

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096484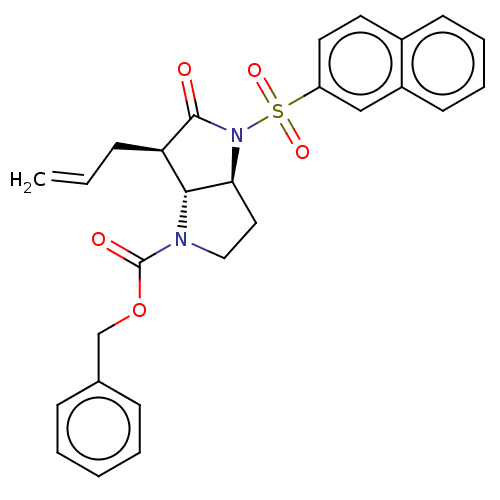 ((4S,6R)-6-Allyl-4-(naphthalene-2-sulfonyl)-5-oxo-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066997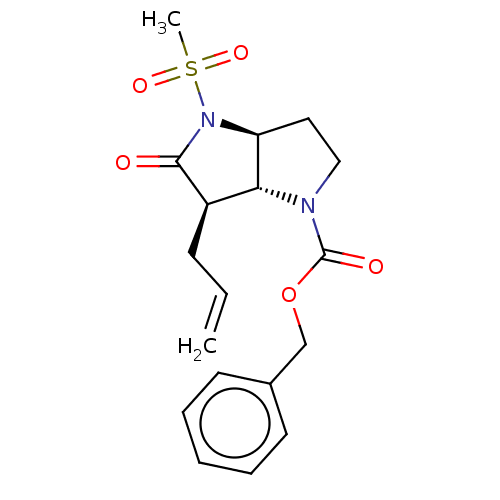 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096486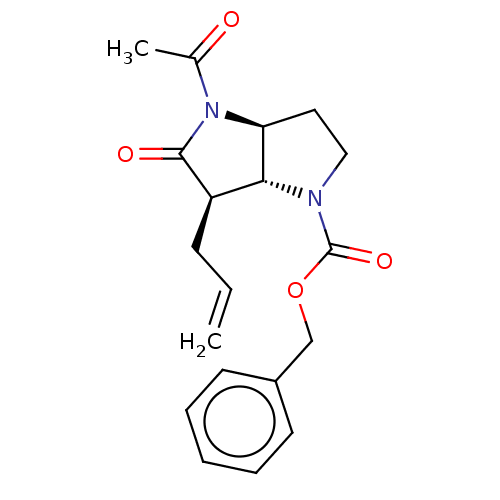 (CHEMBL2367646 | benzyl (3aS,6aR)-4-acetyl-6-allyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096488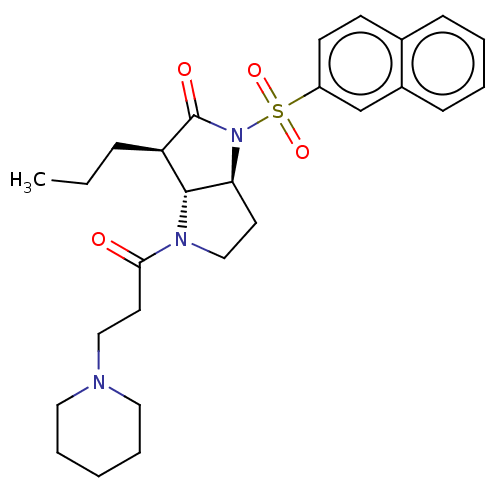 ((1S,3R)-1-(Naphthalene-2-sulfonyl)-4-(3-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4942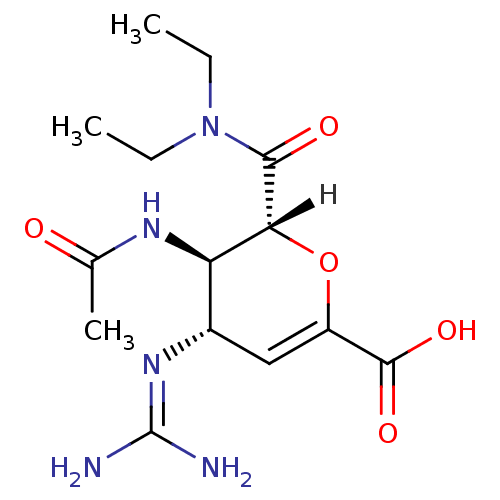 ((2R,3R,4S)-4-carbamimidamido-2-(diethylcarbamoyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096488 ((1S,3R)-1-(Naphthalene-2-sulfonyl)-4-(3-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity for dopamine D4-like receptor labelled with [3H]YM-09151-2 in retina | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4940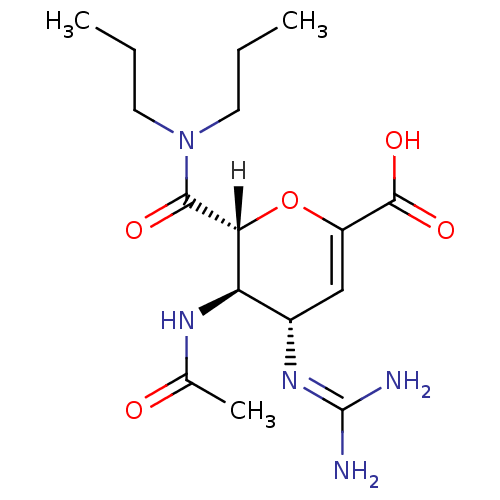 ((2R,3R,4S)-4-carbamimidamido-2-(dipropylcarbamoyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4945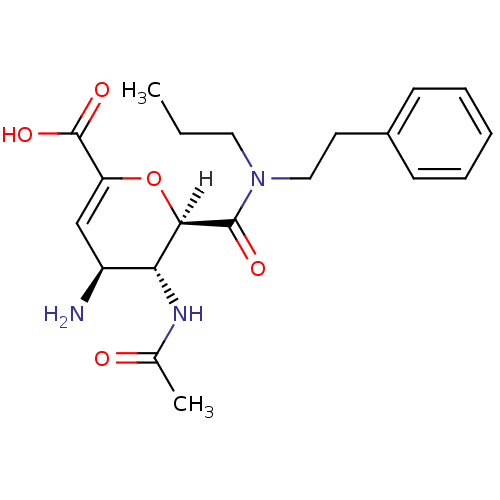 