

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50124564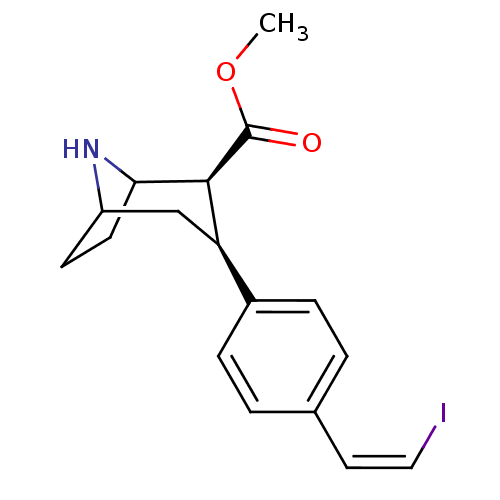 (CHEMBL174088 | methyl 3-{4-[2-iodo-(Z)-1-ethenyl]p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description In vitro competitive binding versus [3H]-citalopram in murine kidney cells transfected with cDNA for human serotonin transporter (SERT) | J Med Chem 46: 925-35 (2003) Article DOI: 10.1021/jm0100180 BindingDB Entry DOI: 10.7270/Q2W66K4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202775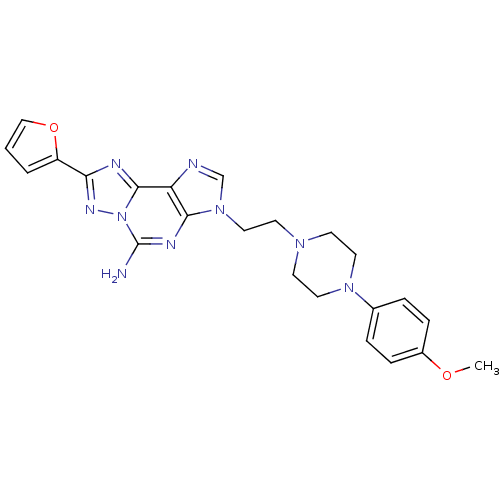 (8-(furan-2-yl)-3-(2-(4-(4-methoxyphenyl)piperazin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50124565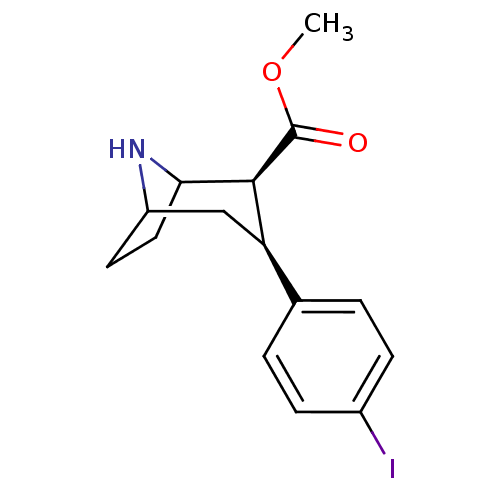 (CHEMBL353333 | methyl 3-(4-iodophenyl)-(2S,3S)-8-a...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Invitro competitive binding versus [N-methyl-3H]-WIN 35428 in murine kidney cells transfected with cDNA for human dopamine transporter (DAT) | J Med Chem 46: 925-35 (2003) Article DOI: 10.1021/jm0100180 BindingDB Entry DOI: 10.7270/Q2W66K4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50163020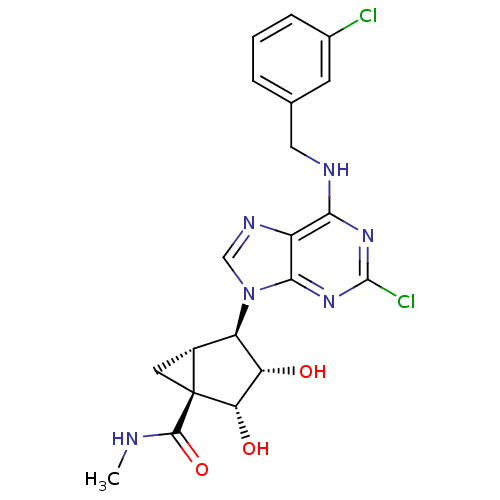 ((1S,2R,3S,4R,5S)-4-(6-(3-chlorobenzylamino)-2-chlo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [125I]-AB-MECA binding to human Adenosine A3 receptor expressed in CHO cells | J Med Chem 48: 1745-58 (2005) Article DOI: 10.1021/jm049580r BindingDB Entry DOI: 10.7270/Q23F4P52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50163020 ((1S,2R,3S,4R,5S)-4-(6-(3-chlorobenzylamino)-2-chlo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 16: 8546-56 (2008) Article DOI: 10.1016/j.bmc.2008.08.007 BindingDB Entry DOI: 10.7270/Q20R9P71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202777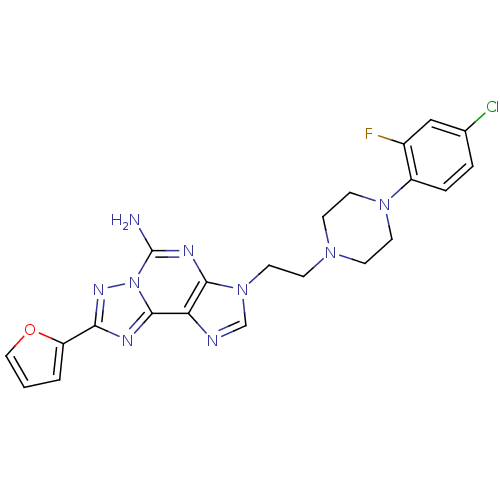 (3-(2-(4-(4-chloro-2-fluorophenyl)piperazin-1-yl)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Binding affinity to orexin receptor 1 (unknown origin) | Bioorg Med Chem Lett 23: 4761-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.057 BindingDB Entry DOI: 10.7270/Q25140NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM104692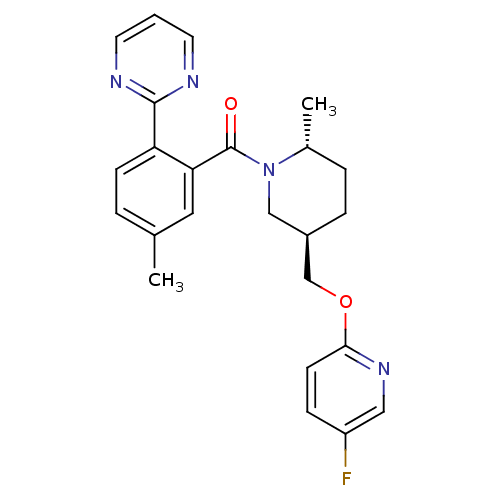 (US8569311, E-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Binding affinity to orexin receptor 2 (unknown origin) | Bioorg Med Chem Lett 23: 4761-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.057 BindingDB Entry DOI: 10.7270/Q25140NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50204794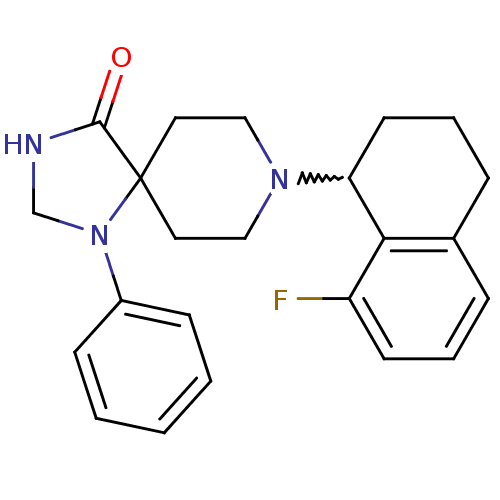 (8-(8-fluoro-1,2,3,4-tetrahydro-naphthalen-1-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 2281-4 (2007) Article DOI: 10.1016/j.bmcl.2007.01.069 BindingDB Entry DOI: 10.7270/Q2T43SRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364980 (CHEMBL1950775) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364980 (CHEMBL1950775) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [125I]-AB-MECA