 Found 790 hits with Last Name = 'sasaki' and Initial = 'k'
Found 790 hits with Last Name = 'sasaki' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769
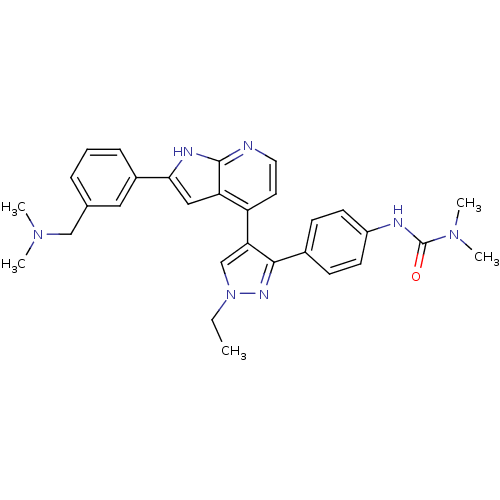
(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora B ATP binding site by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM82372
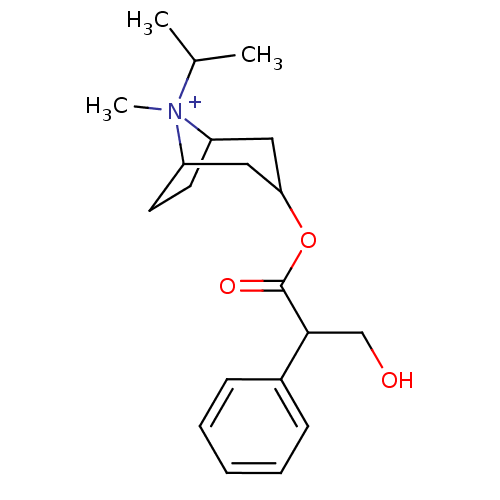
(CAS_22254-24-6 | Ipratropium | NSC_3746)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:1:3:6.7:9.10.11,THB:4:3:6.7:9.10.11,12:10:3:6.7,(-1.51,-1.3,;-.6,-.05,;-1.22,1.36,;.93,-.22,;1.51,1.21,;1.98,-1.04,;1.38,-2.36,;.22,-3.07,;1.19,-1.74,;3.01,-1.74,;3.97,-2.54,;3.71,-1.01,;4.74,-3.87,;3.97,-5.2,;2.43,-5.2,;4.74,-6.54,;6.28,-6.54,;7.05,-7.87,;3.97,-7.87,;4.73,-9.21,;3.96,-10.54,;2.42,-10.54,;1.65,-9.2,;2.43,-7.87,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM82372

(CAS_22254-24-6 | Ipratropium | NSC_3746)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:1:3:6.7:9.10.11,THB:4:3:6.7:9.10.11,12:10:3:6.7,(-1.51,-1.3,;-.6,-.05,;-1.22,1.36,;.93,-.22,;1.51,1.21,;1.98,-1.04,;1.38,-2.36,;.22,-3.07,;1.19,-1.74,;3.01,-1.74,;3.97,-2.54,;3.71,-1.01,;4.74,-3.87,;3.97,-5.2,;2.43,-5.2,;4.74,-6.54,;6.28,-6.54,;7.05,-7.87,;3.97,-7.87,;4.73,-9.21,;3.96,-10.54,;2.42,-10.54,;1.65,-9.2,;2.43,-7.87,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM82372