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-phenylethyl)(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096487 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-pyrrolo[3,2-b]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4937 ((2R,3R,4S)-4-amino-2-(diethylcarbamoyl)-3-acetamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4967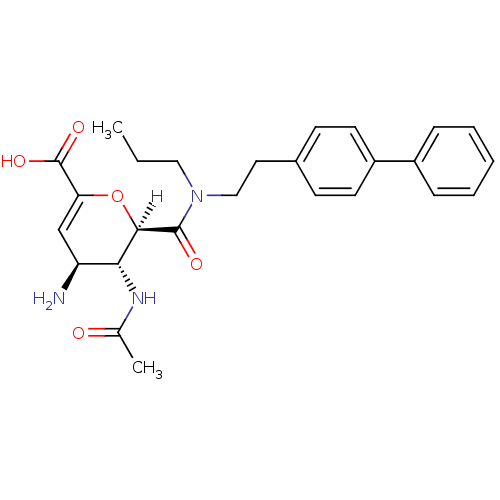 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(4-phenylphen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096483 ((3R,6aS)-1-Methanesulfonyl-4-(3-piperidin-1-yl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 uM | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4941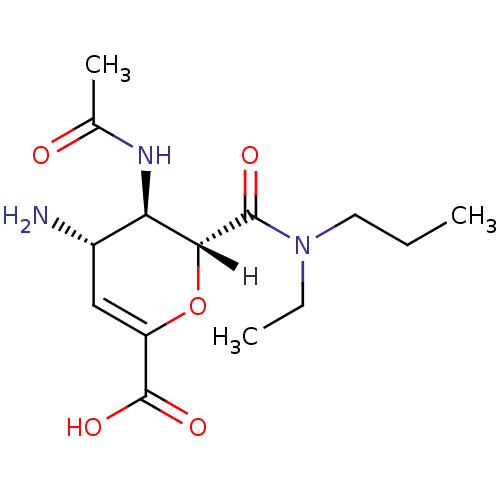 ((2R,3R,4S)-4-amino-3-acetamido-2-[ethyl(propyl)car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4929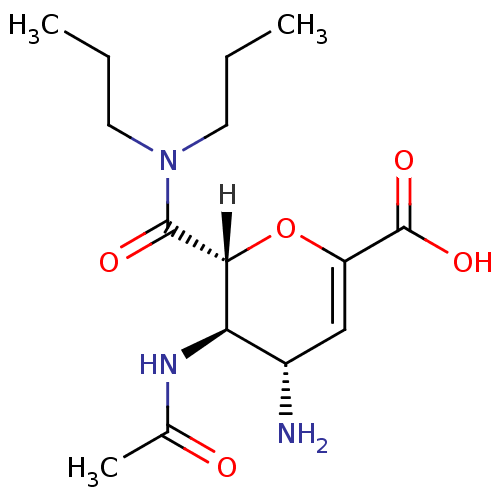 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4933 ((2R,3R,4S)-4-carbamimidamido-3-acetamido-2-[methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza B sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4943 ((2R,3R,4S)-4-amino-2-[butyl(propyl)carbamoyl]-3-ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type B | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4945 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-phenylethyl)(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50288444 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-(phenethyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4952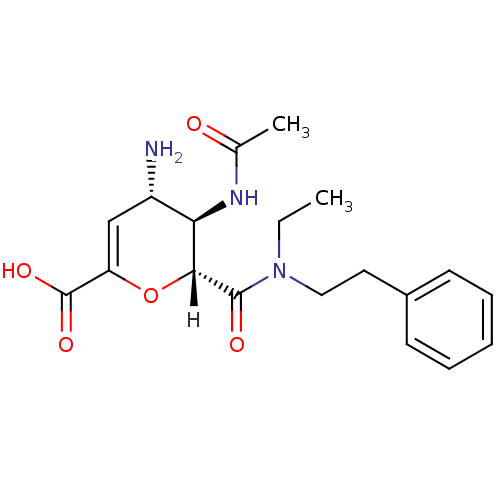 ((2R,3R,4S)-4-amino-3-acetamido-2-[ethyl(2-phenylet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4952 ((2R,3R,4S)-4-amino-3-acetamido-2-[ethyl(2-phenylet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50061024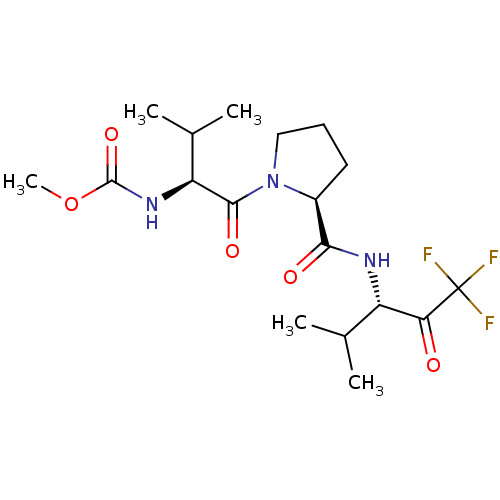 ((2-Methyl-1-{(S)-oxo-[(S)-2-((S)-3,3,3-trifluoro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4958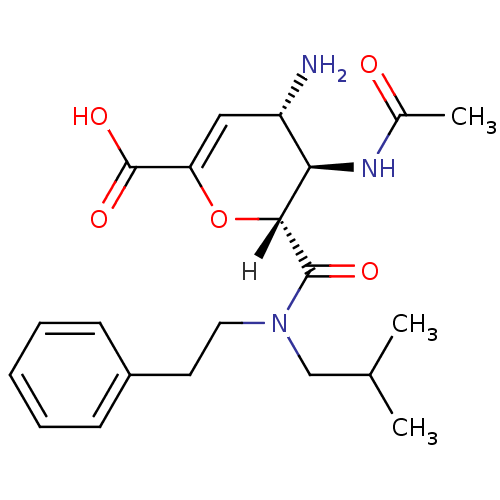 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-methylpropyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50288445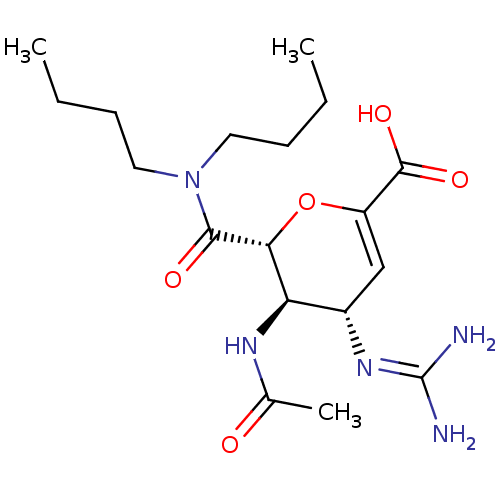 ((4S,5R,6R)-5-Acetylamino-6-dibutylcarbamoyl-4-guan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098095 ((3S,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-5-((E)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against Elastase | Bioorg Med Chem Lett 11: 895-8 (2001) BindingDB