 Found 630 hits with Last Name = 'seo' and Initial = 'sh'
Found 630 hits with Last Name = 'seo' and Initial = 'sh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proteinase-activated receptor 2
(Homo sapiens (Human)) | BDBM50134199
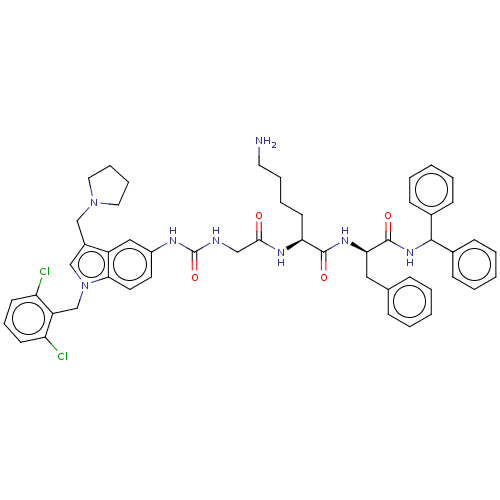
(CHEMBL3735057)Show SMILES NCCCC[C@H](NC(=O)CNC(=O)Nc1ccc2n(Cc3c(Cl)cccc3Cl)cc(CN3CCCC3)c2c1)C(=O)N[C@H](Cc1ccccc1)C(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C51H56Cl2N8O4/c52-42-21-14-22-43(53)41(42)34-61-33-38(32-60-27-12-13-28-60)40-30-39(24-25-46(40)61)56-51(65)55-31-47(62)57-44(23-10-11-26-54)49(63)58-45(29-35-15-4-1-5-16-35)50(64)59-48(36-17-6-2-7-18-36)37-19-8-3-9-20-37/h1-9,14-22,24-25,30,33,44-45,48H,10-13,23,26-29,31-32,34,54H2,(H,57,62)(H,58,63)(H,59,64)(H2,55,56,65)/t44-,45+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 627 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]furoyl-LIGRL-NH2 from human protease-activated receptor 2 in NCTC-2544 cells |
Bioorg Med Chem 23: 7717-27 (2015)
Article DOI: 10.1016/j.bmc.2015.11.016
BindingDB Entry DOI: 10.7270/Q2VH5QP3 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50267969

(Sargahydroquinoic Acid)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1cc(-[#8])cc(-[#6])c1-[#8])-[#6](-[#8])=O Show InChI InChI=1S/C27H38O4/c1-19(2)9-6-13-23(27(30)31)14-8-12-20(3)10-7-11-21(4)15-16-24-18-25(28)17-22(5)26(24)29/h9-10,14-15,17-18,28-29H,6-8,11-13,16H2,1-5H3,(H,30,31)/b20-10+,21-15+,23-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea.
Curated by ChEMBL
| Assay Description
Mixed type inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by Dixon plot analysis |
Bioorg Med Chem 25: 3964-3970 (2017)
Article DOI: 10.1016/j.bmc.2017.05.033
BindingDB Entry DOI: 10.7270/Q2K076RW |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 2
(Homo sapiens (Human)) | BDBM50134200
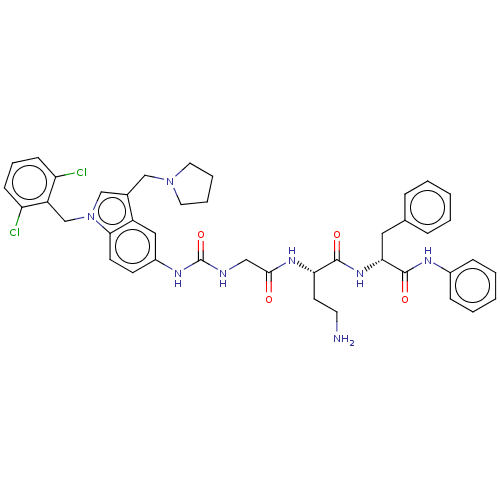
(CHEMBL3735405)Show SMILES NCC[C@H](NC(=O)CNC(=O)Nc1ccc2n(Cc3c(Cl)cccc3Cl)cc(CN3CCCC3)c2c1)C(=O)N[C@H](Cc1ccccc1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C42H46Cl2N8O4/c43-34-14-9-15-35(44)33(34)27-52-26-29(25-51-20-7-8-21-51)32-23-31(16-17-38(32)52)48-42(56)46-24-39(53)49-36(18-19-45)40(54)50-37(22-28-10-3-1-4-11-28)41(55)47-30-12-5-2-6-13-30/h1-6,9-17,23,26,36-37H,7-8,18-22,24-25,27,45H2,(H,47,55)(H,49,53)(H,50,54)(H2,46,48,56)/t36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]furoyl-LIGRL-NH2 from human protease-activated receptor 2 in NCTC-2544 cells |
Bioorg Med Chem 23: 7717-27 (2015)
Article DOI: 10.1016/j.bmc.2015.11.016
BindingDB Entry DOI: 10.7270/Q2VH5QP3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50241345
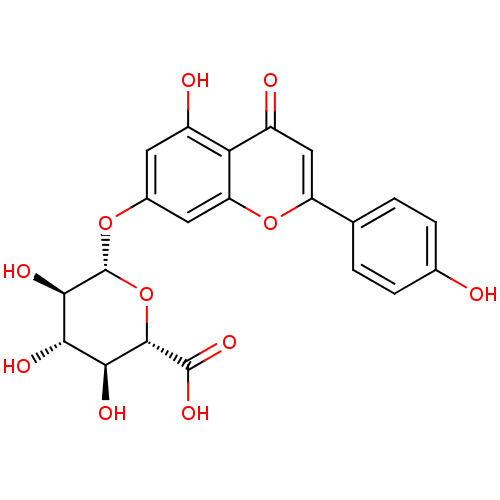