(CAS_22254-24-6 | Ipratropium | NSC_3746)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:1:3:6.7:9.10.11,THB:4:3:6.7:9.10.11,12:10:3:6.7,(-1.51,-1.3,;-.6,-.05,;-1.22,1.36,;.93,-.22,;1.51,1.21,;1.98,-1.04,;1.38,-2.36,;.22,-3.07,;1.19,-1.74,;3.01,-1.74,;3.97,-2.54,;3.71,-1.01,;4.74,-3.87,;3.97,-5.2,;2.43,-5.2,;4.74,-6.54,;6.28,-6.54,;7.05,-7.87,;3.97,-7.87,;4.73,-9.21,;3.96,-10.54,;2.42,-10.54,;1.65,-9.2,;2.43,-7.87,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50109647
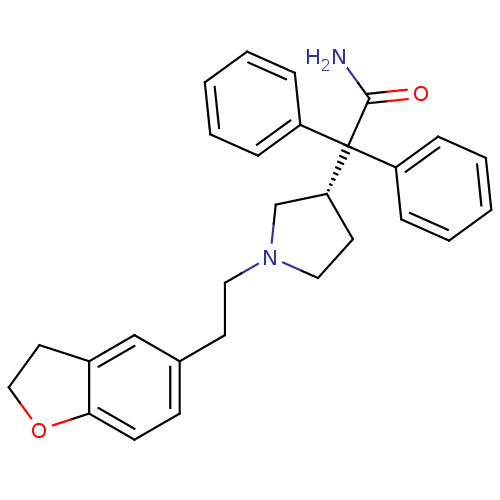
(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM82372

(CAS_22254-24-6 | Ipratropium | NSC_3746)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:1:3:6.7:9.10.11,THB:4:3:6.7:9.10.11,12:10:3:6.7,(-1.51,-1.3,;-.6,-.05,;-1.22,1.36,;.93,-.22,;1.51,1.21,;1.98,-1.04,;1.38,-2.36,;.22,-3.07,;1.19,-1.74,;3.01,-1.74,;3.97,-2.54,;3.71,-1.01,;4.74,-3.87,;3.97,-5.2,;2.43,-5.2,;4.74,-6.54,;6.28,-6.54,;7.05,-7.87,;3.97,-7.87,;4.73,-9.21,;3.96,-10.54,;2.42,-10.54,;1.65,-9.2,;2.43,-7.87,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM82372

(CAS_22254-24-6 | Ipratropium | NSC_3746)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:1:3:6.7:9.10.11,THB:4:3:6.7:9.10.11,12:10:3:6.7,(-1.51,-1.3,;-.6,-.05,;-1.22,1.36,;.93,-.22,;1.51,1.21,;1.98,-1.04,;1.38,-2.36,;.22,-3.07,;1.19,-1.74,;3.01,-1.74,;3.97,-2.54,;3.71,-1.01,;4.74,-3.87,;3.97,-5.2,;2.43,-5.2,;4.74,-6.54,;6.28,-6.54,;7.05,-7.87,;3.97,-7.87,;4.73,-9.21,;3.96,-10.54,;2.42,-10.54,;1.65,-9.2,;2.43,-7.87,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM85787
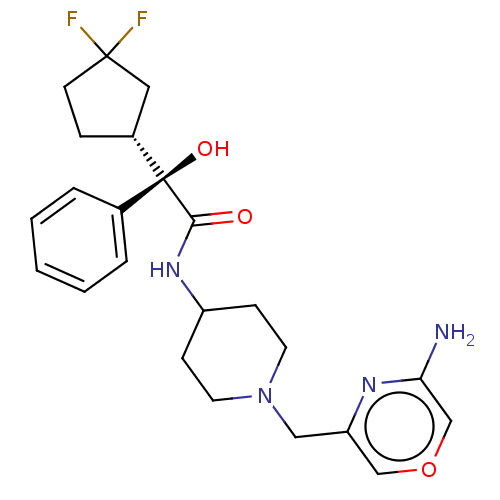
(Compound A)Show SMILES Nc1cocc(CN2CCC(CC2)NC(=O)[C@@](O)([C@@H]2CCC(F)(F)C2)c2ccccc2)n1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50109647

(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM85787

(Compound A)Show SMILES Nc1cocc(CN2CCC(CC2)NC(=O)[C@@](O)([C@@H]2CCC(F)(F)C2)c2ccccc2)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM85787

