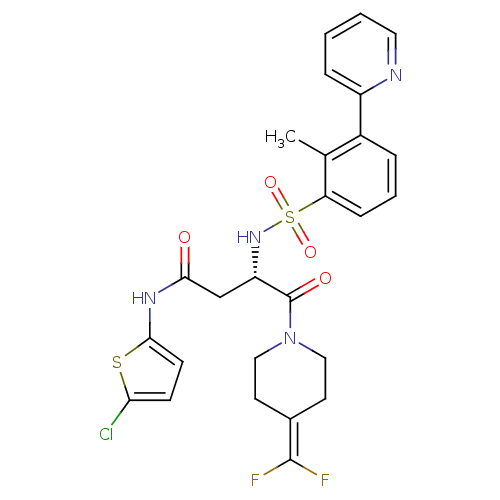
(CHEMBL3091523)Show SMILES [#6]-c1c(cccc1S(=O)(=O)[#7]-[#6@@H](-[#6]-[#6](=O)-[#7]-c1ccc(Cl)s1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](/F)F)-c1ccccn1 |r| Show InChI InChI=1S/C26H25ClF2N4O4S2/c1-16-18(19-6-2-3-12-30-19)5-4-7-21(16)39(36,37)32-20(15-23(34)31-24-9-8-22(27)38-24)26(35)33-13-10-17(11-14-33)25(28)29/h2-9,12,20,32H,10-11,13-15H2,1H3,(H,31,34)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443854
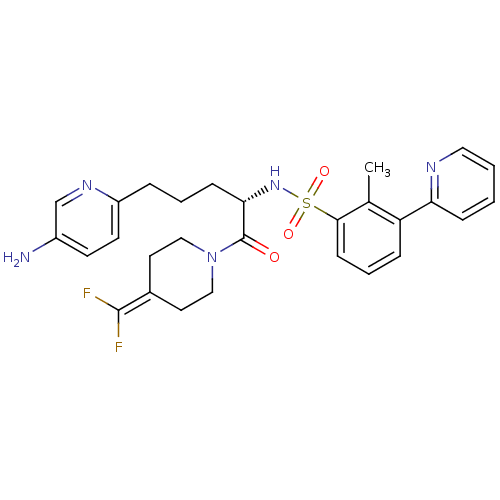
(CHEMBL3091518)Show SMILES [#6]-c1c(cccc1S(=O)(=O)[#7]-[#6@@H](-[#6]-[#6]-[#6]-c1ccc(-[#7])cn1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](/F)F)-c1ccccn1 |r| Show InChI InChI=1S/C28H31F2N5O3S/c1-19-23(24-8-2-3-15-32-24)7-5-10-26(19)39(37,38)34-25(9-4-6-22-12-11-21(31)18-33-22)28(36)35-16-13-20(14-17-35)27(29)30/h2-3,5,7-8,10-12,15,18,25,34H,4,6,9,13-14,16-17,31H2,1H3/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50323006

(1'-(5-(4-chlorophenyl)-4-(2,4-dichlorophenyl)-1-(3...)Show SMILES CS(=O)(=O)NCCCn1c(cc(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)N1CCC(CC1)(N1CCC(F)(F)CC1)C(N)=O Show InChI InChI=1S/C32H36Cl3F2N5O4S/c1-47(45,46)39-13-2-14-42-27(20-25(24-8-7-23(34)19-26(24)35)28(42)21-3-5-22(33)6-4-21)29(43)40-15-9-31(10-16-40,30(38)44)41-17-11-32(36,37)12-18-41/h3-8,19-20,39H,2,9-18H2,1H3,(H2,38,44) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at CB1 receptor in forskolin-stimulated human U373-MG cells assessed as inhibition of CP-55940-induced cAMP accumulation |
Bioorg Med Chem Lett 20: 4573-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.017
BindingDB Entry DOI: 10.7270/Q28G8KV6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data