binding to rat Adenosine A3 receptor expressed in CHO cells | J Med Chem 48: 1745-58 (2005) Article DOI: 10.1021/jm049580r BindingDB Entry DOI: 10.7270/Q23F4P52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364981 (CHEMBL1950776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364981 (CHEMBL1950776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50085072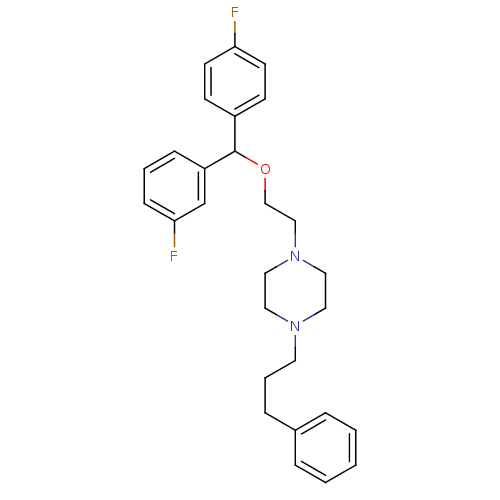 (1-{2-[(3-Fluoro-phenyl)-(4-fluoro-phenyl)-methoxy]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Competitive binding versus [N-methyl-3H]-WIN 35,428 in murine kidney cells transfected with human dopamine transporter | J Med Chem 43: 639-48 (2000) BindingDB Entry DOI: 10.7270/Q27S7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364981 (CHEMBL1950776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364980 (CHEMBL1950775) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50163025 ((1S,2R,3S,4R,5S)-4-(6-(3-bromobenzylamino)-2-chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [125I]-AB-MECA binding to human Adenosine A3 receptor expressed in CHO cells | J Med Chem 48: 1745-58 (2005) Article DOI: 10.1021/jm049580r BindingDB Entry DOI: 10.7270/Q23F4P52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26927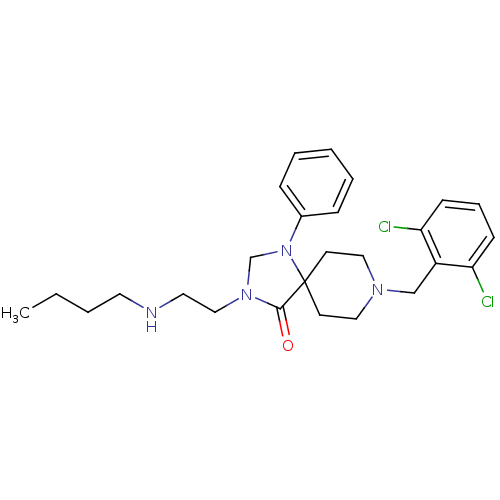 (3-[2-(butylamino)ethyl]-8-[(2,6-dichlorophenyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50371662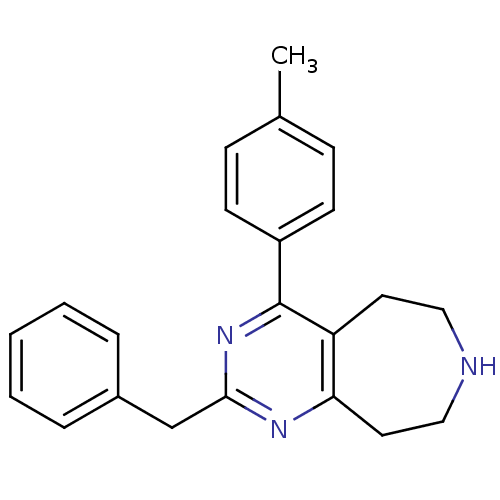 (CHEMBL269974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells | Bioorg Med Chem Lett 18: 2103-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.090 BindingDB Entry DOI: 10.7270/Q2571CW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202771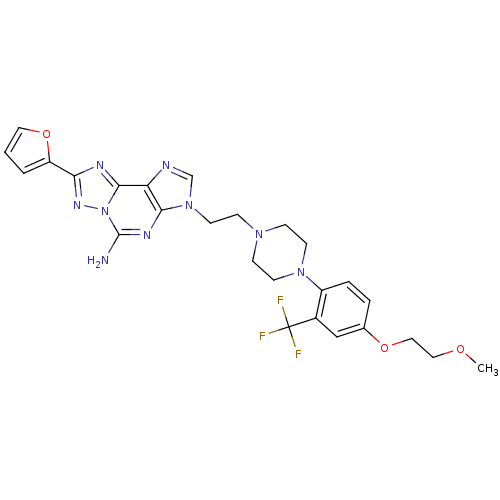 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)-2-(tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Binding affinity to orexin receptor 2 (unknown origin) | Bioorg Med Chem Lett 23: 4761-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.057 BindingDB Entry DOI: 10.7270/Q25140NJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Binding affinity to orexin receptor 2 (unknown origin) | Bioorg Med Chem Lett 23: 4761-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.057 BindingDB Entry DOI: 10.7270/Q25140NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202788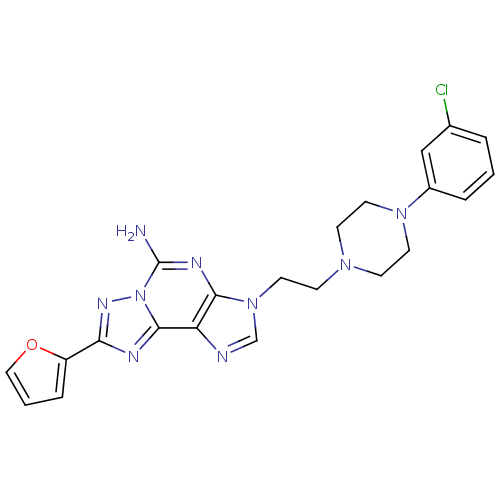 (3-(2-(4-(3-chlorophenyl)piperazin-1-yl)ethyl)-8-(f...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26946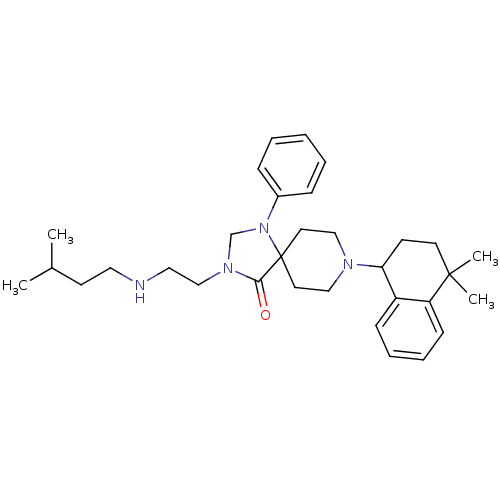 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA or [3H]CGS 21680 from human adenosine A1 receptor in CHO cells | J Med Chem 48: 8103-7 (2005) Article DOI: 10.1021/jm050726b BindingDB Entry DOI: 10.7270/Q2CZ36QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50006774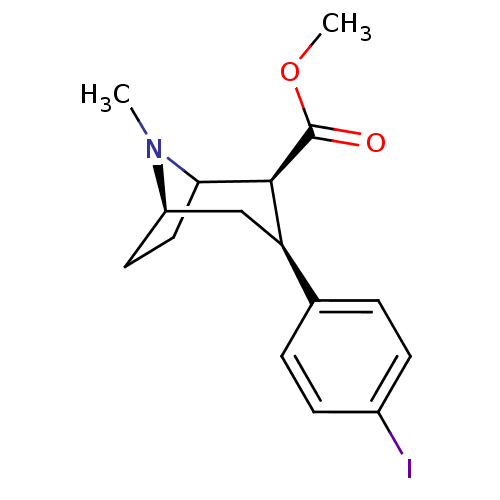 ((2S,3S)-methyl 3-(4-iodophenyl)-8-methyl-8-aza-bic...