(Apigenin 7-O-β-D-glucuronide (9) | CHEMBL2542...)Show SMILES O[C@H]1[C@H](Oc2cc(O)c3c(c2)oc(cc3=O)-c2ccc(O)cc2)O[C@@H]([C@@H](O)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C21H18O11/c22-9-3-1-8(2-4-9)13-7-12(24)15-11(23)5-10(6-14(15)31-13)30-21-18(27)16(25)17(26)19(32-21)20(28)29/h1-7,16-19,21-23,25-27H,(H,28,29)/t16-,17-,18+,19-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
| Assay Description
The reaction mixtures contained in various different concentrations of p-NPP as a PTP1B substrate in the presence or absence of the active compound. ... |
Bioorg Chem 72: 293-300 (2017)
BindingDB Entry DOI: 10.7270/Q2Q52NH6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM4078
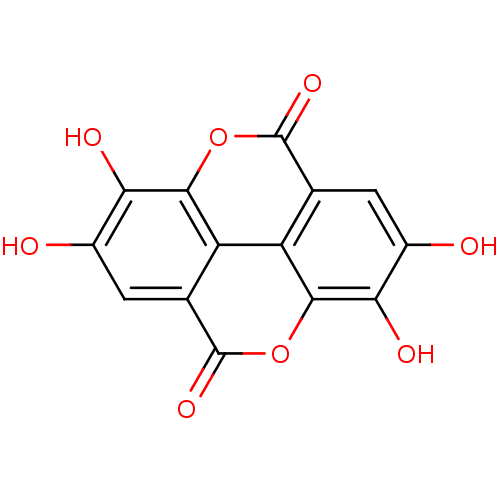
(6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0...)Show InChI InChI=1S/C14H6O8/c15-5-1-3-7-8-4(14(20)22-11(7)9(5)17)2-6(16)10(18)12(8)21-13(3)19/h1-2,15-18H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
| Assay Description
The reaction mixtures contained in various different concentrations of p-NPP as a PTP1B substrate in the presence or absence of the active compound. ... |
Bioorg Chem 72: 293-300 (2017)
BindingDB Entry DOI: 10.7270/Q2Q52NH6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50267968
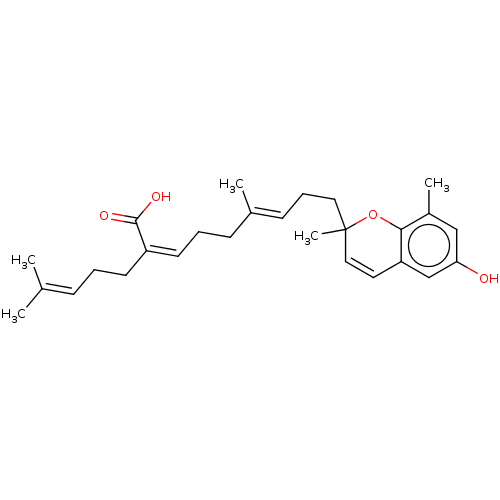
(CHEMBL4085945)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]C1([#6])[#8]-c2c(-[#6])cc(-[#8])cc2-[#6]=[#6]1)-[#6](-[#8])=O |c:27| Show InChI InChI=1S/C27H36O4/c1-19(2)9-6-12-22(26(29)30)13-7-10-20(3)11-8-15-27(5)16-14-23-18-24(28)17-21(4)25(23)31-27/h9,11,13-14,16-18,28H,6-8,10,12,15H2,1-5H3,(H,29,30)/b20-11+,22-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea.
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by Dixon plot analysis |
Bioorg Med Chem 25: 3964-3970 (2017)
Article DOI: 10.1016/j.bmc.2017.05.033
BindingDB Entry DOI: 10.7270/Q2K076RW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM226157

(PTP1B spring 7 (7))Show SMILES COc1ccc(-c2ccc(O)cc2)c(C(c2ccc(O)cc2)c2ccc(O)cc2)c1C#Cc1ccc(O)cc1 Show InChI InChI=1S/C34H26O5/c1-39-32-21-20-30(23-5-13-27(36)14-6-23)34(31(32)19-4-22-2-11-26(35)12-3-22)33(24-7-15-28(37)16-8-24)25-9-17-29(38)18-10-25/h2-3,5-18,20-21,33,35-38H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.00E+3 | -32.8 | 4.80E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Catholic University of Daegu
| Assay Description
In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... |
Bioorg Chem 72: 273-281 (2017)
BindingDB Entry DOI: 10.7270/Q2ZP450S |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50267967
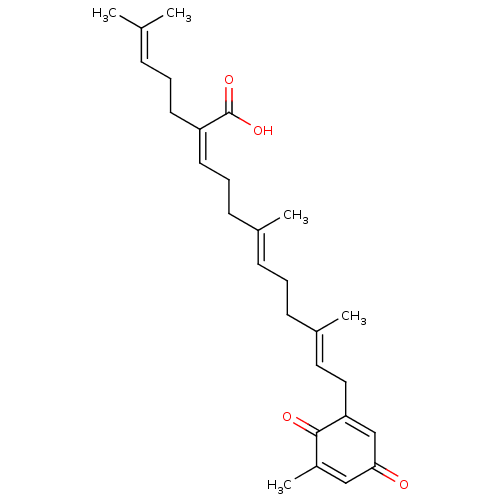
(CHEMBL4064412)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]-1=[#6]-[#6](=O)-[#6]=[#6](-[#6])-[#6]-1=O)-[#6](-[#8])=O |t:19,23| Show InChI InChI=1S/C27H36O4/c1-19(2)9-6-13-23(27(30)31)14-8-12-20(3)10-7-11-21(4)15-16-24-18-25(28)17-22(5)26(24)29/h9-10,14-15,17-18H,6-8,11-13,16H2,1-5H3,(H,30,31)/b20-10+,21-15+,23-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea.
