

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004780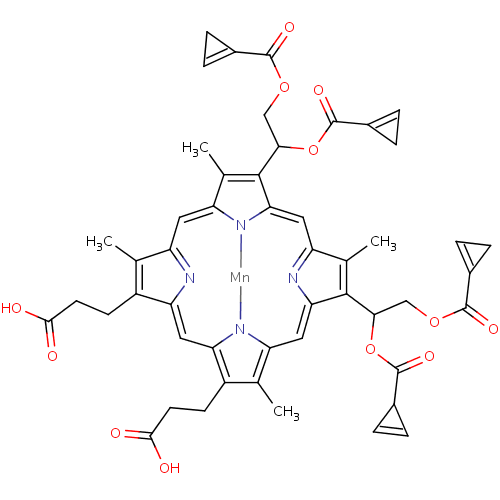 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity against HIV-1 protease | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004781 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity against HIV-1 protease | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50212827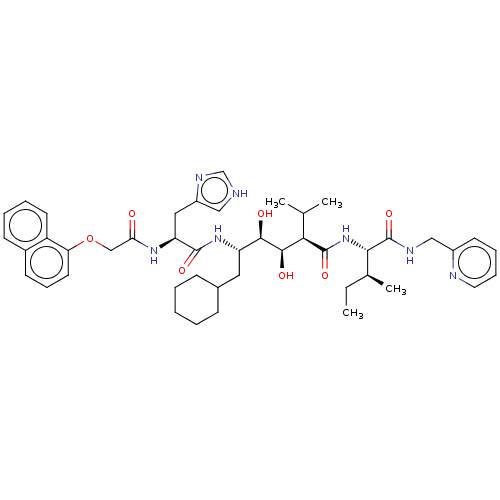 (CHEMBL3350189) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576972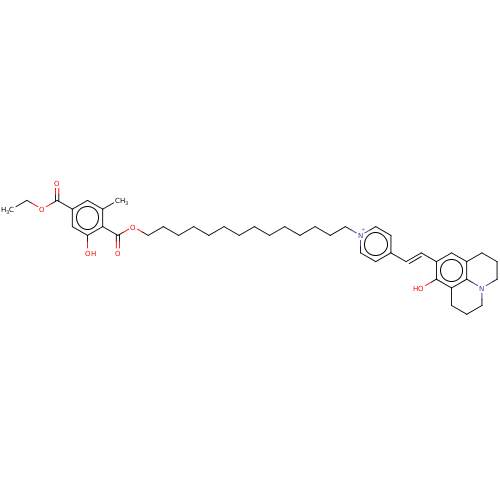 (CHEMBL4879019) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pol polyprotein (Human immunodeficiency virus 2) | BDBM50212827 (CHEMBL3350189) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576974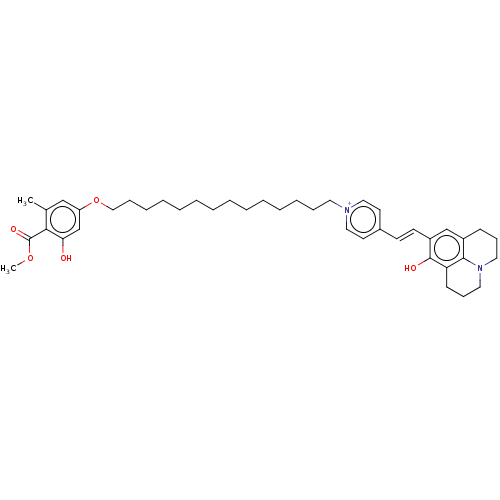 (CHEMBL4872515) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50004778 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 protease | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004778 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 protease in the absence of DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004780 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 protease in the absence of DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576973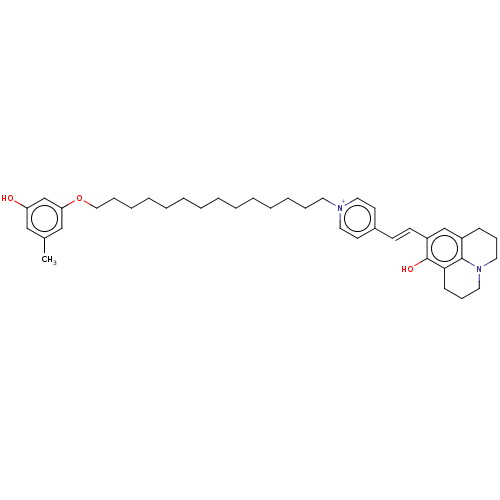 (CHEMBL4857072) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004778 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 protease in the absence of DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004778 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50004778 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-2 protease | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004789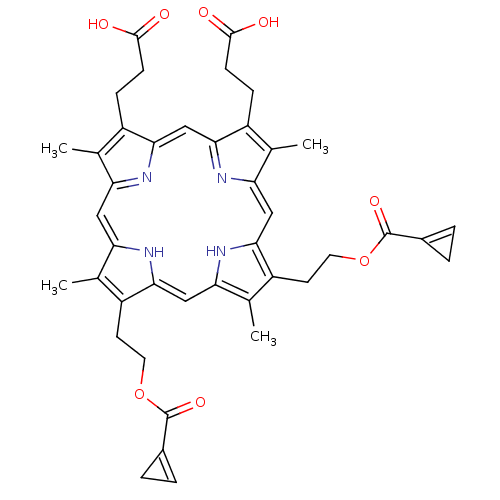 (3-[8,13-Bis-(2-acetoxy-ethyl)-18-(2-carboxy-ethyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576971 (CHEMBL4846979) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004780 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50004781 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-2 protease in the absence of DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50004783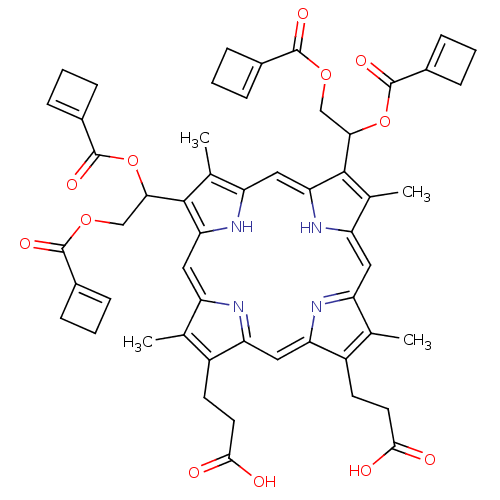 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-2 protease in the absence of DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pol