(Compound A)Show SMILES Nc1cocc(CN2CCC(CC2)NC(=O)[C@@](O)([C@@H]2CCC(F)(F)C2)c2ccccc2)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50109647

(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM85787

(Compound A)Show SMILES Nc1cocc(CN2CCC(CC2)NC(=O)[C@@](O)([C@@H]2CCC(F)(F)C2)c2ccccc2)n1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50109647

(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM85787

(Compound A)Show SMILES Nc1cocc(CN2CCC(CC2)NC(=O)[C@@](O)([C@@H]2CCC(F)(F)C2)c2ccccc2)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50109647

(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM85787

(Compound A)Show SMILES Nc1cocc(CN2CCC(CC2)NC(=O)[C@@](O)([C@@H]2CCC(F)(F)C2)c2ccccc2)n1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora A ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 492 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM85787

(Compound A)Show SMILES Nc1cocc(CN2CCC(CC2)NC(=O)[C@@](O)([C@@H]2CCC(F)(F)C2)c2ccccc2)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50081125
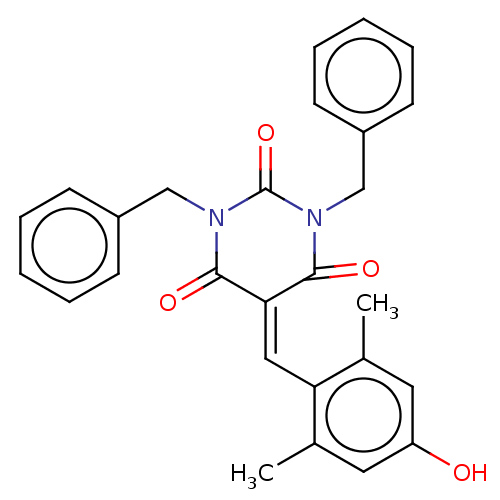
(CHEMBL3421961)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1\[#6]=[#6]-1/[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6]-1=O Show InChI InChI=1S/C27H24N2O4/c1-18-13-22(30)14-19(2)23(18)15-24-25(31)28(16-20-9-5-3-6-10-20)27(33)29(26(24)32)17-21-11-7-4-8-12-21/h3-15,30H,16-17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of P300 (unknown origin) using acetyl CoA as substrate after 15 mins by double reciprocal plot analysis |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50081125

