

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50098203 (1-{4-[2-(1H-Imidazol-4-yl)-ethylsulfanyl]-phenyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of [125I]-iodoproxyfan from rat histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 11: 951-4 (2001) BindingDB Entry DOI: 10.7270/Q28P5ZRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132057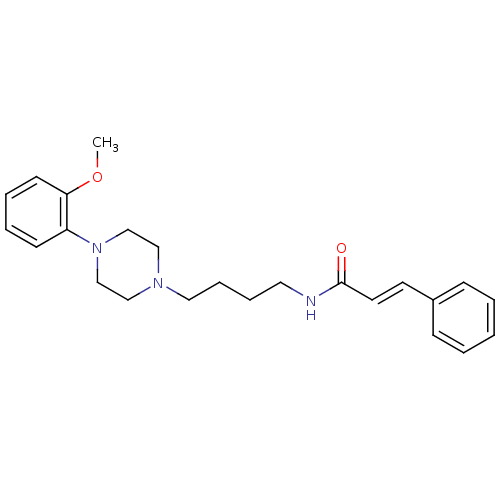 (CHEMBL1180430 | CHEMBL126128 | N-{4-[4-(2-Methoxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132040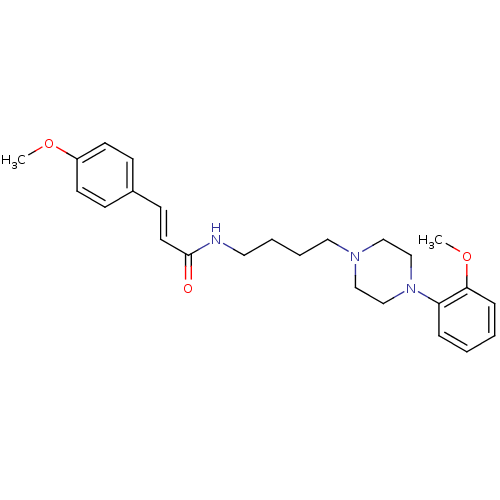 (3-(4-Methoxy-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50077001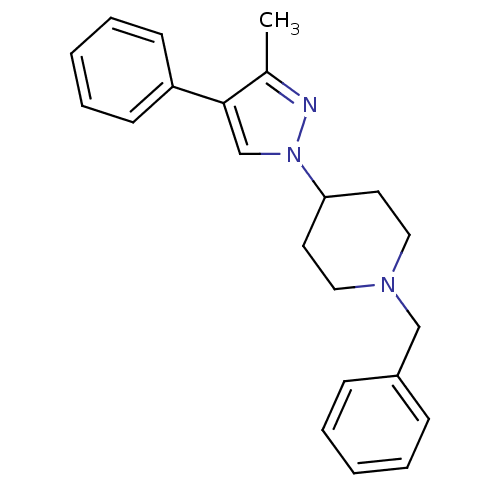 (1-Benzyl-4-(3-methyl-4-phenyl-pyrazol-1-yl)-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity of compound towards human Dopamine receptor D4 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132031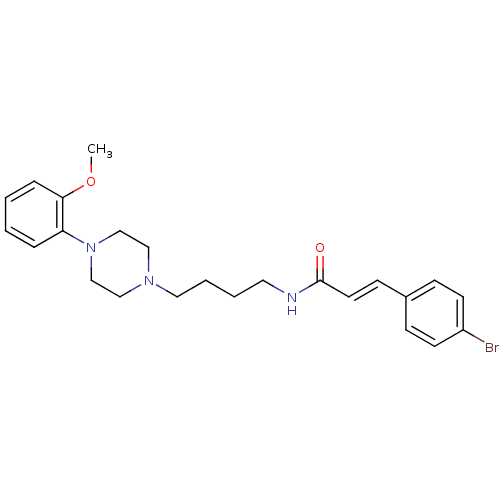 (3-(4-Bromo-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132047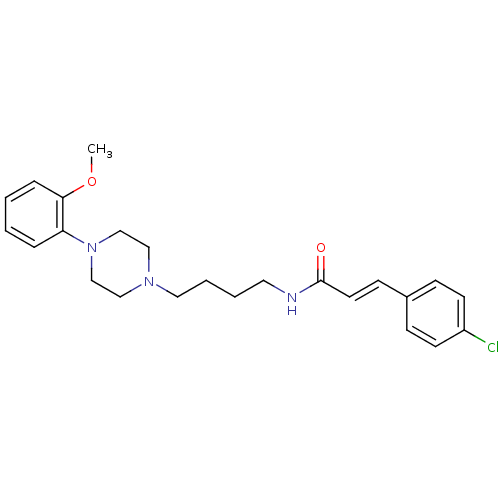 (3-(4-Chloro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132060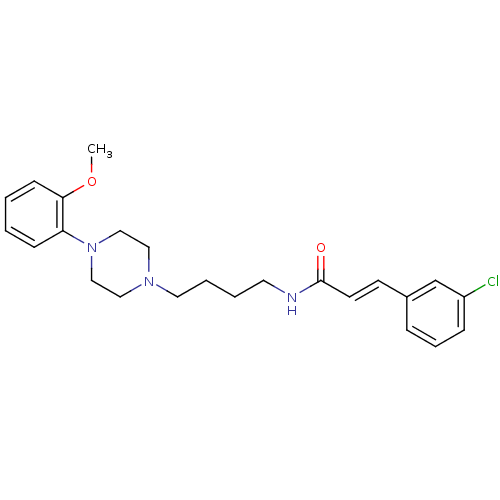 (3-(3-Chloro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132059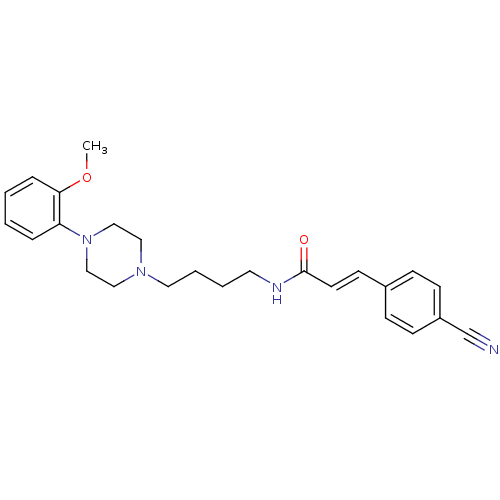 (3-(4-Cyano-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of