Curated by ChEMBL
| Assay Description
Mixed type inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by Dixon plot analysis |
Bioorg Med Chem 25: 3964-3970 (2017)
Article DOI: 10.1016/j.bmc.2017.05.033
BindingDB Entry DOI: 10.7270/Q2K076RW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM226153

(Selaginellin U (2))Show SMILES [#6]-c1ccc(-c2ccc(-[#8])cc2)c(\[#6](=[#6]-2\[#6]=[#6]-[#6](=O)-[#6]=[#6]-2)-c2ccc(-[#8])cc2)c1C#Cc1ccc(-[#8])cc1 |c:16,20| Show InChI InChI=1S/C34H24O4/c1-22-2-20-32(24-6-14-28(36)15-7-24)34(31(22)21-5-23-3-12-27(35)13-4-23)33(25-8-16-29(37)17-9-25)26-10-18-30(38)19-11-26/h2-4,6-20,35-37H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 9.70E+3 | -29.8 | 1.38E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Catholic University of Daegu
| Assay Description
In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... |
Bioorg Chem 72: 273-281 (2017)
BindingDB Entry DOI: 10.7270/Q2ZP450S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM226155
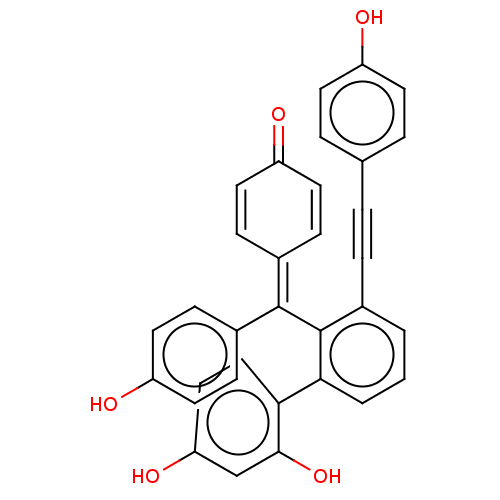
(Selaginellin W (4))Show SMILES [#8]-c1ccc(cc1)C#Cc1cccc(-c2ccc(-[#8])cc2-[#8])c1\[#6](=[#6]-1/[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)-c1ccc(-[#8])cc1 |c:28,32| Show InChI InChI=1S/C33H22O5/c34-25-12-5-21(6-13-25)4-7-22-2-1-3-30(29-19-18-28(37)20-31(29)38)33(22)32(23-8-14-26(35)15-9-23)24-10-16-27(36)17-11-24/h1-3,5-6,8-20,34-35,37-38H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.11E+4 | -29.4 | 1.46E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Catholic University of Daegu
| Assay Description
In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... |
Bioorg Chem 72: 273-281 (2017)
BindingDB Entry DOI: 10.7270/Q2ZP450S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM226154

(Selaginellin V (3))Show SMILES [#6]-[#8]-c1cc(ccc1-[#8])C#Cc1c(-[#6]-[#8])ccc(-c2ccc(-[#8])cc2)c1\[#6](=[#6]-1\[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)-c1ccc(-[#8])cc1 |c:31,35| Show InChI InChI=1S/C35H26O6/c1-41-33-20-22(3-19-32(33)40)2-17-31-26(21-36)10-18-30(23-4-11-27(37)12-5-23)35(31)34(24-6-13-28(38)14-7-24)25-8-15-29(39)16-9-25/h3-16,18-20,36-38,40H,21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.13E+4 | -29.4 | 1.45E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Catholic University of Daegu
| Assay Description
In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... |
Bioorg Chem 72: 273-281 (2017)
BindingDB Entry DOI: 10.7270/Q2ZP450S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM226156
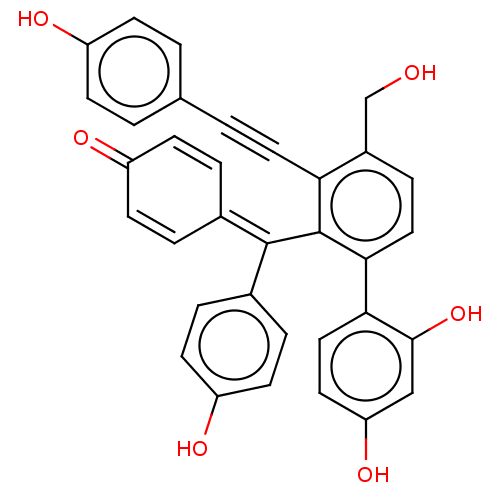
(PTP1B spring 5 (5))Show SMILES [#8]-[#6]-c1ccc(-c2ccc(-[#8])cc2-[#8])c(\[#6](=[#6]-2/[#6]=[#6]-[#6](=O)-[#6]=[#6]-2)-c2ccc(-[#8])cc2)c1C#Cc1ccc(-[#8])cc1 |c:18,22| Show InChI InChI=1S/C34H24O6/c35-20-24-8-17-31(30-18-15-28(39)19-32(30)40)34(29(24)16-3-21-1-9-25(36)10-2-21)33(22-4-11-26(37)12-5-22)23-6-13-27(38)14-7-23/h1-2,4-15,17-19,35-37,39-40H,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 1.39E+4 | -28.8 | 1.59E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Catholic University of Daegu
| Assay Description
In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... |
Bioorg Chem 72: 273-281 (2017)
BindingDB Entry DOI: 10.7270/Q2ZP450S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50093523
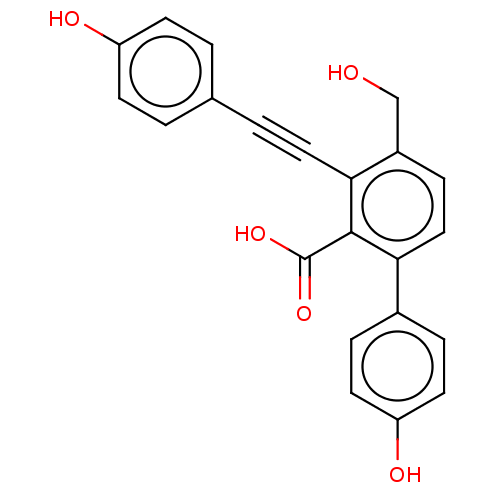