(CHEMBL3421961)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1\[#6]=[#6]-1/[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6]-1=O Show InChI InChI=1S/C27H24N2O4/c1-18-13-22(30)14-19(2)23(18)15-24-25(31)28(16-20-9-5-3-6-10-20)27(33)29(26(24)32)17-21-11-7-4-8-12-21/h3-15,30H,16-17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of P300 (unknown origin) using biotinylated H3 as substrate after 15 mins by double reciprocal plot analysis |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM50189415
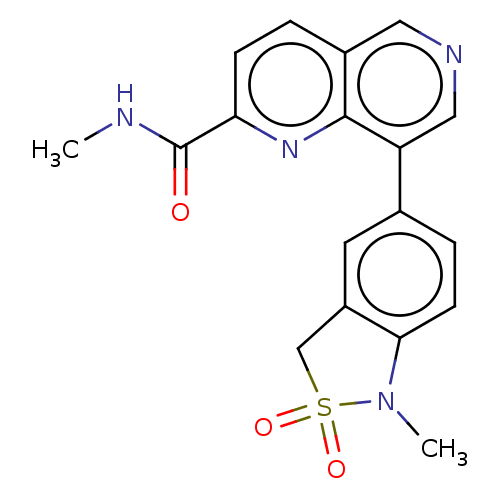
(CHEMBL3828637)Show SMILES CNC(=O)c1ccc2cncc(-c3ccc4N(C)S(=O)(=O)Cc4c3)c2n1 Show InChI InChI=1S/C18H16N4O3S/c1-19-18(23)15-5-3-12-8-20-9-14(17(12)21-15)11-4-6-16-13(7-11)10-26(24,25)22(16)2/h3-9H,10H2,1-2H3,(H,19,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of CDK8 (unknown origin) |
ACS Med Chem Lett 11: 127-132 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00480
BindingDB Entry DOI: 10.7270/Q20Z76K1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-C
(Homo sapiens (Human)) | BDBM50509567
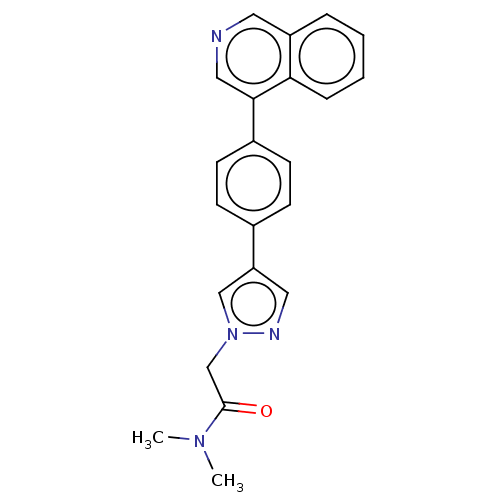
(CHEMBL4455382 | US11213520, Example I-003)Show SMILES CN(C)C(=O)Cn1cc(cn1)-c1ccc(cc1)-c1cncc2ccccc12 Show InChI InChI=1S/C22H20N4O/c1-25(2)22(27)15-26-14-19(12-24-26)16-7-9-17(10-8-16)21-13-23-11-18-5-3-4-6-20(18)21/h3-14H,15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of CDK8/CyclinC (unknown origin) |
ACS Med Chem Lett 11: 127-132 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00480
BindingDB Entry DOI: 10.7270/Q20Z76K1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50241089
