

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194104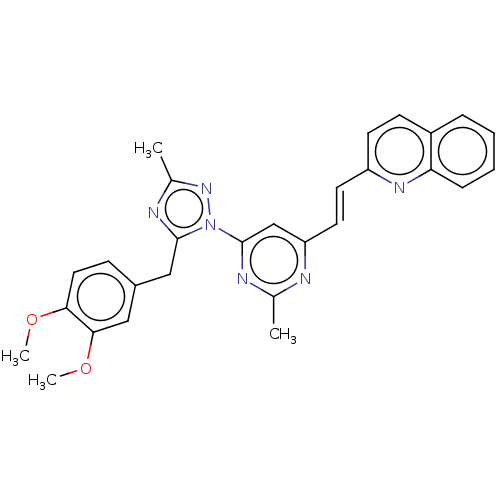 (US9200001, 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194105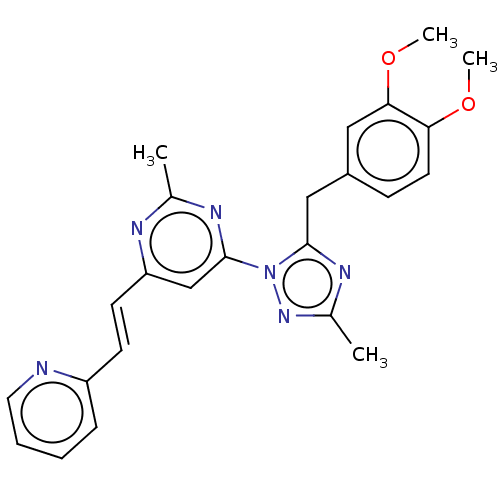 (US9200001, 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194113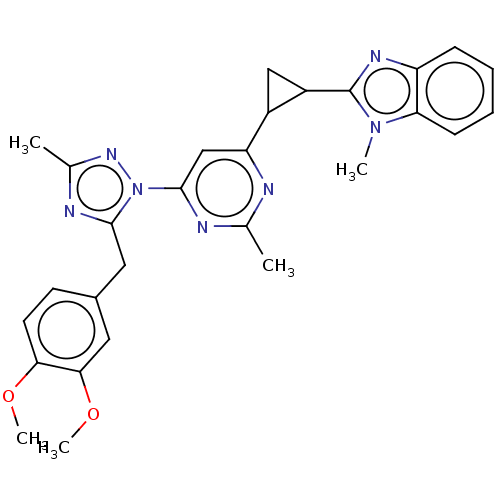 (US9200001, 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194090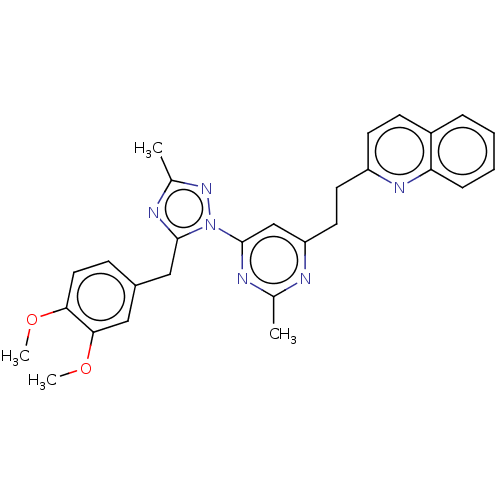 (US9200001, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194122 (US9200001, 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194223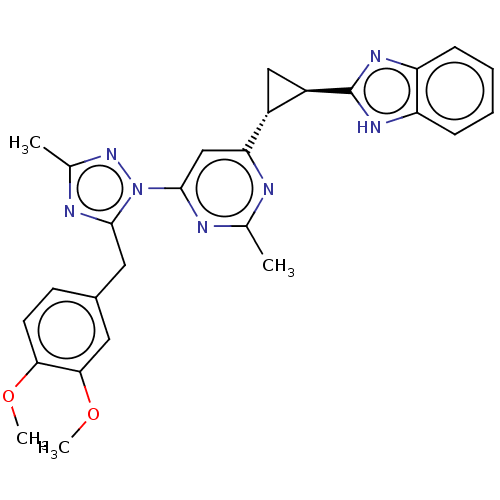 (US9200001, 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194099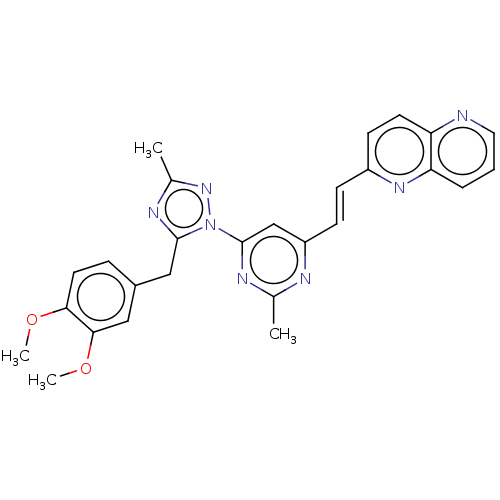 (US9200001, 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243536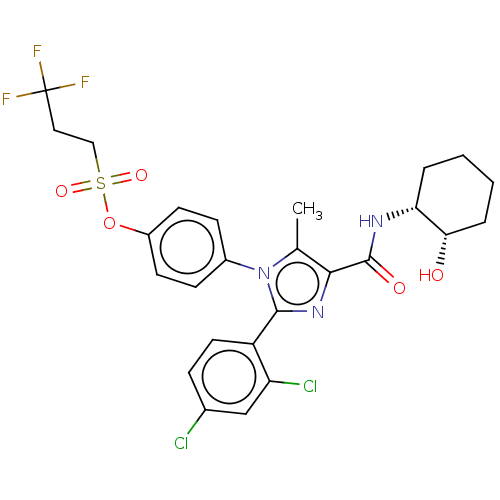 (CHEMBL4062749) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243536 (CHEMBL4062749) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50379086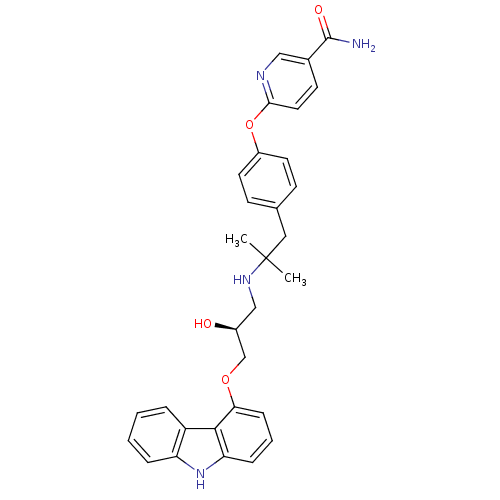 (CHEMBL2012521 | CHEMBL2012522 | LY-377604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting | ACS Med Chem Lett 2: 583-586 (2011) Article DOI: 