(CHEMBL3585679 | PTP1B spring 6 (6))Show SMILES OCc1ccc(-c2ccc(O)cc2)c(C(O)=O)c1C#Cc1ccc(O)cc1 Show InChI InChI=1S/C22H16O5/c23-13-16-6-12-19(15-4-9-18(25)10-5-15)21(22(26)27)20(16)11-3-14-1-7-17(24)8-2-14/h1-2,4-10,12,23-25H,13H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 1.45E+4 | -28.7 | 1.32E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Catholic University of Daegu
| Assay Description
In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... |
Bioorg Chem 72: 273-281 (2017)
BindingDB Entry DOI: 10.7270/Q2ZP450S |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50333656
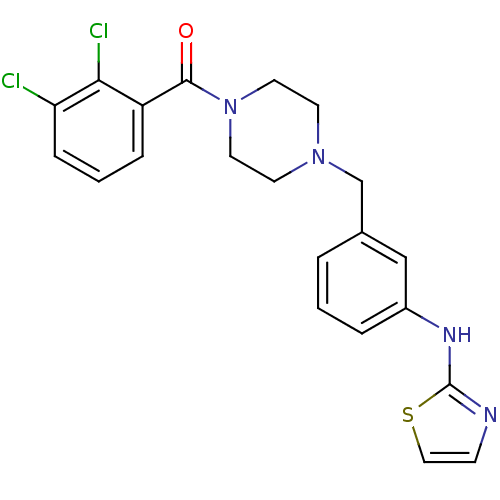
((2,3-dichlorophenyl)(4-(3-(thiazol-2-ylamino)benzy...)Show SMILES Clc1cccc(C(=O)N2CCN(Cc3cccc(Nc4nccs4)c3)CC2)c1Cl Show InChI InChI=1S/C21H20Cl2N4OS/c22-18-6-2-5-17(19(18)23)20(28)27-10-8-26(9-11-27)14-15-3-1-4-16(13-15)25-21-24-7-12-29-21/h1-7,12-13H,8-11,14H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged Aurora A kinase expressed in insect cells |
Bioorg Med Chem 19: 907-16 (2011)
Article DOI: 10.1016/j.bmc.2010.11.064
BindingDB Entry DOI: 10.7270/Q2V40VGC |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM14192
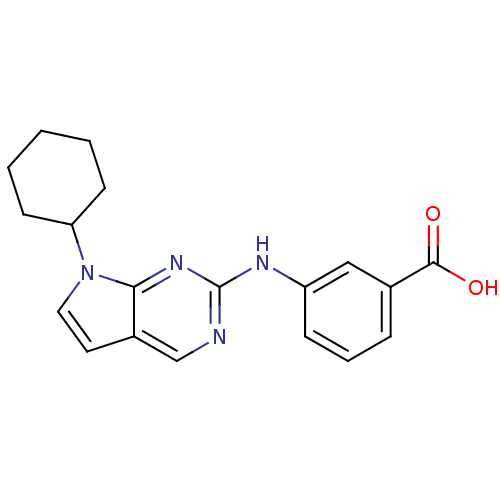
(3-({7-cyclohexyl-7H-pyrrolo[2,3-d]pyrimidin-2-yl}a...)Show InChI InChI=1S/C19H20N4O2/c24-18(25)13-5-4-6-15(11-13)21-19-20-12-14-9-10-23(17(14)22-19)16-7-2-1-3-8-16/h4-6,9-12,16H,1-3,7-8H2,(H,24,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged Aurora A kinase expressed in insect cells |
Bioorg Med Chem 19: 907-16 (2011)
Article DOI: 10.1016/j.bmc.2010.11.064
BindingDB Entry DOI: 10.7270/Q2V40VGC |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged Aurora A kinase expressed in insect cells |
Bioorg Med Chem 19: 907-16 (2011)
Article DOI: 10.1016/j.bmc.2010.11.064
BindingDB Entry DOI: 10.7270/Q2V40VGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50333657
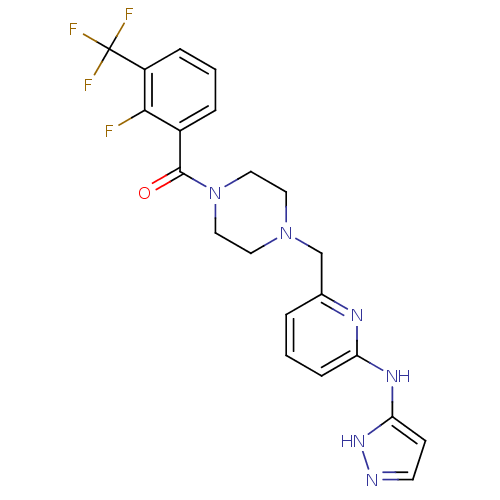
((4-((6-(1H-pyrazol-3-ylamino)pyridin-2-yl)methyl)p...)Show SMILES Fc1c(cccc1C(F)(F)F)C(=O)N1CCN(Cc2cccc(Nc3ccn[nH]3)n2)CC1 Show InChI InChI=1S/C21H20F4N6O/c22-19-15(4-2-5-16(19)21(23,24)25)20(32)31-11-9-30(10-12-31)13-14-3-1-6-17(27-14)28-18-7-8-26-29-18/h1-8H,9-13H2,(H2,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged Aurora A kinase expressed in insect cells |
Bioorg Med Chem 19: 907-16 (2011)
Article DOI: 10.1016/j.bmc.2010.11.064
BindingDB Entry DOI: 10.7270/Q2V40VGC |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FMS (unknown origin) in presence of ATP |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112710
BindingDB Entry DOI: 10.7270/Q2W38122 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Src (unknown origin) in presence of ATP |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112710
BindingDB Entry DOI: 10.7270/Q2W38122 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of human ERG assessed as reduction in channel current by automated patch-clamp electrophysiology assay |
Eur J Med Chem 115: 201-16 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.039
BindingDB Entry DOI: 10.7270/Q27947QQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FLT3 (unknown origin) in presence of ATP |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112710
BindingDB Entry DOI: 10.7270/Q2W38122 |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50565746