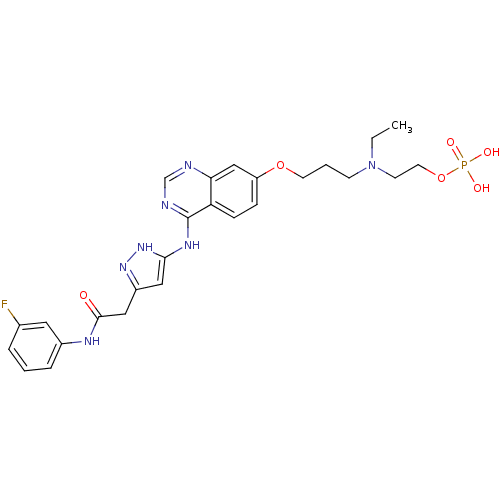
(2-(ethyl(3-(4-(5-(2-(3-fluorophenylamino)-2-oxoeth...)Show SMILES CCN(CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)n[nH]3)ncnc2c1)CCOP(O)(O)=O Show InChI InChI=1S/C26H31FN7O6P/c1-2-34(10-12-40-41(36,37)38)9-4-11-39-21-7-8-22-23(16-21)28-17-29-26(22)31-24-14-20(32-33-24)15-25(35)30-19-6-3-5-18(27)13-19/h3,5-8,13-14,16-17H,2,4,9-12,15H2,1H3,(H,30,35)(H2,36,37,38)(H2,28,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316497
(CHEMBL1097106 | N-{4-[1-(2,3-Dihydroxypropyl)-4-(1...)Show SMILES OCC(O)Cn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C26H24N6O3/c33-16-20(34)14-32-15-23(21-10-12-27-25-22(21)11-13-28-25)24(31-32)17-6-8-19(9-7-17)30-26(35)29-18-4-2-1-3-5-18/h1-13,15,20,33-34H,14,16H2,(H,27,28)(H2,29,30,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50376383
(CHEMBL259972)Show InChI InChI=1S/C19H17NO5/c1-23-14-7-3-12(4-8-14)18-16(11-17(21)22)25-20-19(18)13-5-9-15(24-2)10-6-13/h3-10H,11H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Med Chem 51: 2400-11 (2008)
Article DOI: 10.1021/jm701191z
BindingDB Entry DOI: 10.7270/Q2Z0391M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50288232
(2-[2-(carboxymethoxy)-4-(2-{4H,5H,6H,7H-thieno[2,3...)Show SMILES OC(=O)COc1ccc(cc1OCC(O)=O)C(=O)CNC(=O)c1cc2CCNCc2s1 Show InChI InChI=1S/C20H20N2O8S/c23-13(7-22-20(28)16-6-12-3-4-21-8-17(12)31-16)11-1-2-14(29-9-18(24)25)15(5-11)30-10-19(26)27/h1-2,5-6,21H,3-4,7-10H2,(H,22,28)(H,24,25)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Vitronectin binding to GPIIb/IIIIa Vitronectin receptor |