10.1021/ml200071k BindingDB Entry DOI: 10.7270/Q20R9QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194089 (US9200001, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50379086 (CHEMBL2012521 | CHEMBL2012522 | LY-377604) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]Iodocyanopindolol from human adrenergic beta1 receptor expressed in insect sf9 cells by scintillation counting | ACS Med Chem Lett 2: 583-586 (2011) Article DOI: 10.1021/ml200071k BindingDB Entry DOI: 10.7270/Q20R9QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50315401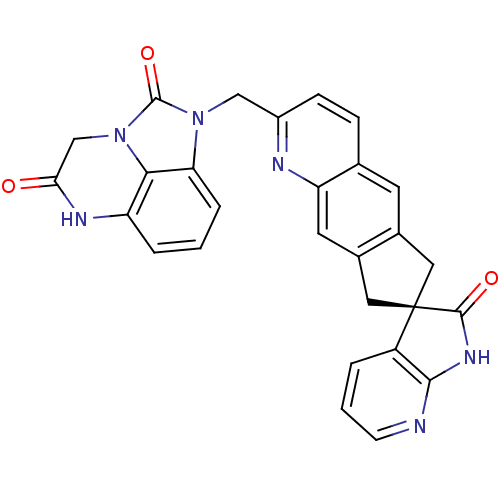 ((R)-1-((2'-oxo-1',2',6,8-tetrahydrospiro[cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Displacement of [125I]human CLR from human CGRP expressed in HEK293 cells coexpressing human RAMP1 | Bioorg Med Chem Lett 20: 2572-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.086 BindingDB Entry DOI: 10.7270/Q2765FFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076227 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194111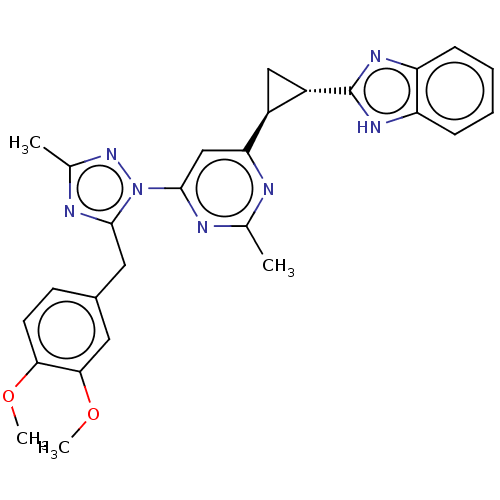 (US9200001, 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194102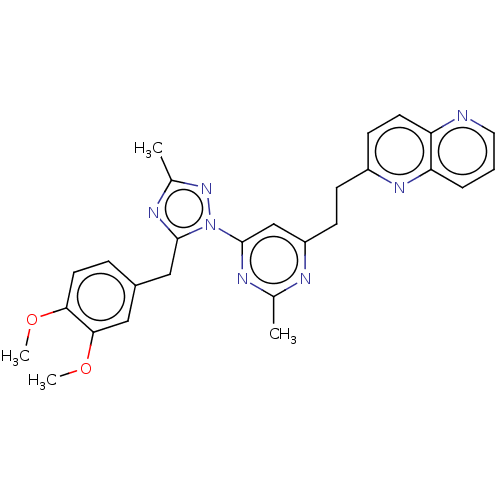 (US9200001, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194095 (US9200001, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085666 ((2S,3S,4R,5R)-5-{6-(2,2-Diphenyl-ethylamino)-2-[2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50254445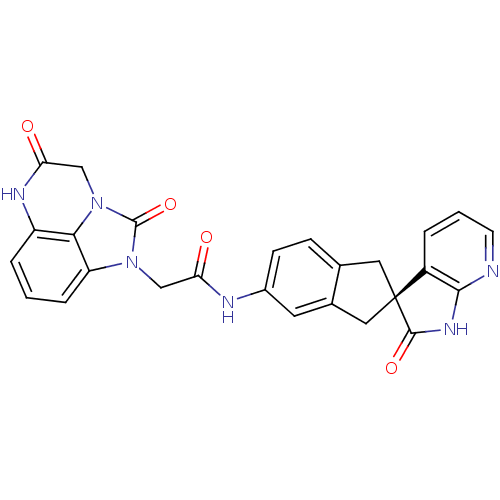 ((S)-2-(2,5-dioxo-2,4,5,6-tetrahydro-1H-imidazo[1,5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]human CGRP from human CLR expressed in HEK293 cells coexpressing human RAMP1 | Bioorg Med Chem Lett 19: 4740-2 (2009) Article DOI: 10.1016/j.bmcl.2009.06.057 BindingDB Entry DOI: 10.7270/Q2QV3MJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50254445 ((S)-2-(2,5-dioxo-2,4,5,6-tetrahydro-1H-imidazo[1,5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Displacement of [125I]human CLR from human CGRP expressed in HEK293 cells coexpressing human RAMP1 | Bioorg Med Chem Lett 20: 2572-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.086 BindingDB Entry DOI: 10.7270/Q2765FFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085674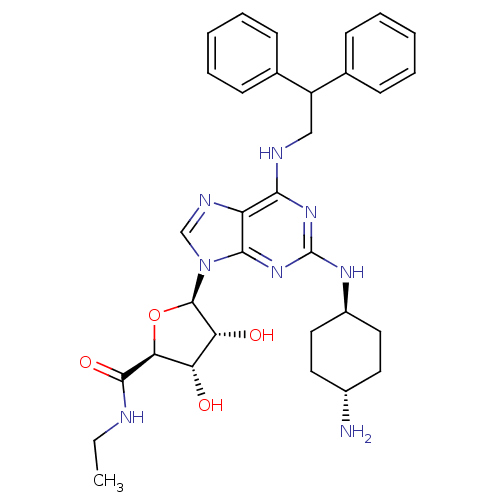 ((2S,3S,4R,5R)-5-[2-(4-Amino-cyclohexylamino)-6-(2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194101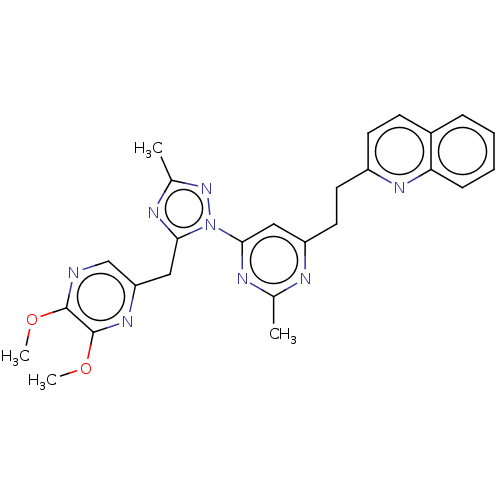 (US9200001, 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM236019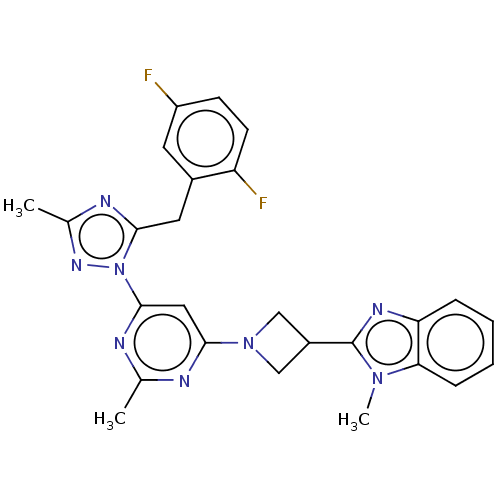 (US9365562, 57) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0650 | -58.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, S... | US Patent US9365562 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194119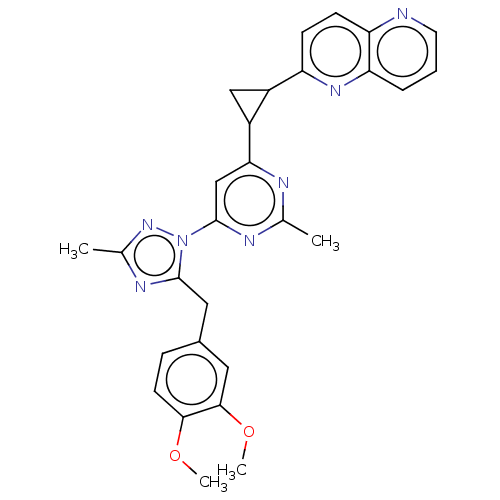 (US9200001, 34) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50144508 (4-Methyl-1-(5-phenyl-pentyl)-piperidine | CHEMBL30...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand | Bioorg Med Chem Lett 14: 2217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.02.018 BindingDB Entry DOI: 10.7270/Q2R210T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076227 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50315409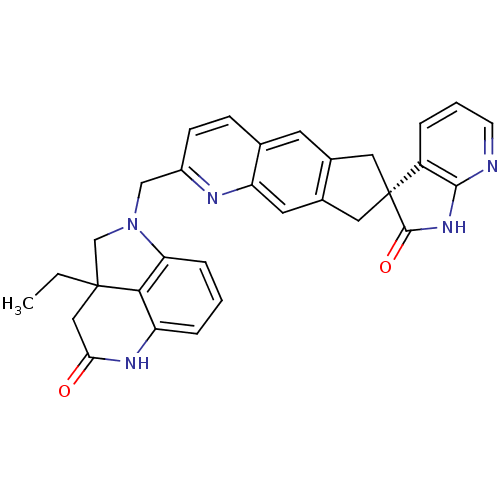 (2a-ethyl-1-(((R)-2'-oxo-1',2',6,8-tetrahydrospiro[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Displacement of [125I]human CLR from human CGRP expressed in HEK293 cells coexpressing human RAMP1 | Bioorg Med Chem Lett 20: 2572-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.086 BindingDB