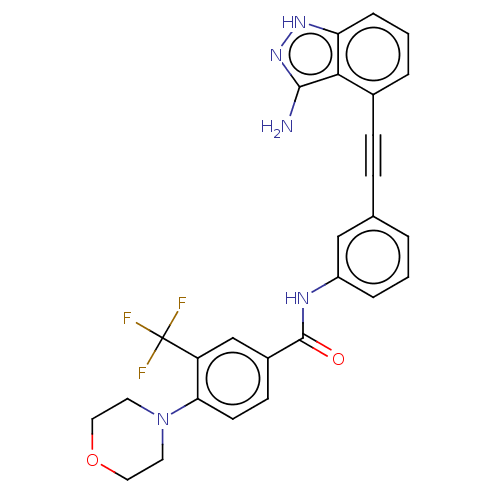
(CHEMBL4793906)Show SMILES Nc1n[nH]c2cccc(C#Cc3cccc(NC(=O)c4ccc(N5CCOCC5)c(c4)C(F)(F)F)c3)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type BCR-ABL (unknown origin) incubated for 40 mins in presence of [gamma33P]ATP by scintillation method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112710
BindingDB Entry DOI: 10.7270/Q2W38122 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12103
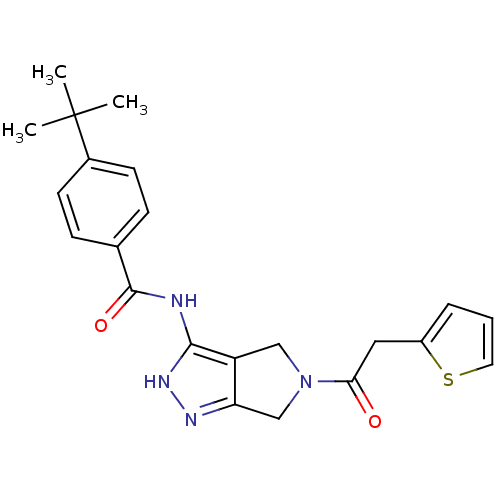
(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 11 | 4-te...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)Cc1cccs1 Show InChI InChI=1S/C22H24N4O2S/c1-22(2,3)15-8-6-14(7-9-15)21(28)23-20-17-12-26(13-18(17)24-25-20)19(27)11-16-5-4-10-29-16/h4-10H,11-13H2,1-3H3,(H2,23,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged Aurora A kinase expressed in insect cells |
Bioorg Med Chem 19: 907-16 (2011)
Article DOI: 10.1016/j.bmc.2010.11.064
BindingDB Entry DOI: 10.7270/Q2V40VGC |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of human C-RAF measured after 40 mins by scintillation counting |
Eur J Med Chem 115: 201-16 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.039
BindingDB Entry DOI: 10.7270/Q27947QQ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50345949
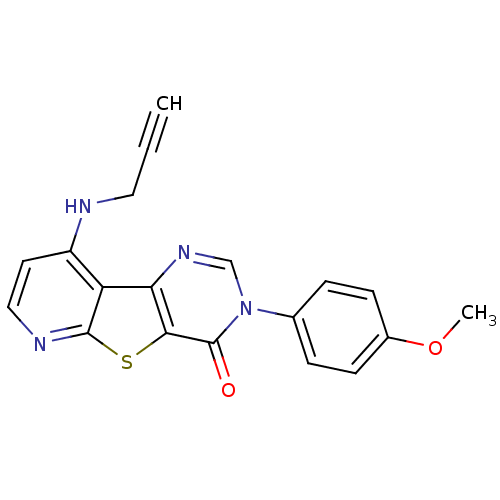
(3-(4-Methoxy-phenyl)-9-prop-2-ynylamino-3H-pyrido[...)Show SMILES COc1ccc(cc1)-n1cnc2c(sc3nccc(NCC#C)c23)c1=O Show InChI InChI=1S/C19H14N4O2S/c1-3-9-20-14-8-10-21-18-15(14)16-17(26-18)19(24)23(11-22-16)12-4-6-13(25-2)7-5-12/h1,4-8,10-11H,9H2,2H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50222901

(CHEMBL1163059)Show SMILES CSCC[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O7S2/c1-30-3-2-7(16)14(25)21-31(26,27)28-4-8-10(23)11(24)15(29-8)22-6-20-9-12(17)18-5-19-13(9)22/h5-8,10-11,15,23-24H,2-4,16H2,1H3,(H,21,25)(H2,17,18,19)/t7-,8+,10+,11+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli Methionyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 13: 1087-92 (2003)
BindingDB Entry DOI: 10.7270/Q29K4BRS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50117930
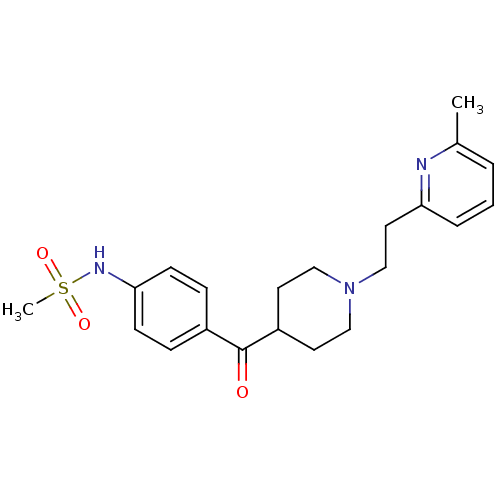
((4-{1-[2-(6-Methyl-pyridin-2-yl)-ethyl]-piperidine...)Show SMILES Cc1cccc(CCN2CCC(CC2)C(=O)c2ccc(NS(C)(=O)=O)cc2)n1 Show InChI InChI=1S/C21H27N3O3S/c1-16-4-3-5-19(22-16)12-15-24-13-10-18(11-14-24)21(25)17-6-8-20(9-7-17)23-28(2,26)27/h3-9,18,23H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG incubated for 4 hrs by competitive fluorescence tracer binding based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112710
BindingDB Entry DOI: 10.7270/Q2W38122 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50085415
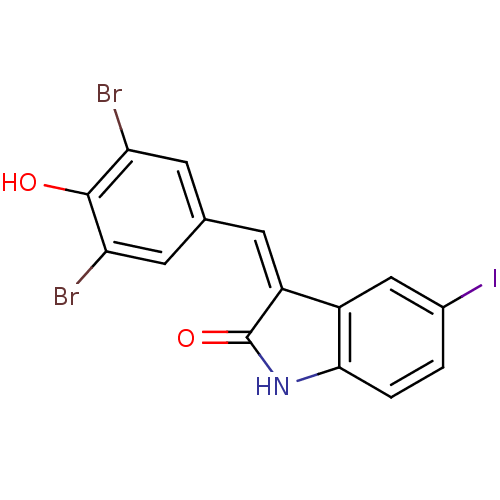
(3-(3,5-Dibromo-4-hydroxy-benzylidene)-5-iodo-1,3-d...)Show InChI InChI=1S/C15H8Br2INO2/c16-11-4-7(5-12(17)14(11)20)3-10-9-6-8(18)1-2-13(9)19-15(10)21/h1-6,20H,(H,19,21)/b10-3- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) in presence of ATP |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112710
BindingDB Entry DOI: 10.7270/Q2W38122 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50366870
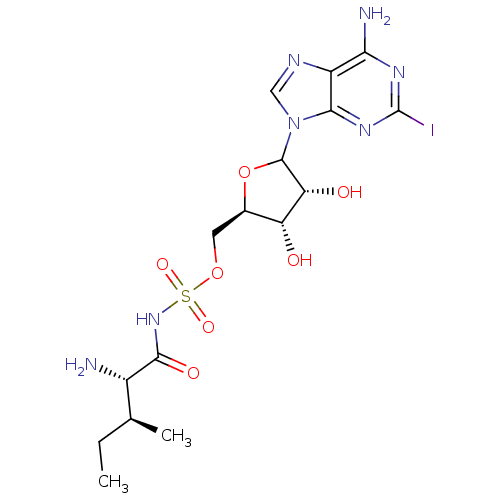