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description In vitro competitive binding versus [N-methyl-3H]-WIN 35428 in murine kidney cells transfected with cDNA for human dopamine transporter (DAT) | J Med Chem 46: 925-35 (2003) Article DOI: 10.1021/jm0100180 BindingDB Entry DOI: 10.7270/Q2W66K4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50085071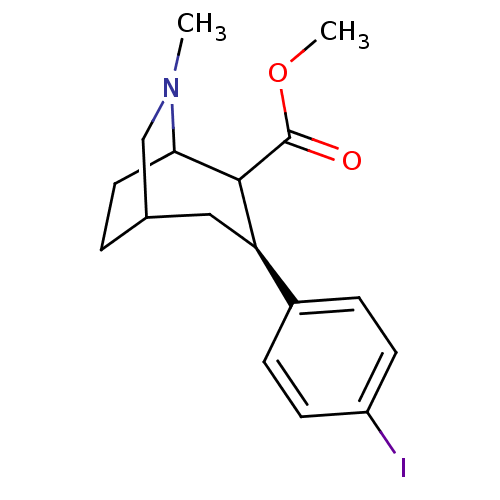 (3-(4-Iodo-phenyl)-6-methyl-6-aza-bicyclo[3.2.2]non...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description In vitro affinity determined using [3H]-WIN- 35428 in murine kidney cells transfected with human dopamine transporter (DAT) | J Med Chem 43: 639-48 (2000) BindingDB Entry DOI: 10.7270/Q27S7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50179182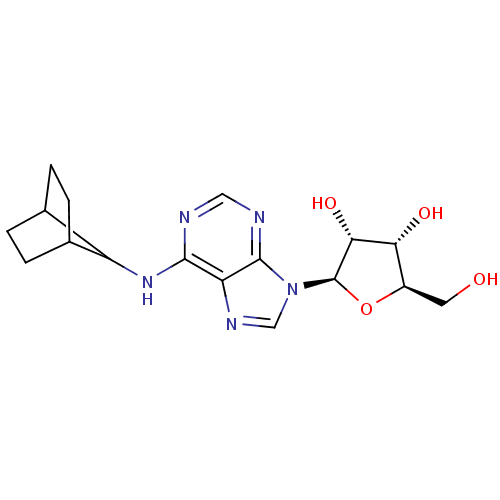 ((2R,3R,4S,5R)-2-(6-(bicyclo[2.2.1]heptan-7-ylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA or [3H]CGS 21680 from human adenosine A1 receptor in CHO cells | J Med Chem 48: 8103-7 (2005) Article DOI: 10.1021/jm050726b BindingDB Entry DOI: 10.7270/Q2CZ36QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Invitro competitive binding versus [N-methyl-3H]-WIN 35428 in murine kidney cells transfected with cDNA for human dopamine transporter (DAT) | J Med Chem 46: 925-35 (2003) Article DOI: 10.1021/jm0100180 BindingDB Entry DOI: 10.7270/Q2W66K4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50438824 (CHEMBL2413365) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Binding affinity