human full length PKMYT1 expressed in HEK293 cells using EFS (247 to 259 residues) as substrate after 1 hr by fluorescence polarization... | Bioorg Med Chem 26: 4014-4024 (2018) Article DOI: 10.1016/j.bmc.2018.06.027 BindingDB Entry DOI: 10.7270/Q29Z97K0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132012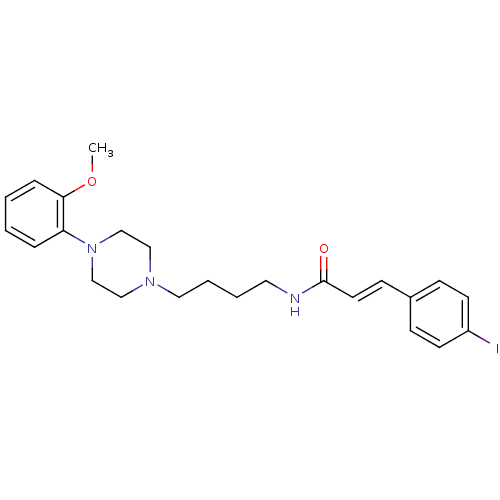 ((E)-3-(4-Iodo-phenyl)-N-{4-[4-(2-methoxy-phenyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase (Homo sapiens (Human)) | BDBM50506980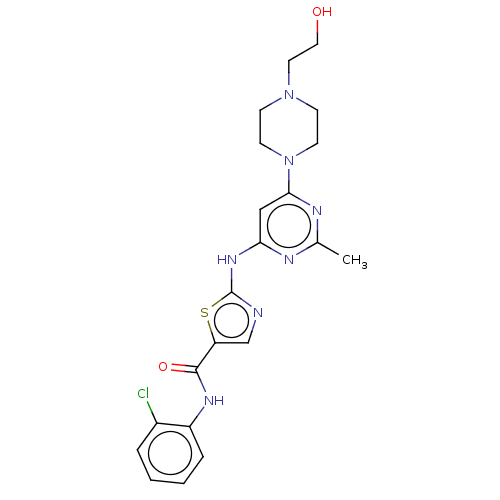 (CHEMBL4583196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Displacement of (6-FAM)KI(pY)VV from full length PKMYT1 (unknown origin) by fluorescence polarization immuno assay | Eur J Med Chem 161: 479-492 (2019) Article DOI: 10.1016/j.ejmech.2018.10.050 BindingDB Entry DOI: 10.7270/Q2C53Q5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132013 (CHEMBL341542 | N-{4-[4-(2-Methoxy-phenyl)-piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50007182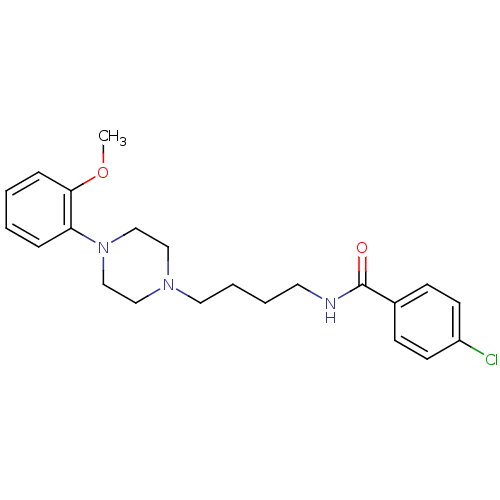 (4-Chloro-N-{4-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132027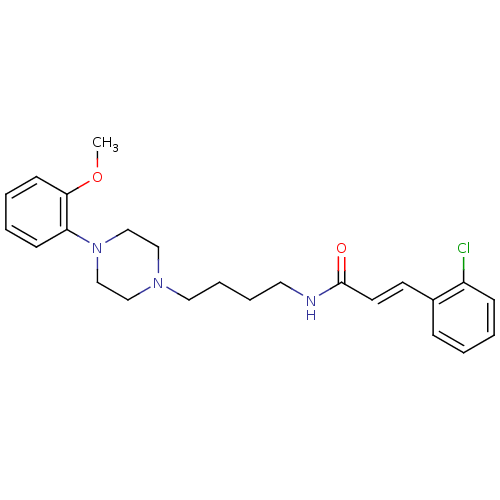 (3-(2-Chloro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132076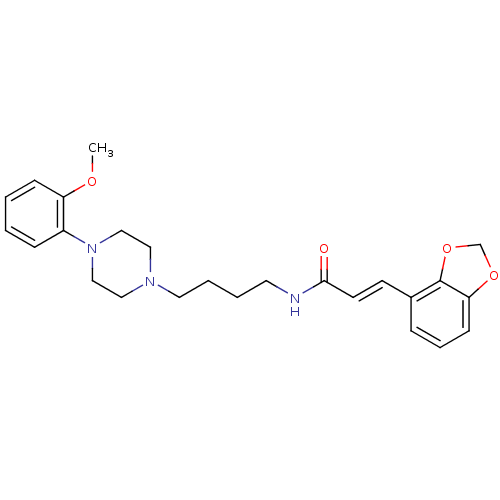 (3-Benzo[1,3]dioxol-4-yl-N-{4-[4-(2-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132019 (3-(2-Methoxy-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132070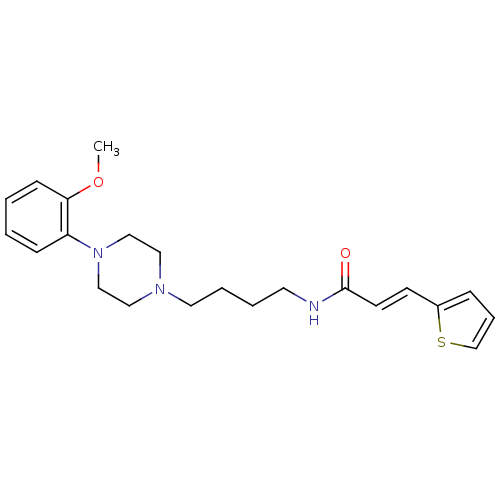 (CHEMBL338552 | N-{4-[4-(2-Methoxy-phenyl)-piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132055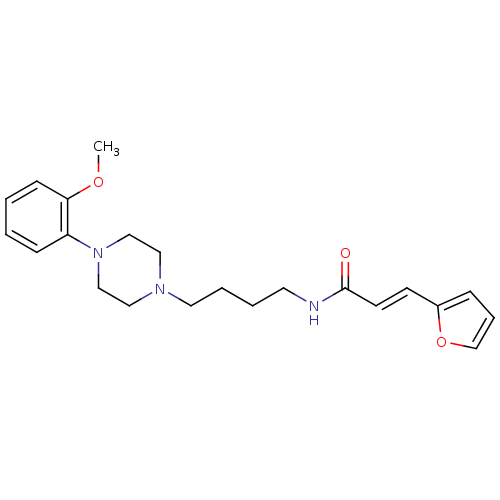 (3-Furan-2-yl-N-{4-[4-(2-methoxy-phenyl)-piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132039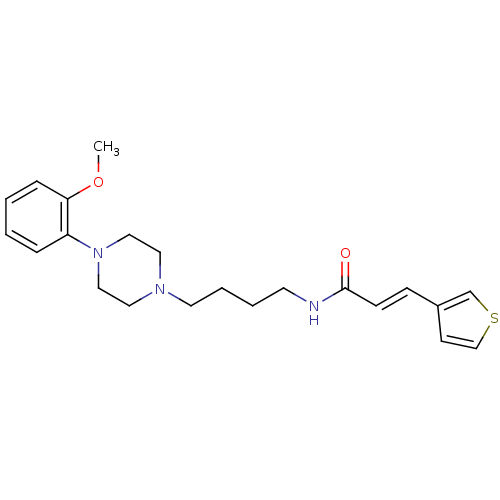 (CHEMBL126280 | N-{4-[4-(2-Methoxy-phenyl)-piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132050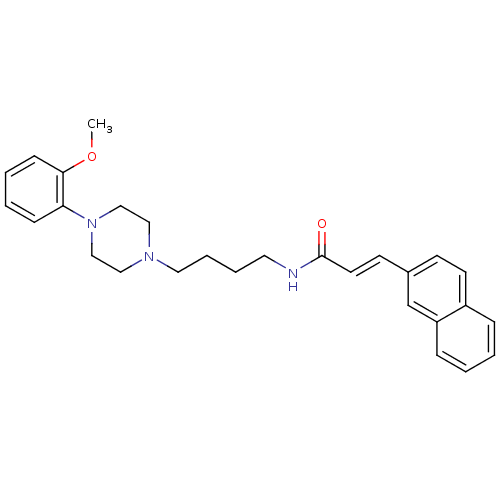 (CHEMBL126749 | N-{4-[4-(2-Methoxy-phenyl)-piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132069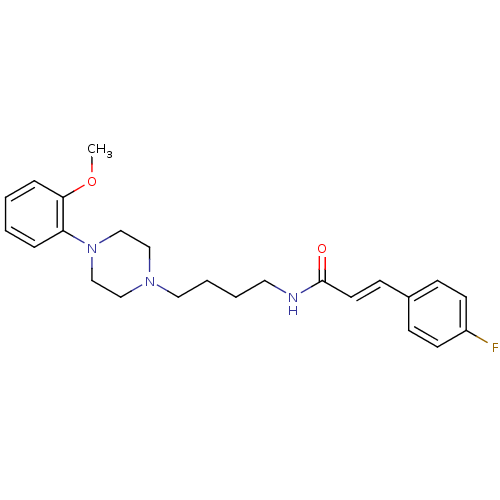 (3-(4-Fluoro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132072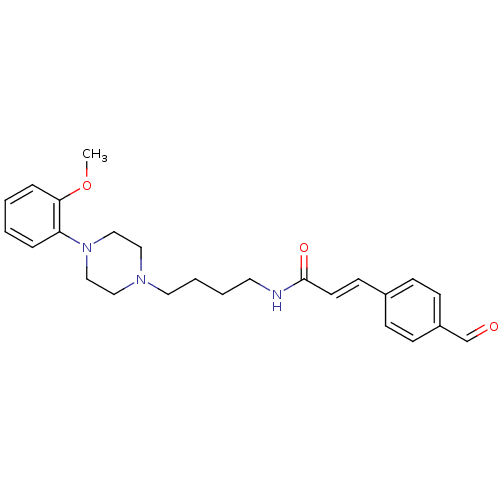 (3-(4-Formyl-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132067 (CHEMBL129465 | N-{4-[4-(2-Methoxy-phenyl)-piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132053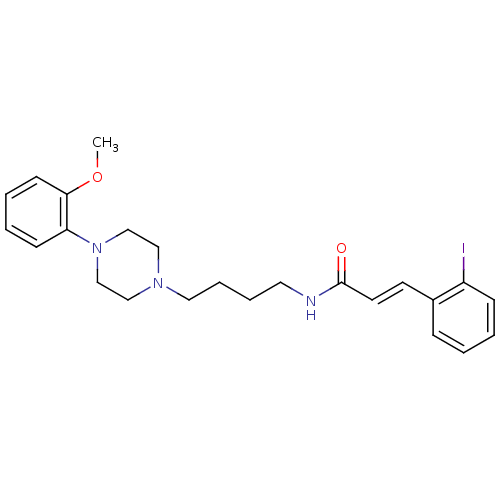 (3-(2-Iodo-phenyl)-N-{4-[4-(2-methoxy-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase (Homo sapiens (Human)) | BDBM3096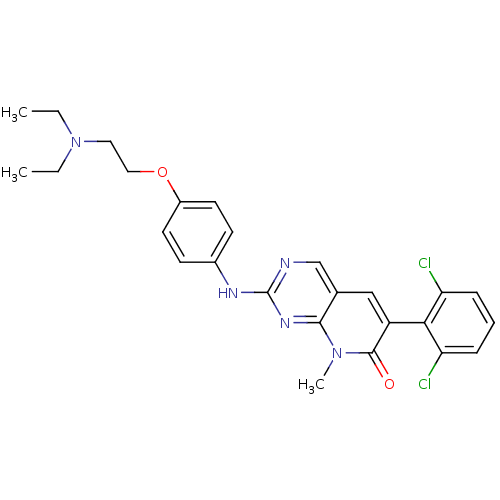 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Displacement