(CHEMBL605376)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)nc(I)nc12 |r| Show InChI InChI=1S/C16H24IN7O7S/c1-3-6(2)8(18)14(27)23-32(28,29)30-4-7-10(25)11(26)15(31-7)24-5-20-9-12(19)21-16(17)22-13(9)24/h5-8,10-11,15,25-26H,3-4,18H2,1-2H3,(H,23,27)(H2,19,21,22)/t6-,7+,8-,10+,11+,15?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli Isoleucyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 13: 1087-92 (2003)
BindingDB Entry DOI: 10.7270/Q29K4BRS |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50500971
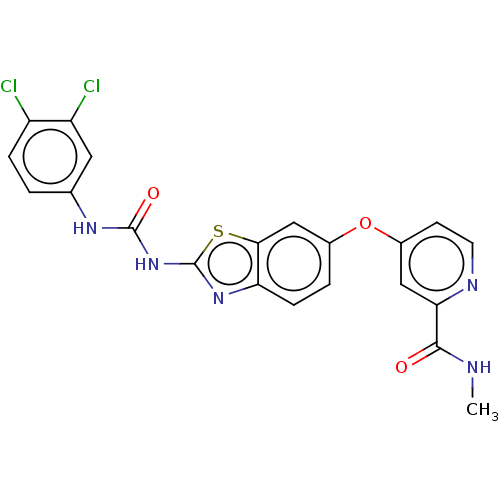
(CHEMBL3798983)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(NC(=O)Nc4ccc(Cl)c(Cl)c4)sc3c2)ccn1 Show InChI InChI=1S/C21H15Cl2N5O3S/c1-24-19(29)17-9-13(6-7-25-17)31-12-3-5-16-18(10-12)32-21(27-16)28-20(30)26-11-2-4-14(22)15(23)8-11/h2-10H,1H3,(H,24,29)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of human C-RAF measured after 40 mins by scintillation counting |
Eur J Med Chem 115: 201-16 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.039
BindingDB Entry DOI: 10.7270/Q27947QQ |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50565741
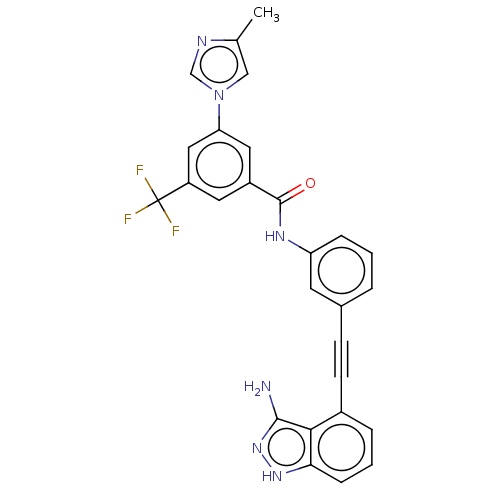
(CHEMBL4784922)Show SMILES Cc1cn(cn1)-c1cc(cc(c1)C(F)(F)F)C(=O)Nc1cccc(c1)C#Cc1cccc2[nH]nc(N)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type BCR-ABL (unknown origin) incubated for 40 mins in presence of [gamma33P]ATP by scintillation method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112710
BindingDB Entry DOI: 10.7270/Q2W38122 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50565746

(CHEMBL4793906)Show SMILES Nc1n[nH]c2cccc(C#Cc3cccc(NC(=O)c4ccc(N5CCOCC5)c(c4)C(F)(F)F)c3)c12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FMS (unknown origin) in presence of ATP |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112710
BindingDB Entry DOI: 10.7270/Q2W38122 |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50565749
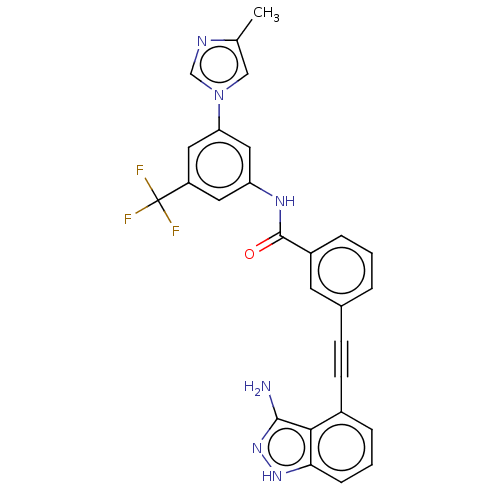
(CHEMBL4794093)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2cccc(c2)C#Cc2cccc3[nH]nc(N)c23)cc(c1)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type BCR-ABL (unknown origin) incubated for 40 mins in presence of [gamma33P]ATP by scintillation method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112710
BindingDB Entry DOI: 10.7270/Q2W38122 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM20800
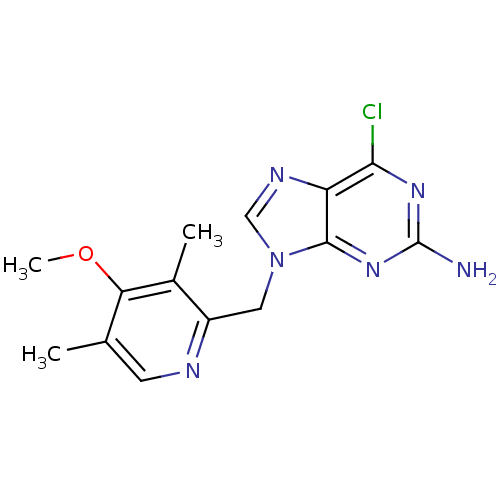
(2-amino-6-halopurine analogue, 20 | 6-chloro-9-[(4...)Show InChI InChI=1S/C14H15ClN6O/c1-7-4-17-9(8(2)11(7)22-3)5-21-6-18-10-12(15)19-14(16)20-13(10)21/h4,6H,5H2,1-3H3,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of FITC-labeled geldanamycin binding to human Hsp90alpha by fluorescence polarization assay |
Bioorg Med Chem Lett 27: 237-241 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.062
BindingDB Entry DOI: 10.7270/Q28W3G91 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM206763
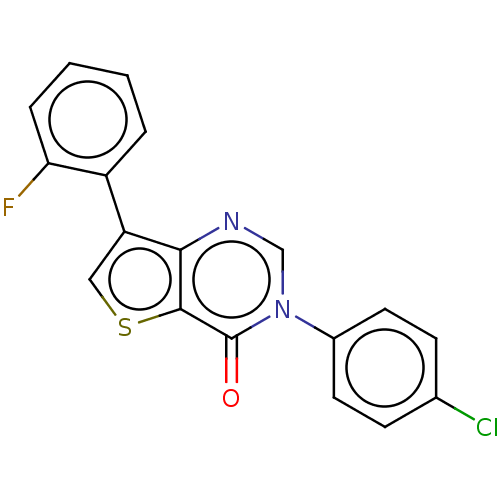
(US9260448, 32)Show InChI InChI=1S/C18H10ClFN2OS/c19-11-5-7-12(8-6-11)22-10-21-16-14(9-24-17(16)18(22)23)13-3-1-2-4-15(13)20/h1-10H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
| Assay Description
Cells of Chem3 Cell Line (HTS145C:Millipore) in which mGluR1 was stably expressed were adjusted to a density of 2×106/ml. 50 μl of the cells wer... |
US Patent US9260448 (2016)
BindingDB Entry DOI: 10.7270/Q2H9941W |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50207416