to orexin receptor 1 (unknown origin) | Bioorg Med Chem Lett 23: 4761-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.057 BindingDB Entry DOI: 10.7270/Q25140NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26942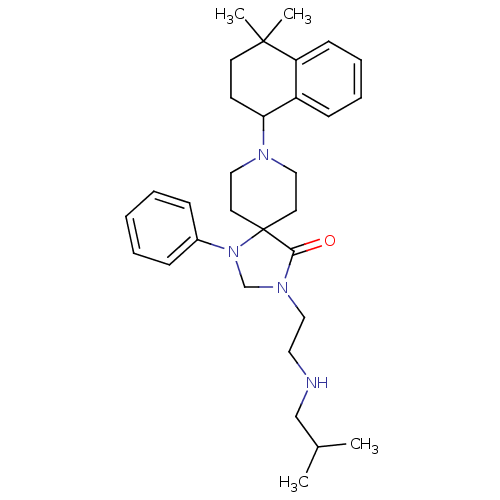 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26924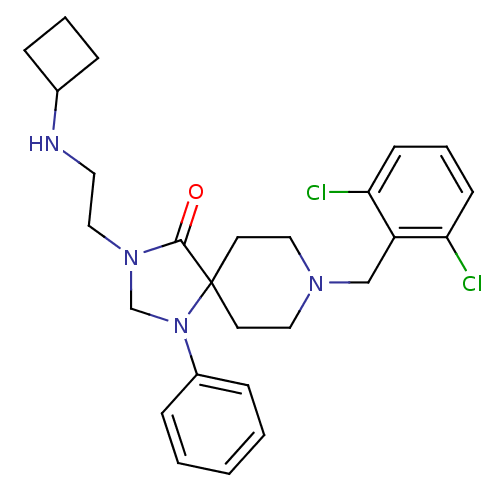 (3-[2-(cyclobutylamino)ethyl]-8-[(2,6-dichloropheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26926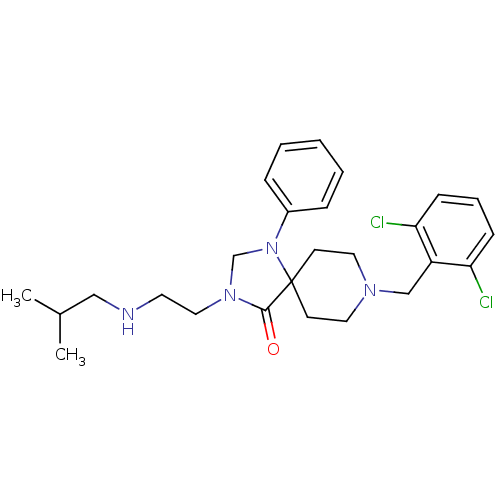 (8-[(2,6-dichlorophenyl)methyl]-3-{2-[(2-methylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26923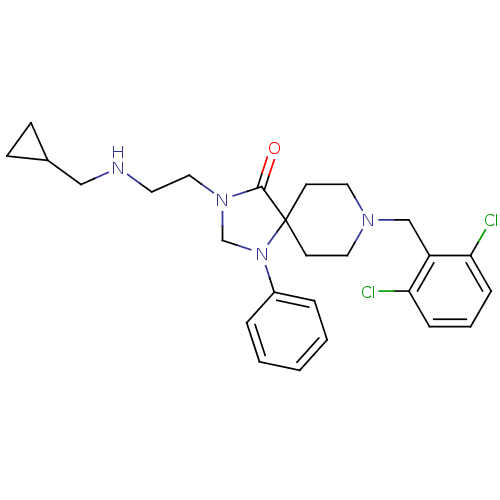 (3-{2-[(cyclopropylmethyl)amino]ethyl}-8-[(2,6-dich...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50028059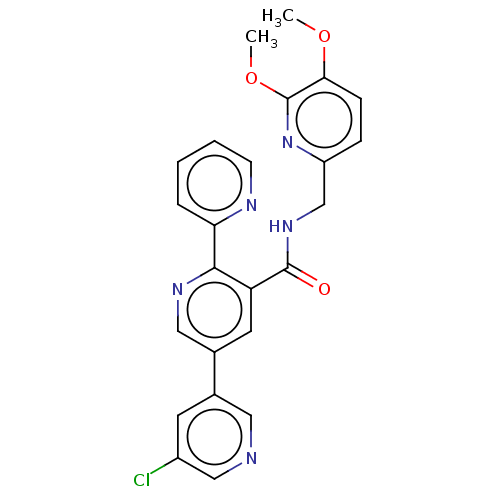 (CHEMBL3338866) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec... | J Med Chem 58: 5620-36 (2015) Article DOI: 10.1021/acs.jmedchem.5b00742 BindingDB Entry DOI: 10.7270/Q2QR4ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26945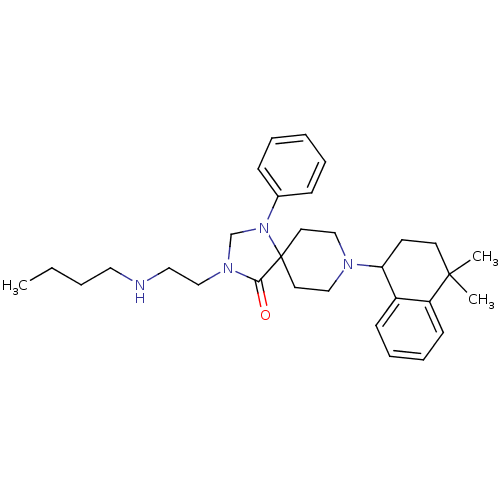 (3-[2-(butylamino)ethyl]-8-(4,4-dimethyl-1,2,3,4-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50163029 (1N-methyl-4-[2-chloro-6-(5-iodo-2-methoxybenzylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [125I]-AB-MECA binding to human Adenosine A3 receptor expressed in CHO cells | J Med Chem 48: 1745-58 (2005) Article DOI: 10.1021/jm049580r BindingDB Entry DOI: 