Bioorg Med Chem Lett 6: 2601-2606 (1996)
Article DOI: 10.1016/0960-894X(96)00476-3
BindingDB Entry DOI: 10.7270/Q28C9W76 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316473
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1cccc(CN2CCCC2)c1 Show InChI InChI=1S/C32H35N7O/c1-3-33-32(40)35-25-12-10-23(11-13-25)30-28(21-39(4-2)37-30)26-14-15-34-31-27(26)19-29(36-31)24-9-7-8-22(18-24)20-38-16-5-6-17-38/h7-15,18-19,21H,3-6,16-17,20H2,1-2H3,(H,34,36)(H2,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316475
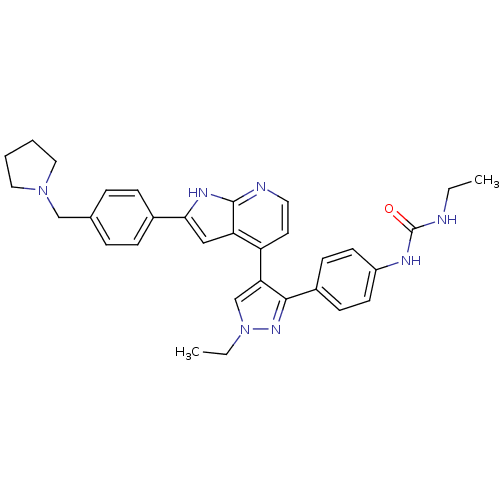
(CHEMBL1097191 | N-Ethyl-N'-[4-(1-ethyl-4-{2-[4-(1-...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1ccc(CN2CCCC2)cc1 Show InChI InChI=1S/C32H35N7O/c1-3-33-32(40)35-25-13-11-24(12-14-25)30-28(21-39(4-2)37-30)26-15-16-34-31-27(26)19-29(36-31)23-9-7-22(8-10-23)20-38-17-5-6-18-38/h7-16,19,21H,3-6,17-18,20H2,1-2H3,(H,34,36)(H2,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
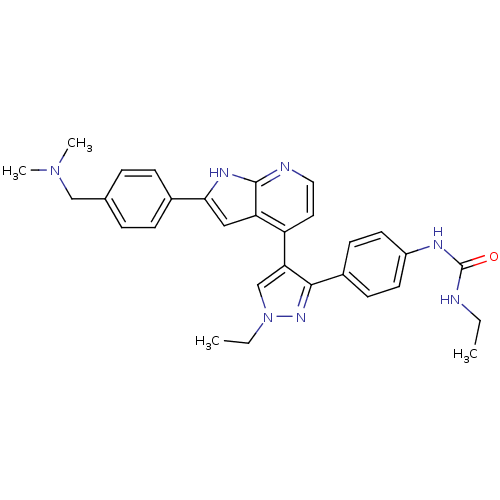
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316471
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1ccc(CN(C)C)cc1 Show InChI InChI=1S/C30H33N7O/c1-5-31-30(38)33-23-13-11-22(12-14-23)28-26(19-37(6-2)35-28)24-15-16-32-29-25(24)17-27(34-29)21-9-7-20(8-10-21)18-36(3)4/h7-17,19H,5-6,18H2,1-4H3,(H,32,34)(H2,31,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
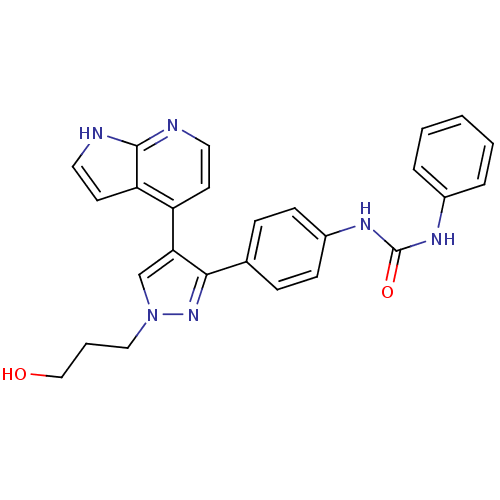
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316498