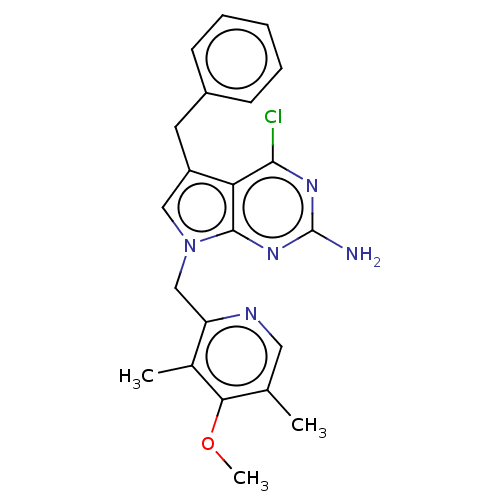
(CHEMBL3891745)Show SMILES COc1c(C)cnc(Cn2cc(Cc3ccccc3)c3c(Cl)nc(N)nc23)c1C Show InChI InChI=1S/C22H22ClN5O/c1-13-10-25-17(14(2)19(13)29-3)12-28-11-16(9-15-7-5-4-6-8-15)18-20(23)26-22(24)27-21(18)28/h4-8,10-11H,9,12H2,1-3H3,(H2,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of FITC-labeled geldanamycin binding to human Hsp90alpha by fluorescence polarization assay |
Bioorg Med Chem Lett 27: 237-241 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.062
BindingDB Entry DOI: 10.7270/Q28W3G91 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) measured after 40 mins by scintillation counting |
Eur J Med Chem 115: 201-16 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.039
BindingDB Entry DOI: 10.7270/Q27947QQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50366862

(CHEMBL605592)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)nc(nc12)C#C |r| Show InChI InChI=1S/C18H25N7O7S/c1-4-8(3)11(19)17(28)24-33(29,30)31-6-9-13(26)14(27)18(32-9)25-7-21-12-15(20)22-10(5-2)23-16(12)25/h2,7-9,11,13-14,18,26-27H,4,6,19H2,1,3H3,(H,24,28)(H2,20,22,23)/t8-,9+,11-,13+,14+,18?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli Isoleucyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 13: 1087-92 (2003)
BindingDB Entry DOI: 10.7270/Q29K4BRS |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058042
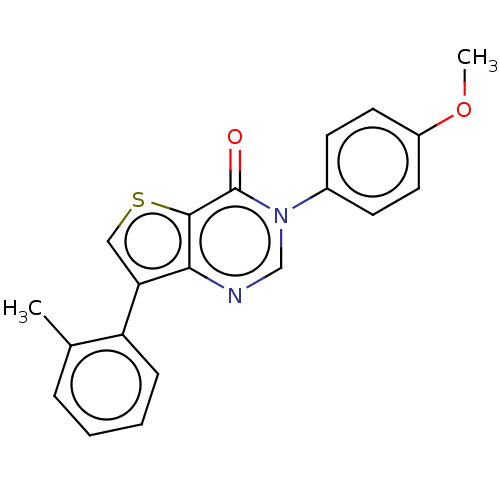
(CHEMBL3330823)Show InChI InChI=1S/C20H16N2O2S/c1-13-5-3-4-6-16(13)17-11-25-19-18(17)21-12-22(20(19)23)14-7-9-15(24-2)10-8-14/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50207419
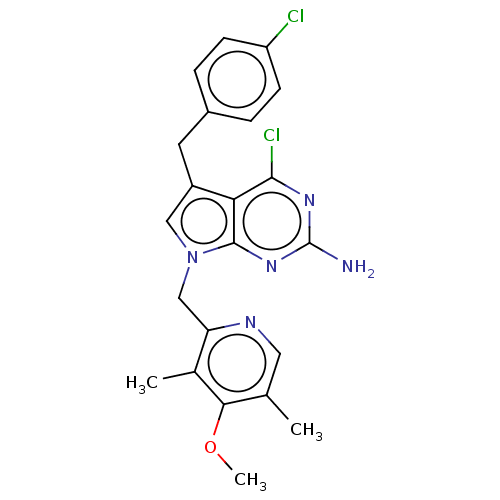
(CHEMBL3940517)Show SMILES COc1c(C)cnc(Cn2cc(Cc3ccc(Cl)cc3)c3c(Cl)nc(N)nc23)c1C Show InChI InChI=1S/C22H21Cl2N5O/c1-12-9-26-17(13(2)19(12)30-3)11-29-10-15(8-14-4-6-16(23)7-5-14)18-20(24)27-22(25)28-21(18)29/h4-7,9-10H,8,11H2,1-3H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of FITC-labeled geldanamycin binding to human Hsp90alpha by fluorescence polarization assay |
Bioorg Med Chem Lett 27: 237-241 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.062
BindingDB Entry DOI: 10.7270/Q28W3G91 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058048

(CHEMBL3330811)Show InChI InChI=1S/C19H14N2O2S/c1-23-15-9-7-14(8-10-15)21-12-20-17-16(11-24-18(17)19(21)22)13-5-3-2-4-6-13/h2-12H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50180302
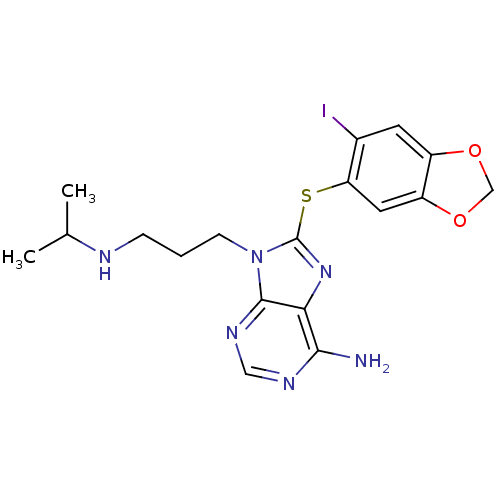
(8-(6-iodo-benzo[1,3]dioxol-5-ylsulfanyl)-9-(3-isop...)Show InChI InChI=1S/C18H21IN6O2S/c1-10(2)21-4-3-5-25-17-15(16(20)22-8-23-17)24-18(25)28-14-7-13-12(6-11(14)19)26-9-27-13/h6-8,10,21H,3-5,9H2,1-2H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of FITC-labeled geldanamycin binding to human Hsp90alpha by fluorescence polarization assay |
Bioorg Med Chem Lett 27: 237-241 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.062
BindingDB Entry DOI: 10.7270/Q28W3G91 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50207421
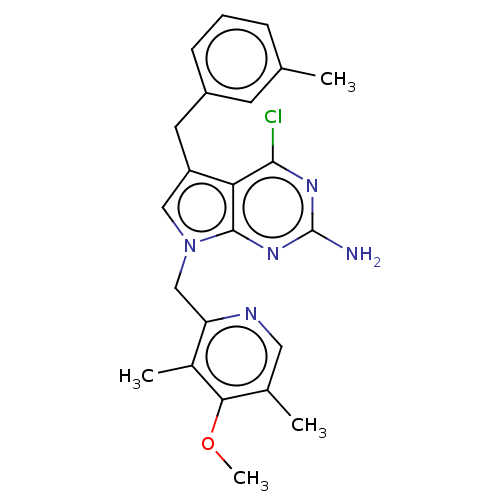
(CHEMBL3910684)Show SMILES COc1c(C)cnc(Cn2cc(Cc3cccc(C)c3)c3c(Cl)nc(N)nc23)c1C Show InChI InChI=1S/C23H24ClN5O/c1-13-6-5-7-16(8-13)9-17-11-29(22-19(17)21(24)27-23(25)28-22)12-18-15(3)20(30-4)14(2)10-26-18/h5-8,10-11H,9,12H2,1-4H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of FITC-labeled geldanamycin binding to human Hsp90alpha by fluorescence polarization assay |