Entry DOI: 10.7270/Q2765FFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076224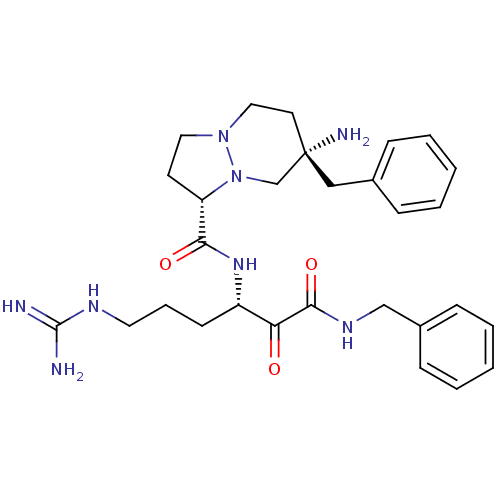 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194094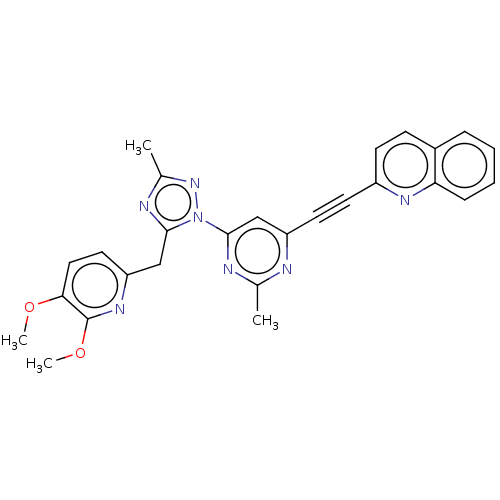 (US9200001, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076224 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085671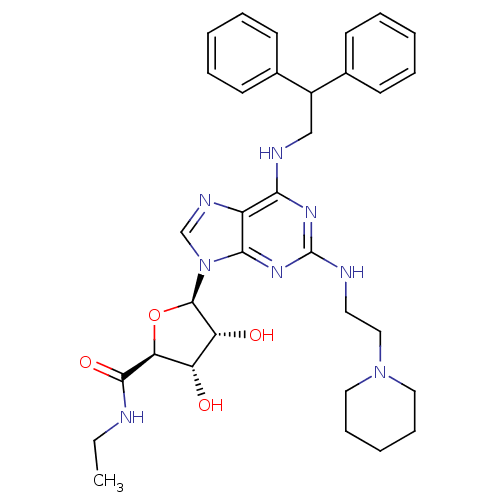 ((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-(2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194145 (US9200001, 61) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50144518 (1-(5,5-Diphenyl-pentyl)-4-methyl-piperidine | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand | Bioorg Med Chem Lett 14: 2217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.02.018 BindingDB Entry DOI: 10.7270/Q2R210T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243625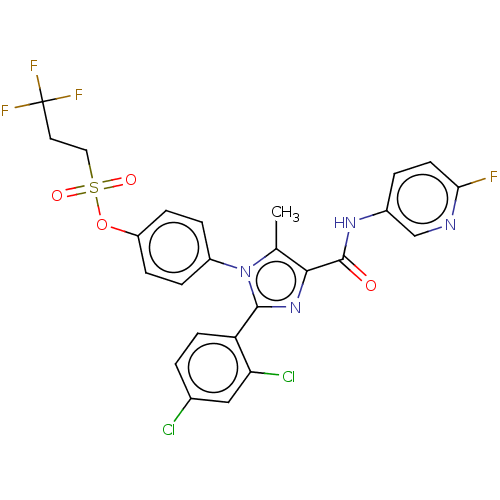 (CHEMBL4094098) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243545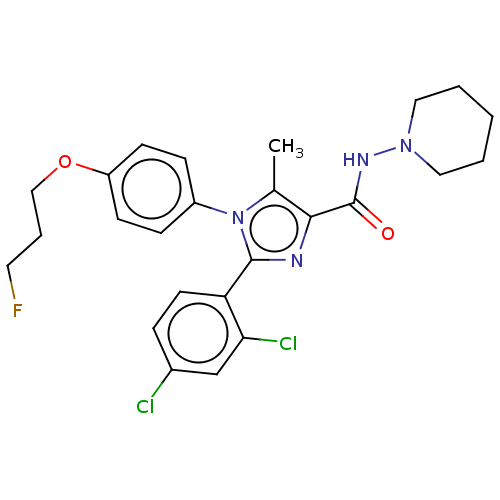 (CHEMBL4078689) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50246967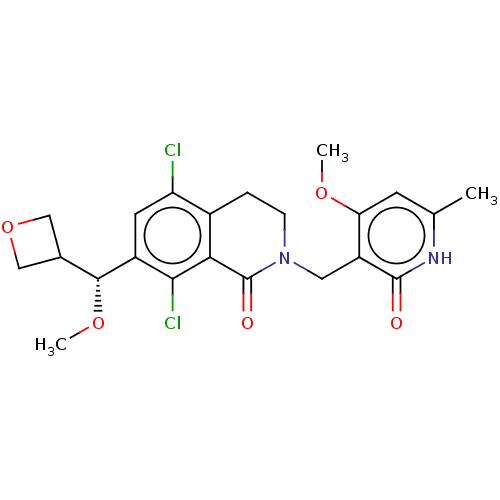 (CHEMBL4080228 | US10570121, Example 81) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Curated by ChEMBL | Assay Description Binding affinity to EZH2 (unknown origin) | J Med Chem 61: 650-665 (2018) Article DOI: 10.1021/acs.jmedchem.7b01375 BindingDB Entry DOI: 10.7270/Q2X069G8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085668 ((2S,3S,4R,5R)-5-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243608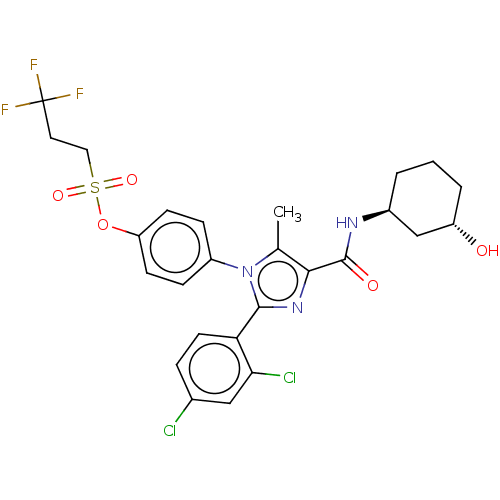 (CHEMBL4100882) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243608 (CHEMBL4100882) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243625 (CHEMBL4094098) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243545 (CHEMBL4078689) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243589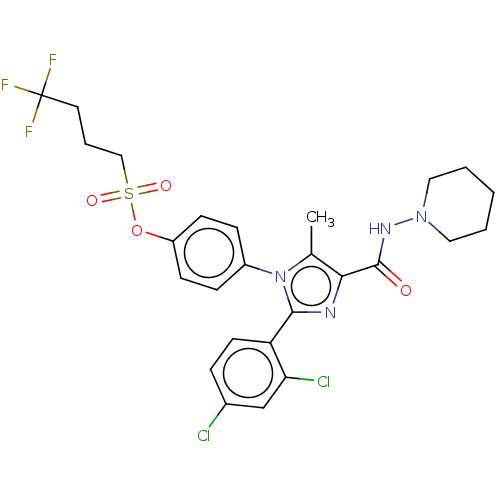 (CHEMBL4095223) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243589 (CHEMBL4095223) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194106 (US9200001, 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM236020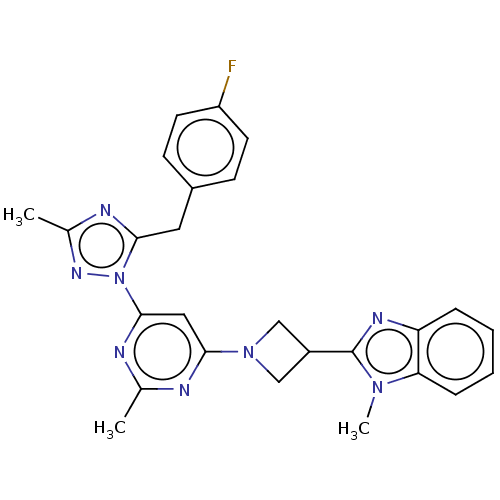 (US9365562, 58) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, S... | US Patent US9365562 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50296785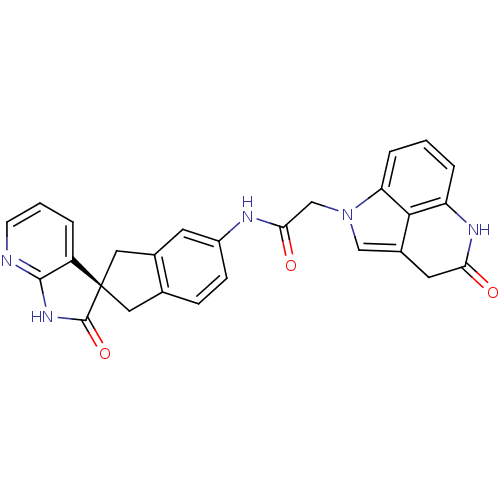 ((S)-N-(2'-oxo-1,1',2',3-tetrahydrospiro[indene-2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]human CGRP from human CLR expressed in HEK293 cells coexpressing human RAMP1 | Bioorg Med Chem Lett 19: 4740-2 (2009) Article DOI: 10.1016/j.bmcl.2009.06.057 BindingDB Entry DOI: 10.7270/Q2QV3MJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50315407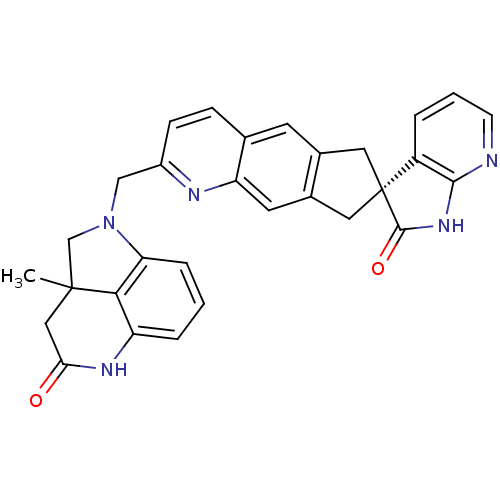 (2a-methyl-1-(((R)-2'-oxo-1',2',6,8-tetrahydrospiro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Displacement of [125I]human CLR from human CGRP expressed in HEK293 cells coexpressing human RAMP1 | Bioorg Med Chem Lett 20: 2572-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.086 BindingDB Entry DOI: 10.7270/Q2765FFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243645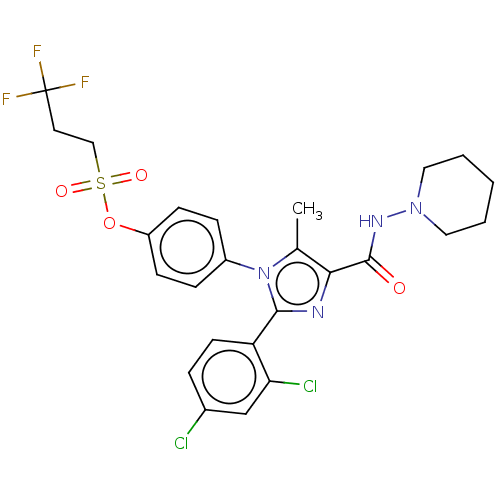 (CHEMBL4087520) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243627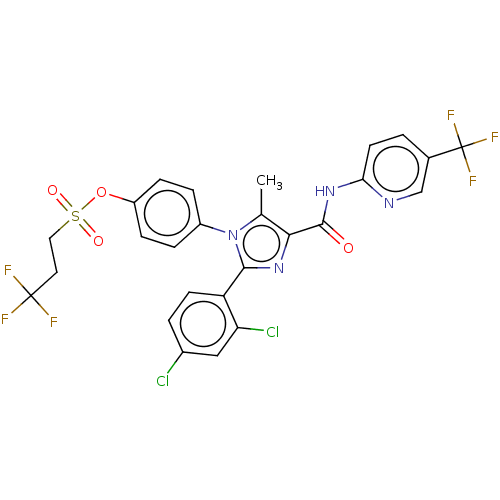 (CHEMBL4068708) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243588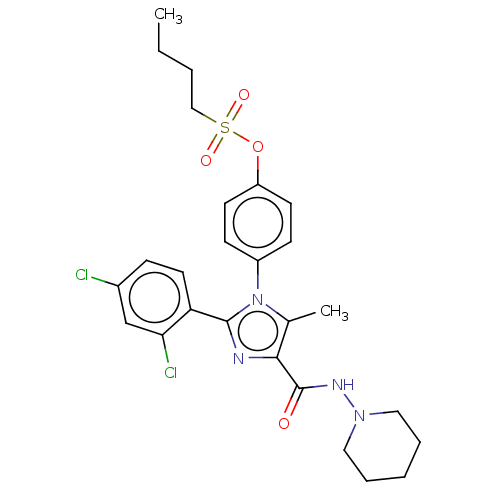 (CHEMBL4067800) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 20145 total ) | Next | Last >> |