polyprotein (Human immunodeficiency virus 2) | BDBM50004778 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-2 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pol polyprotein (Human immunodeficiency virus 2) | BDBM50004778 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-2 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50004780 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 10% fetal calf serum(FCS) | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004783 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576979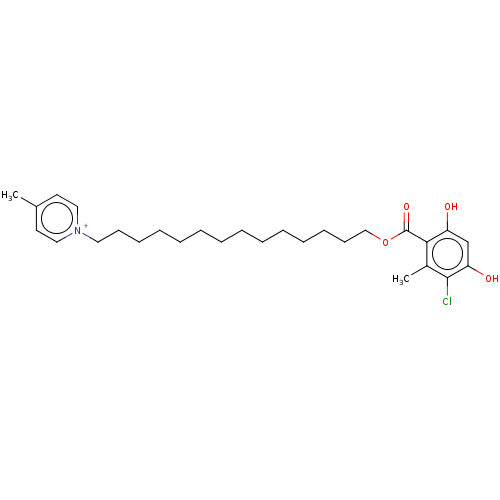 (CHEMBL4878802) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004781 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 725 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50212827 (CHEMBL3350189) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 10% fetal calf serum(FCS) | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576976 (CHEMBL4857860) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576978 (CHEMBL4846471) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004781 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 975 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 10% fetal calf serum(FCS) | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pol polyprotein (Human immunodeficiency virus 2) | BDBM50004789 (3-[8,13-Bis-(2-acetoxy-ethyl)-18-(2-carboxy-ethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-2 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50004780 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description In vitro Inhibitory activity against HIV-2 protease in the presence of 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pol polyprotein (Human immunodeficiency virus 2) | BDBM50004788 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-2 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pol polyprotein (Human immunodeficiency virus 2) | BDBM50004783 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-2 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576980 (CHEMBL4846671) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pol polyprotein (Human immunodeficiency virus 2) | BDBM50004781 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-2 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004788 