of (6-FAM)KI(pY)VV from full length PKMYT1 (unknown origin) by fluorescence polarization immuno assay | Eur J Med Chem 161: 479-492 (2019) Article DOI: 10.1016/j.ejmech.2018.10.050 BindingDB Entry DOI: 10.7270/Q2C53Q5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase (Homo sapiens (Human)) | BDBM3096 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of human full length PKMYT1 expressed in HEK293 cells using EFS (247 to 259 residues) as substrate after 1 hr by fluorescence polarization... | Bioorg Med Chem 26: 4014-4024 (2018) Article DOI: 10.1016/j.bmc.2018.06.027 BindingDB Entry DOI: 10.7270/Q29Z97K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50119380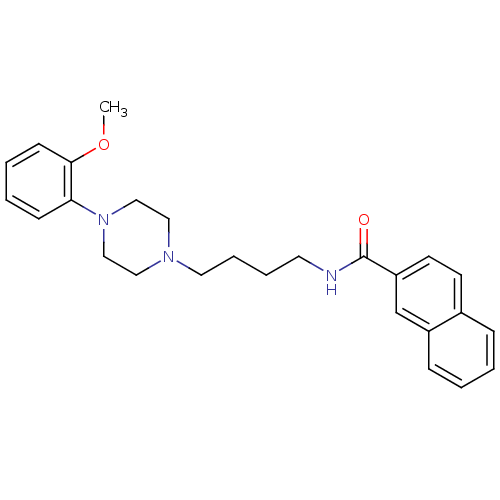 (CHEMBL25236 | CHEMBL540612 | N-(4-(4-(2-methoxyphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132042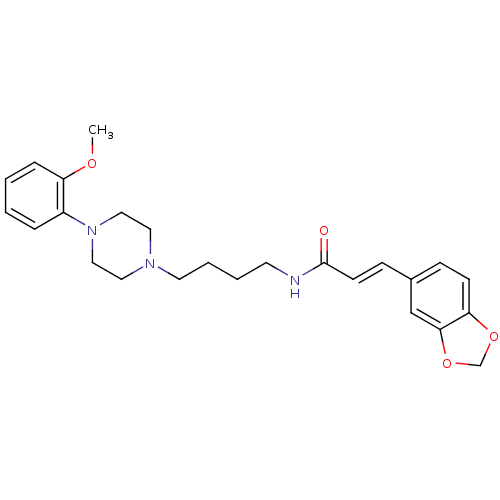 (3-Benzo[1,3]dioxol-5-yl-N-{4-[4-(2-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132078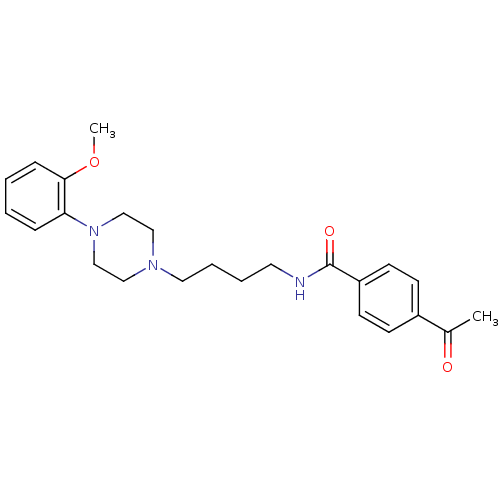 (4-Acetyl-N-{4-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132022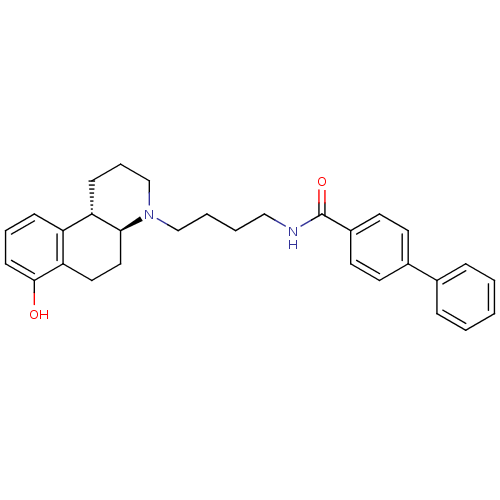 (Biphenyl-4-carboxylic acid [4-((4aS,10bS)-7-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50098203 (1-{4-[2-(1H-Imidazol-4-yl)-ethylsulfanyl]-phenyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of [125I]-iodoproxyfan binding to human histamine H3 receptor of CHO-K1 cells | Bioorg Med Chem Lett 11: 951-4 (2001) BindingDB Entry DOI: 10.7270/Q28P5ZRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132024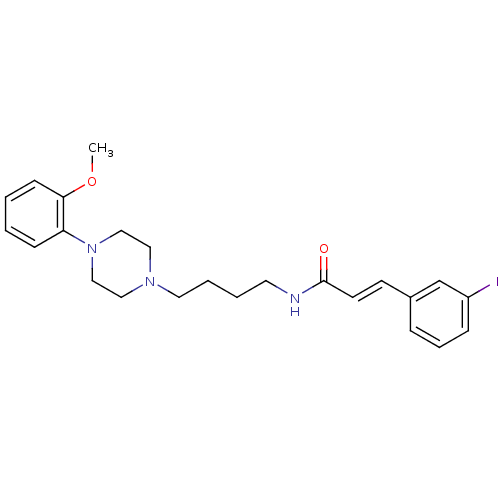 (3-(3-Iodo-phenyl)-N-{4-[4-(2-methoxy-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132081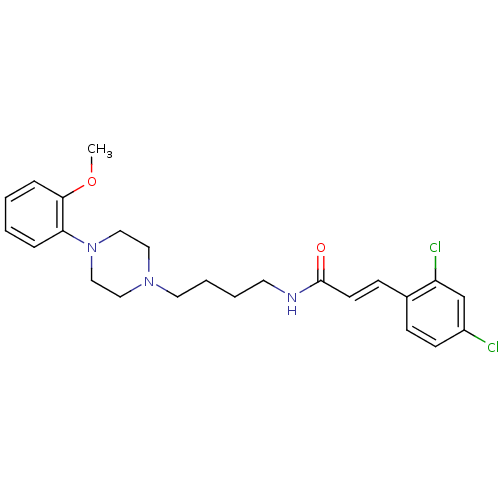 (3-(2,4-Dichloro-phenyl)-N-{4-[4-(2-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132061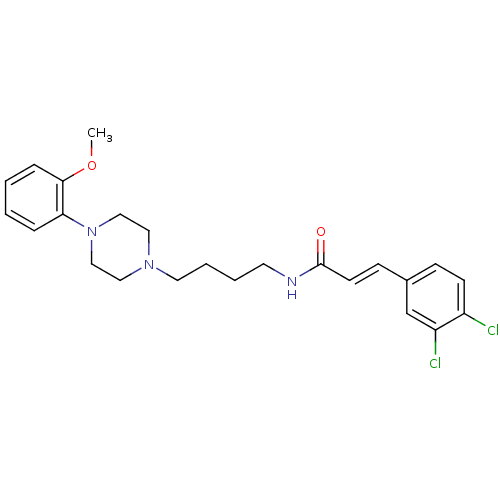 (3-(3,4-Dichloro-phenyl)-N-{4-[4-(2-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of [125I]-iodoproxyfan from rat histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 11: 951-4 (2001) BindingDB Entry DOI: 10.7270/Q28P5ZRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50131926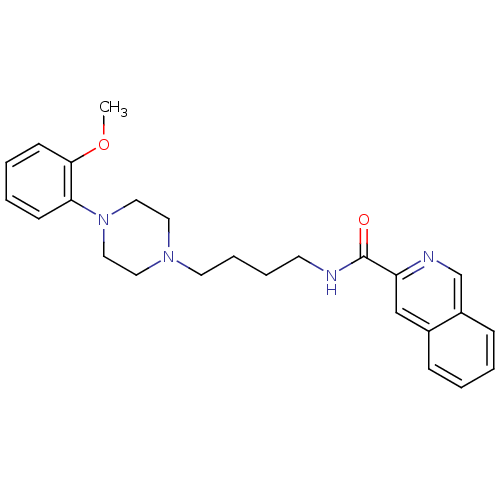 (CHEMBL127400 | CHEMBL129022 | Isoquinoline-3-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132038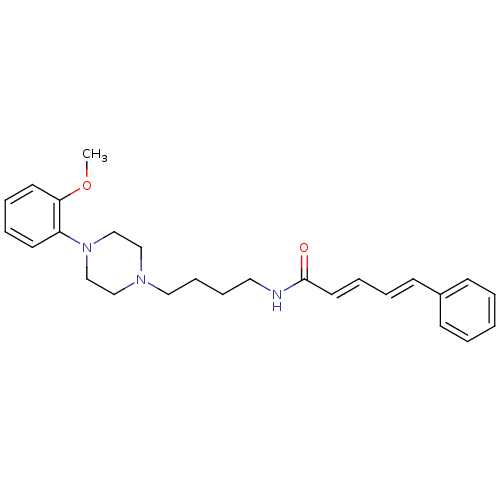 (5-Phenyl-penta-2,4-dienoic acid {4-[4-(2-methoxy-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50499215 (CHEMBL3735947) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Munster Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil from Glun2B receptor (unknown origin) expressed in mouse L(tk-) cell membranes after 120 mins by scintillation countin... | Bioorg Med Chem Lett 25: 5748-51 (2015) Article DOI: 10.1016/j.bmcl.2015.10.076 BindingDB Entry DOI: 10.7270/Q2639SQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132025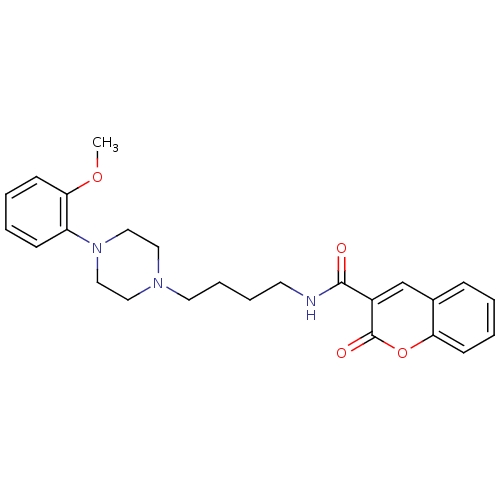 (2-Oxo-2H-chromene-3-carboxylic acid {4-[4-(2-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50551241 (CHEMBL4746028) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-(+)-pentazocine from Sigma1 receptor in guinea pig cortex membranes incubated for 120 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112138 BindingDB Entry DOI: 10.7270/Q2D50RKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase (Homo sapiens (Human)) | BDBM3096 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Displacement of (6-FAM)KI(pY)VV from PKMYT1 kinase domain (unknown origin) by fluorescence polarization binding assay | Eur J Med Chem 161: 479-492 (2019) Article DOI: 10.1016/j.ejmech.2018.10.050 BindingDB Entry DOI: 10.7270/Q2C53Q5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase (Homo sapiens (Human)) | BDBM3096 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Displacement of (6-FAM)KI(pY)VV from PKMYT1 kinase domain (unknown origin) by fluorescence polarization binding