Bioorg Med Chem Lett 27: 237-241 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.062
BindingDB Entry DOI: 10.7270/Q28W3G91 |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BCR-ABL T315I mutant (unknown origin) incubated for 40 mins in presence of [gamma33P]ATP by scintillation method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112710
BindingDB Entry DOI: 10.7270/Q2W38122 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50008057
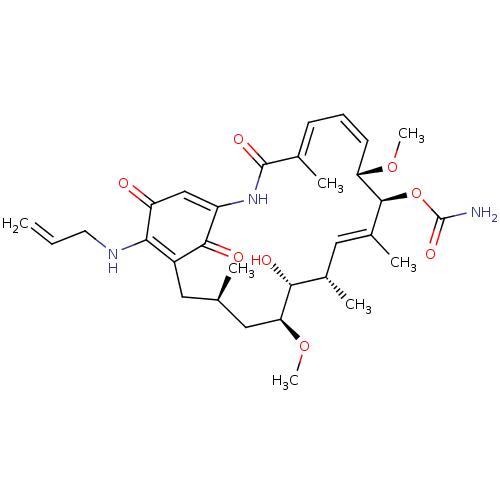
(BMS-722782 | CHEBI:64153 | TANESPIMYCIN)Show SMILES CO[C@H]1C[C@H](C)CC2=C(NCC=C)C(=O)C=C(NC(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)C2=O |r,c:7,23,t:15,21,34| Show InChI InChI=1S/C31H43N3O8/c1-8-12-33-26-21-13-17(2)14-25(41-7)27(36)19(4)15-20(5)29(42-31(32)39)24(40-6)11-9-10-18(3)30(38)34-22(28(21)37)16-23(26)35/h8-11,15-17,19,24-25,27,29,33,36H,1,12-14H2,2-7H3,(H2,32,39)(H,34,38)/b11-9-,18-10+,20-15+/t17-,19+,24+,25+,27-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of FITC-labeled geldanamycin binding to human Hsp90alpha by fluorescence polarization assay |
Bioorg Med Chem Lett 27: 237-241 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.062
BindingDB Entry DOI: 10.7270/Q28W3G91 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058044

(CHEMBL3330817)Show InChI InChI=1S/C19H13FN2O2S/c1-24-13-8-6-12(7-9-13)22-11-21-17-15(10-25-18(17)19(22)23)14-4-2-3-5-16(14)20/h2-11H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50565750
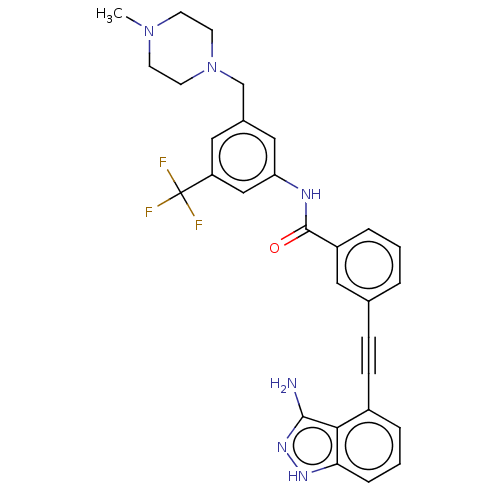
(CHEMBL4780226)Show SMILES CN1CCN(Cc2cc(NC(=O)c3cccc(c3)C#Cc3cccc4[nH]nc(N)c34)cc(c2)C(F)(F)F)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type BCR-ABL (unknown origin) incubated for 40 mins in presence of [gamma33P]ATP by scintillation method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112710
BindingDB Entry DOI: 10.7270/Q2W38122 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50207414
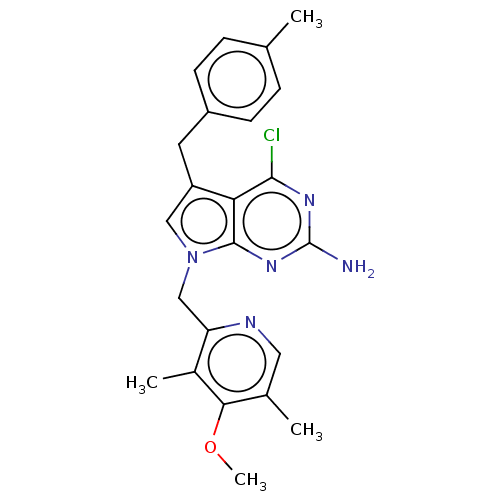
(CHEMBL3929614)Show SMILES COc1c(C)cnc(Cn2cc(Cc3ccc(C)cc3)c3c(Cl)nc(N)nc23)c1C Show InChI InChI=1S/C23H24ClN5O/c1-13-5-7-16(8-6-13)9-17-11-29(22-19(17)21(24)27-23(25)28-22)12-18-15(3)20(30-4)14(2)10-26-18/h5-8,10-11H,9,12H2,1-4H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of FITC-labeled geldanamycin binding to human Hsp90alpha by fluorescence polarization assay |
Bioorg Med Chem Lett 27: 237-241 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.062
BindingDB Entry DOI: 10.7270/Q28W3G91 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058056

(CHEMBL3330801)Show InChI InChI=1S/C18H11FN2OS/c19-13-7-4-8-14(9-13)21-11-20-16-15(10-23-17(16)18(21)22)12-5-2-1-3-6-12/h1-11H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058053

(CHEMBL3330804 | US9260448, 6)Show InChI InChI=1S/C18H11ClN2OS/c19-13-7-4-8-14(9-13)21-11-20-16-15(10-23-17(16)18(21)22)12-5-2-1-3-6-12/h1-11H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
| Assay Description
Cells of Chem3 Cell Line (HTS145C:Millipore) in which mGluR1 was stably expressed were adjusted to a density of 2×106/ml. 50 μl of the cells wer... |
US Patent US9260448 (2016)
BindingDB Entry DOI: 10.7270/Q2H9941W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data