10.7270/Q23F4P52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50371682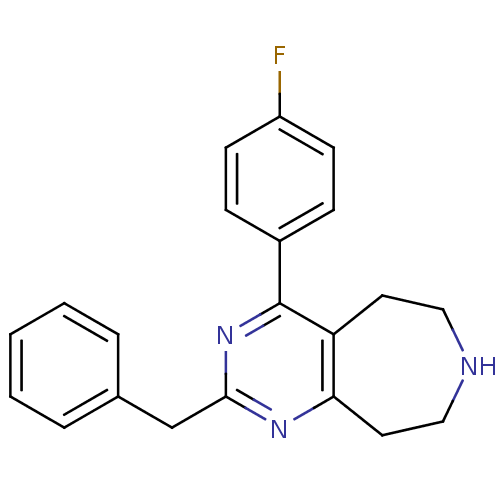 (CHEMBL270188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells | Bioorg Med Chem Lett 18: 2103-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.090 BindingDB Entry DOI: 10.7270/Q2571CW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Binding affinity to orexin receptor 1 (unknown origin) | Bioorg Med Chem Lett 23: 4761-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.057 BindingDB Entry DOI: 10.7270/Q25140NJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26925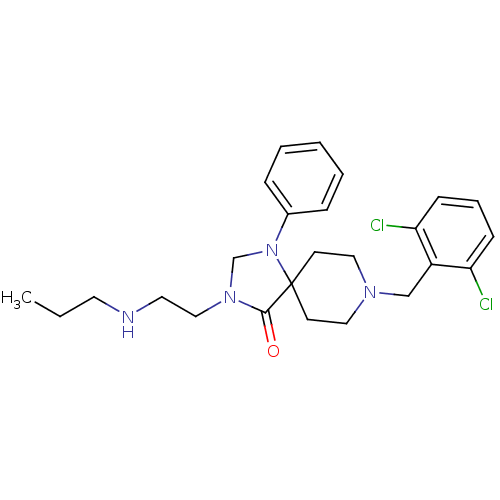 (8-[(2,6-dichlorophenyl)methyl]-1-phenyl-3-[2-(prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26954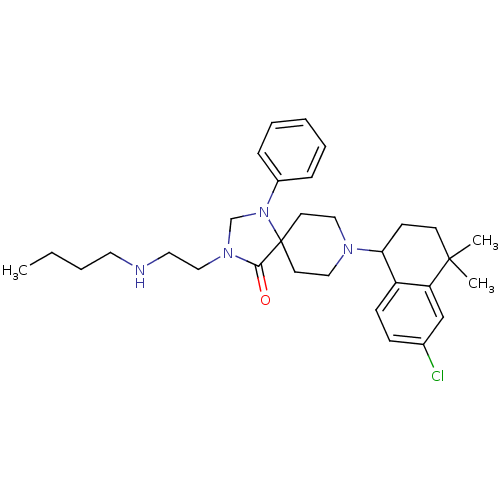 (3-[2-(butylamino)ethyl]-8-(6-chloro-4,4-dimethyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50085071 (3-(4-Iodo-phenyl)-6-methyl-6-aza-bicyclo[3.2.2]non...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description In vitro affinity determined using [3H]-citalopram in murine kidney cells transfected with human serotonin transporter (SERT) | J Med Chem 43: 639-48 (2000) BindingDB Entry DOI: 10.7270/Q27S7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50006774 ((2S,3S)-methyl 3-(4-iodophenyl)-8-methyl-8-aza-bic...