assay | Eur J Med Chem 161: 479-492 (2019) Article DOI: 10.1016/j.ejmech.2018.10.050 BindingDB Entry DOI: 10.7270/Q2C53Q5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase (Homo sapiens (Human)) | BDBM3096 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of N-[3',6'-dihydroxy-3-oxo-3H-spiro(2-benzofuran-1,9'-xanthen)-5-yl]-N'-[2-(4-{4-[N-(2-chloro-6-methylphenyl)-5-carboxamido]-thiazol-2-yl... | Bioorg Med Chem 26: 4014-4024 (2018) Article DOI: 10.1016/j.bmc.2018.06.027 BindingDB Entry DOI: 10.7270/Q29Z97K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50071197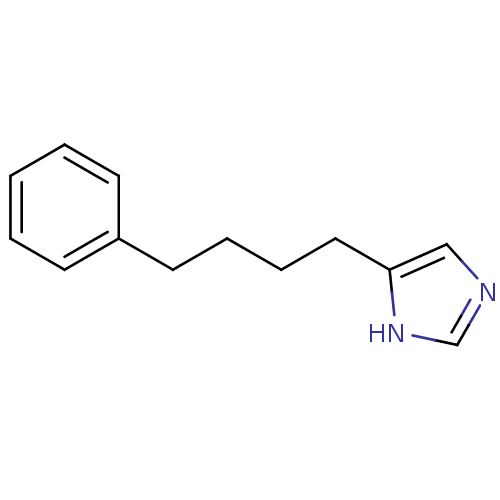 (4-(4-Phenyl-butyl)-1H-imidazole | CHEMBL296450) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of [125I]-iodoproxyfan binding to human histamine H3 receptor of CHO-K1 cells | Bioorg Med Chem Lett 11: 951-4 (2001) BindingDB Entry DOI: 10.7270/Q28P5ZRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50551242 (CHEMBL4740593) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DTG from sigma 2 receptor in rat liver membranes incubated for 120 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112138 BindingDB Entry DOI: 10.7270/Q2D50RKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132041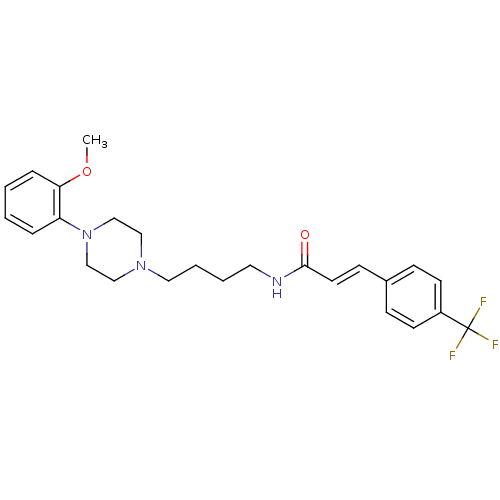 (CHEMBL129429 | N-{4-[4-(2-Methoxy-phenyl)-piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase (Homo sapiens (Human)) | BDBM4213 (6-(2,6-dichlorophenyl)-8-methyl-2-{[4-(morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Displacement of (6-FAM)KI(pY)VV from full length PKMYT1 (unknown origin) by fluorescence polarization immuno assay | Eur J Med Chem 161: 479-492 (2019) Article DOI: 10.1016/j.ejmech.2018.10.050 BindingDB Entry DOI: 10.7270/Q2C53Q5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase (Homo sapiens (Human)) | BDBM4213 (6-(2,6-dichlorophenyl)-8-methyl-2-{[4-(morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of human full length PKMYT1 expressed in HEK293 cells using EFS (247 to 259 residues) as substrate after 1 hr by fluorescence polarization... | Bioorg Med Chem 26: 4014-4024 (2018) Article DOI: 10.1016/j.bmc.2018.06.027 BindingDB Entry DOI: 10.7270/Q29Z97K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of [125I]-iodoproxyfan binding to human histamine H3 receptor of CHO-K1 cells | Bioorg Med Chem Lett 11: 951-4 (2001) BindingDB Entry DOI: 10.7270/Q28P5ZRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132030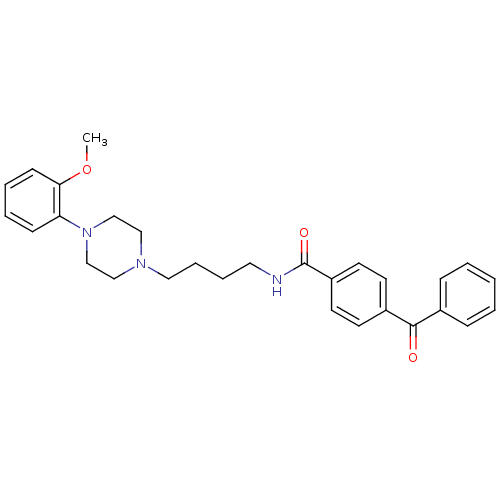 (4-Benzoyl-N-{4-[4-(2-methoxy-phenyl)-piperazin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2334 total ) | Next | Last >> |