(CHEMBL1097454 | N-{4-[1-(3-Hydroxypropyl)-4-(1H-py...)Show SMILES OCCCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C26H24N6O2/c33-16-4-15-32-17-23(21-11-13-27-25-22(21)12-14-28-25)24(31-32)18-7-9-20(10-8-18)30-26(34)29-19-5-2-1-3-6-19/h1-3,5-14,17,33H,4,15-16H2,(H,27,28)(H2,29,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
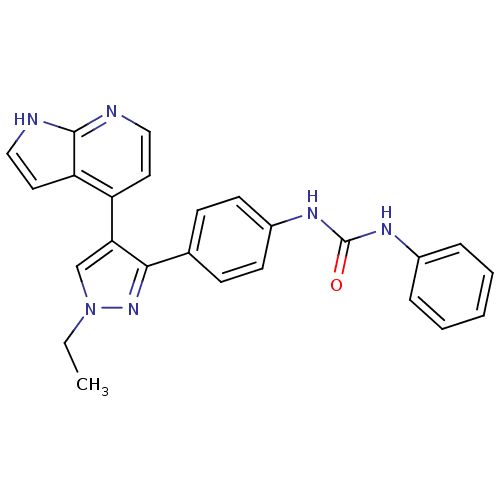
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316492
(1-(4-(1-ethyl-4-(1H-pyrrolo[2,3-b]pyridin-4-yl)-1H...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C25H22N6O/c1-2-31-16-22(20-12-14-26-24-21(20)13-15-27-24)23(30-31)17-8-10-19(11-9-17)29-25(32)28-18-6-4-3-5-7-18/h3-16H,2H2,1H3,(H,26,27)(H2,28,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257167
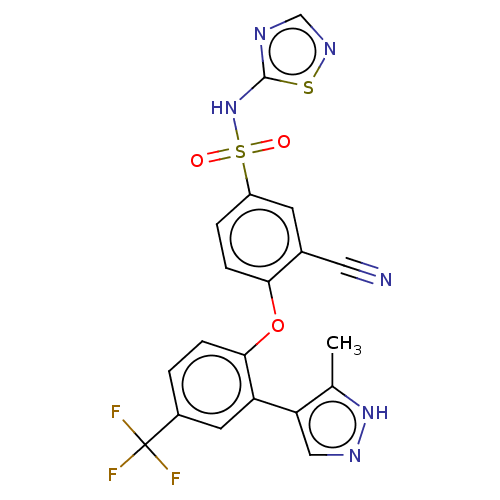
(CHEMBL2325619)Show SMILES Cc1[nH]ncc1-c1cc(ccc1Oc1ccc(cc1C#N)S(=O)(=O)Nc1ncns1)C(F)(F)F Show InChI InChI=1S/C20H13F3N6O3S2/c1-11-16(9-26-28-11)15-7-13(20(21,22)23)2-4-18(15)32-17-5-3-14(6-12(17)8-24)34(30,31)29-19-25-10-27-33-19/h2-7,9-10H,1H3,(H,26,28)(H,25,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316474
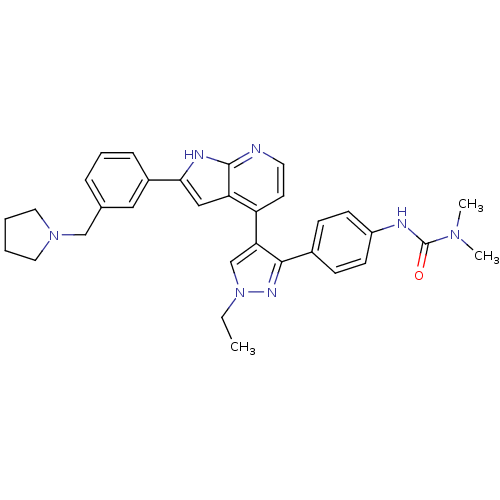
(4-[3-(4-N,N-Dimethylcarbamylaminophenyl)-1-ethyl-1...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN2CCCC2)c1 Show InChI InChI=1S/C32H35N7O/c1-4-39-21-28(30(36-39)23-10-12-25(13-11-23)34-32(40)37(2)3)26-14-15-33-31-27(26)19-29(35-31)24-9-7-8-22(18-24)20-38-16-5-6-17-38/h7-15,18-19,21H,4-6,16-17,20H2,1-3H3,(H,33,35)(H,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257179
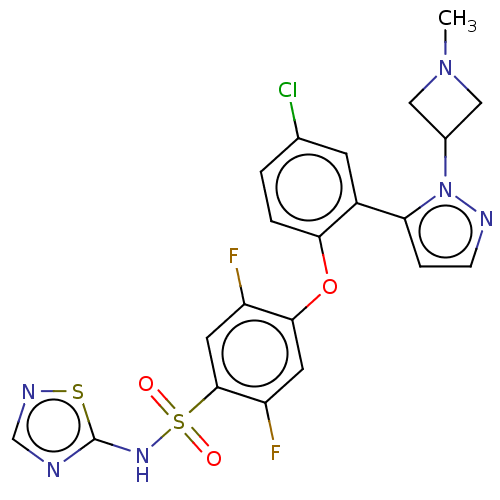