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description In vitro competitive binding versus [3H]-citalopram in murine kidney cells transfected with cDNA for human serotonin transporter (SERT) | J Med Chem 46: 925-35 (2003) Article DOI: 10.1021/jm0100180 BindingDB Entry DOI: 10.7270/Q2W66K4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50163031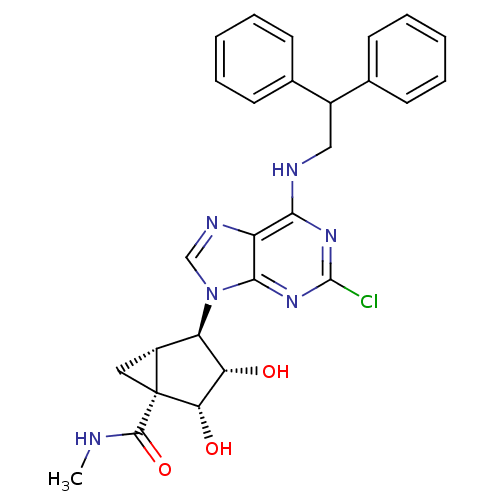 ((1S,2R,3S,4R,5S)-4-(2-chloro-6-(2,2-diphenylethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [125I]-AB-MECA binding to human Adenosine A3 receptor expressed in CHO cells | J Med Chem 48: 1745-58 (2005) Article DOI: 10.1021/jm049580r BindingDB Entry DOI: 10.7270/Q23F4P52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202790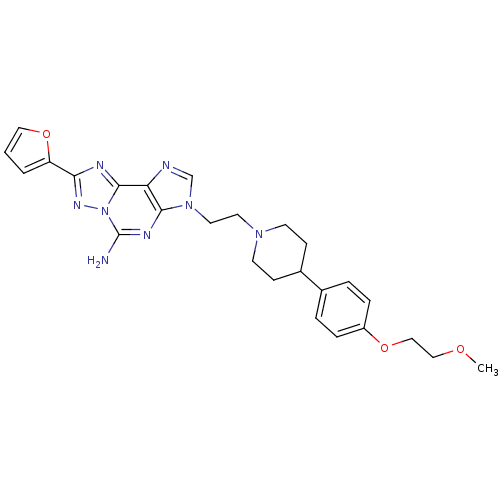 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50371669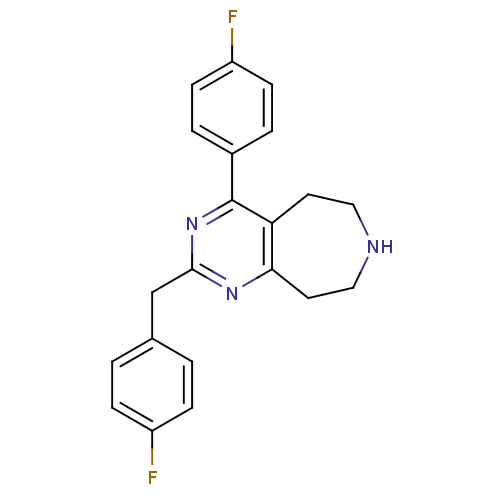 (CHEMBL402164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells | Bioorg Med Chem Lett 18: 2103-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.090 BindingDB Entry DOI: 10.7270/Q2571CW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26922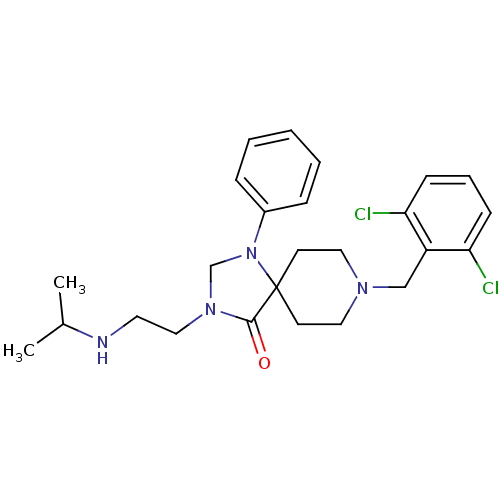 (8-[(2,6-dichlorophenyl)methyl]-1-phenyl-3-[2-(prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202779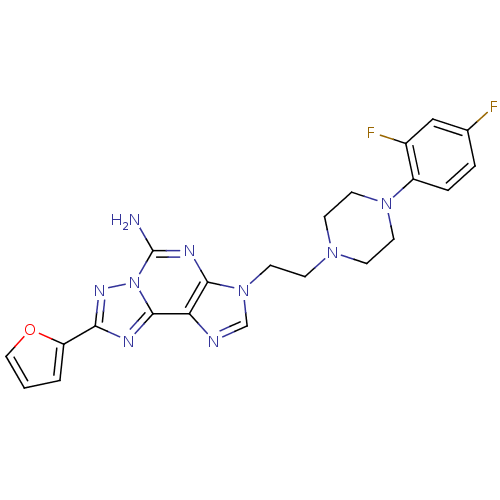 (3-(2-(4-(2,4-difluorophenyl)piperazin-1-yl)ethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26921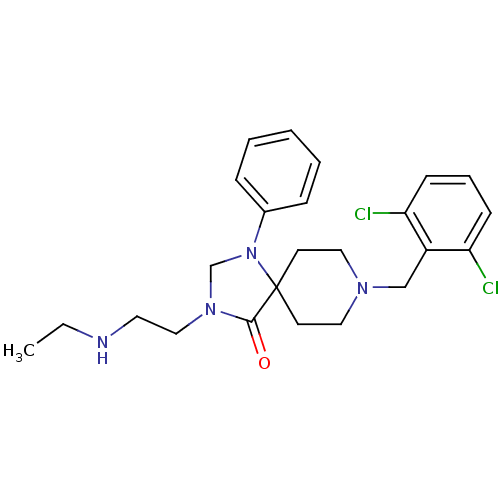 (8-[(2,6-dichlorophenyl)methyl]-3-[2-(ethylamino)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 18025 total ) | Next | Last >> |