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50004778 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against renin | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A-5 (Homo sapiens (Human)) | BDBM50004778 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against pepsin | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576975 (CHEMBL4873996) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004786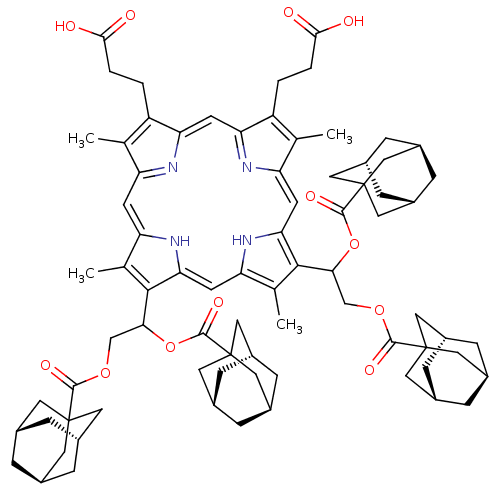 (CHEMBL268410 | Porphyrin analogue) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576977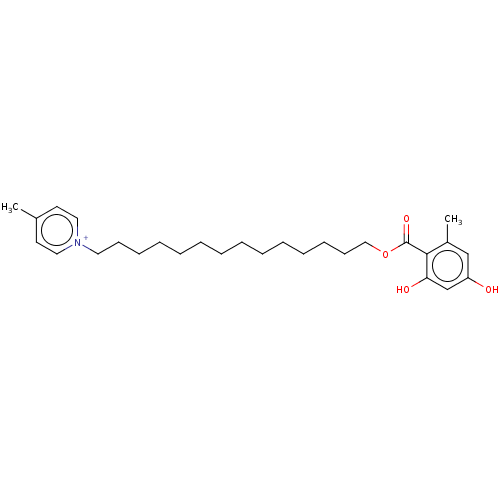 (CHEMBL4866499) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004787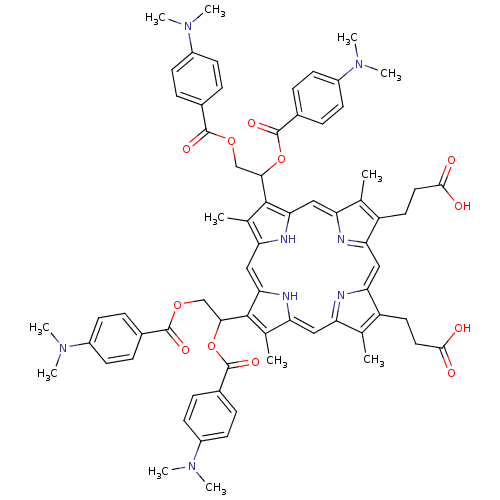 (CHEMBL442513 | Porphyrin analogue) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity against HIV-1 protease | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50004778 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004783 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 10% fetal calf serum(FCS) | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004783 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-2 protease | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pol polyprotein (Human immunodeficiency virus 2) | BDBM50004786 (CHEMBL268410 | Porphyrin analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-2 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004790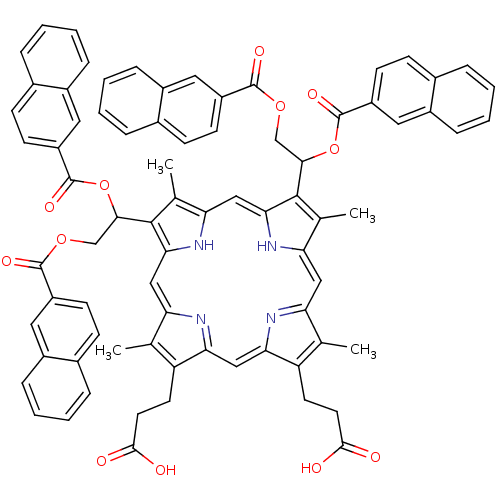 (CHEMBL384612 | Porphyrin analogue) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004785 (CHEMBL217693 | Porphyrin analogue) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pol polyprotein (Human immunodeficiency virus 2) | BDBM50004787 (CHEMBL442513 | Porphyrin analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-2 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50040573 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description In vitro inhibition of recombinant HIV-1 protease expressed in E. coli strain D1210 | J Med Chem 37: 665-73 (1994) BindingDB Entry DOI: 10.7270/Q2WM1CG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pol polyprotein (Human immunodeficiency virus 2) | BDBM50004790 (CHEMBL384612 | Porphyrin analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-2 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 83 total ) | Next | Last >> |