(CHEMBL2325622)Show SMILES CN1CC(C1)n1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C21H17ClF2N6O3S2/c1-29-9-13(10-29)30-17(4-5-26-30)14-6-12(22)2-3-18(14)33-19-7-16(24)20(8-15(19)23)35(31,32)28-21-25-11-27-34-21/h2-8,11,13H,9-10H2,1H3,(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316470
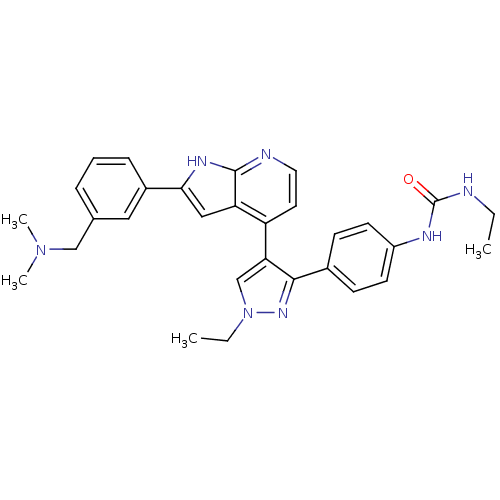
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-5-31-30(38)33-23-12-10-21(11-13-23)28-26(19-37(6-2)35-28)24-14-15-32-29-25(24)17-27(34-29)22-9-7-8-20(16-22)18-36(3)4/h7-17,19H,5-6,18H2,1-4H3,(H,32,34)(H2,31,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316496
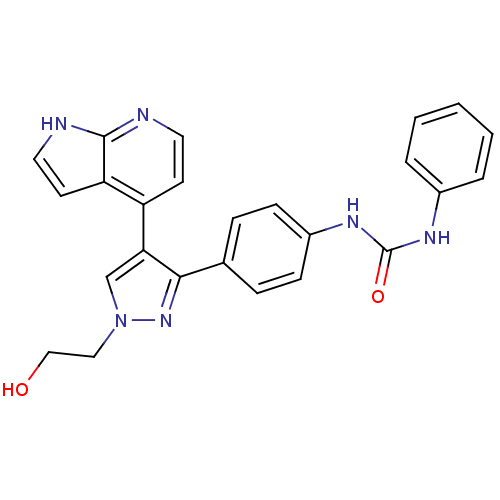
(CHEMBL1099010 | N-{4-[1-(2-Hydroxyethyl)-4-(1H-pyr...)Show SMILES OCCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C25H22N6O2/c32-15-14-31-16-22(20-10-12-26-24-21(20)11-13-27-24)23(30-31)17-6-8-19(9-7-17)29-25(33)28-18-4-2-1-3-5-18/h1-13,16,32H,14-15H2,(H,26,27)(H2,28,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316472
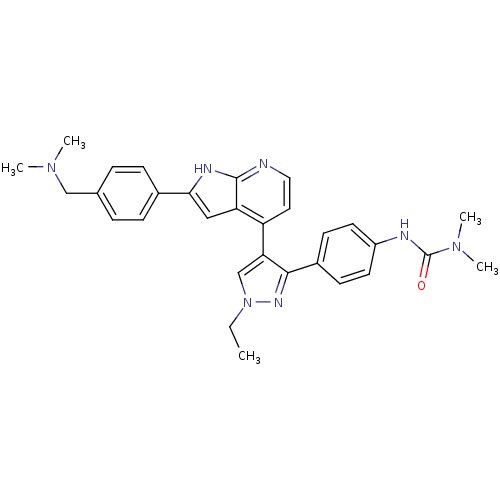
(4-[3-(4-N,N-Dimethylcarbamylaminophenyl)-1-ethyl-1...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1ccc(CN(C)C)cc1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)22-11-13-23(14-12-22)32-30(38)36(4)5)24-15-16-31-29-25(24)17-27(33-29)21-9-7-20(8-10-21)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50257166
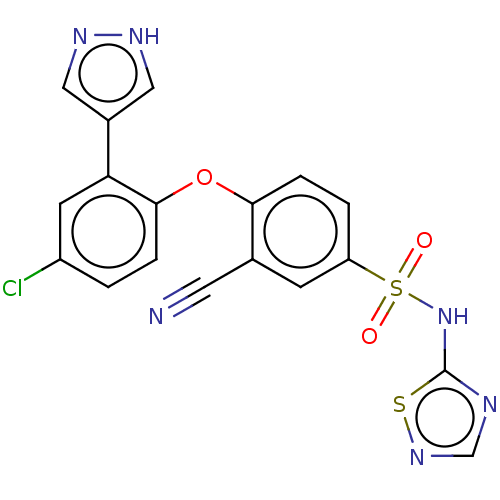
(CHEMBL2325330)Show SMILES Clc1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1cn[nH]c1 Show InChI InChI=1S/C18H11ClN6O3S2/c19-13-1-3-17(15(6-13)12-8-22-23-9-12)28-16-4-2-14(5-11(16)7-20)30(26,27)25-18-21-10-24-29-18/h1-6,8-10H,(H,22,23)(H,21,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data