Entry DOI: 10.7270/Q29C6XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4948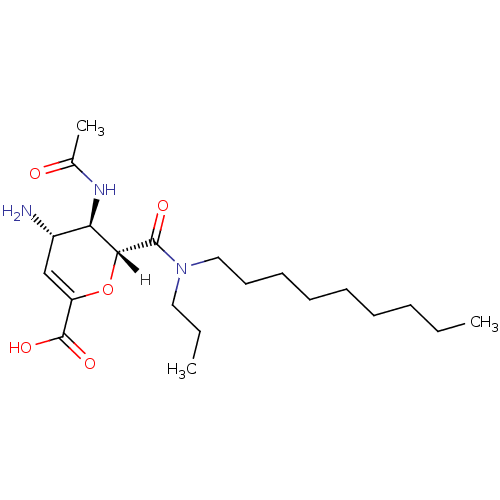 ((2R,3R,4S)-4-amino-3-acetamido-2-[nonyl(propyl)car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4932 ((2R,3R,4S)-4-carbamimidamido-2-(dimethylcarbamoyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098094 ((3R,6aR)-3-Isopropyl-1-methanesulfonyl-5-((E)-4-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against Elastase | Bioorg Med Chem Lett 11: 895-8 (2001) BindingDB Entry DOI: 10.7270/Q29C6XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096490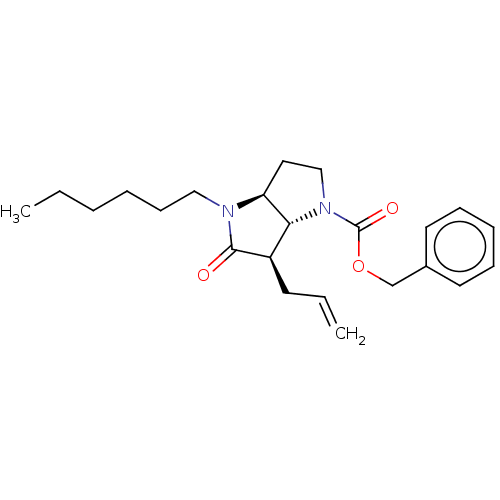 ((3aS,6R)-6-Allyl-4-hexyl-5-oxo-hexahydro-pyrrolo[3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096485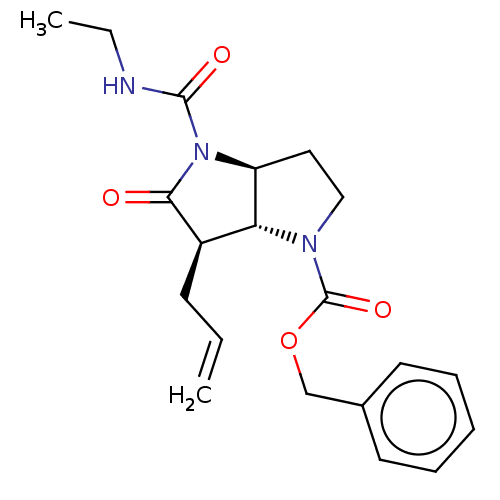 ((3aS,6R)-6-Allyl-4-ethylcarbamoyl-5-oxo-hexahydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096489 ((3aS,6R)-6-Allyl-4-formyl-5-oxo-hexahydro-pyrrolo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098097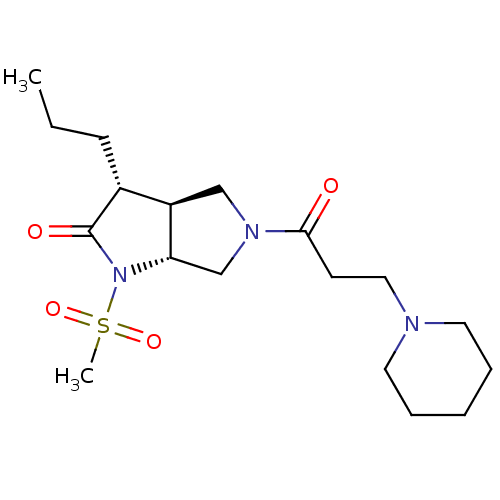 ((3R,3aS,6aR)-1-Methanesulfonyl-5-(3-piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against Elastase | Bioorg Med Chem Lett 11: 895-8 (2001) BindingDB Entry DOI: 10.7270/Q29C6XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096483 ((3R,6aS)-1-Methanesulfonyl-4-(3-piperidin-1-yl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 uM | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4928 ((2R,3R,4S)-4-amino-3-acetamido-2-[methyl(propyl)ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098096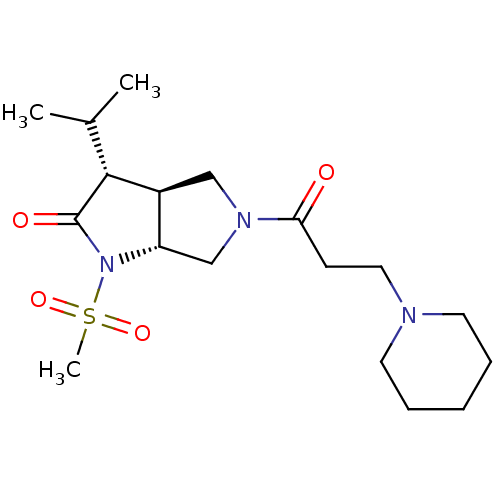 ((3R,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-5-(3-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against Elastase | Bioorg Med Chem Lett 11: 895-8 (2001) BindingDB Entry DOI: 10.7270/Q29C6XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098098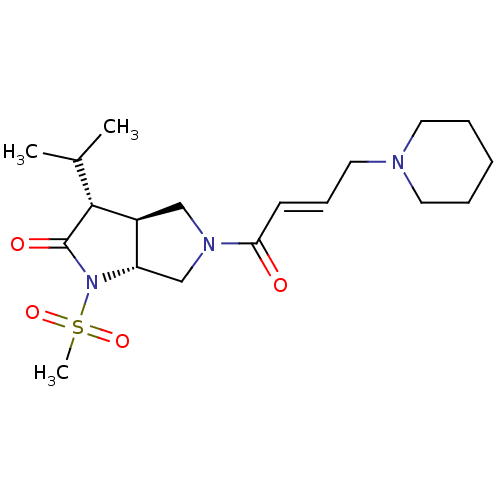 ((3R,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-5-((E)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 274 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against Elastase | Bioorg Med Chem Lett 11: 895-8 (2001) BindingDB Entry DOI: 10.7270/Q29C6XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50063303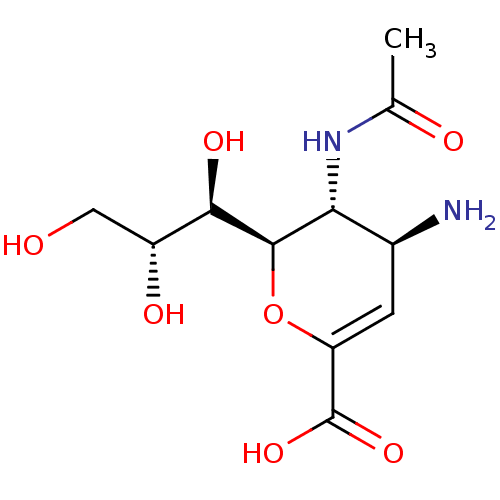 ((2R,3R,4S)-3-acetamido-4-amino-2-((1R,2R)-1,2,3-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4951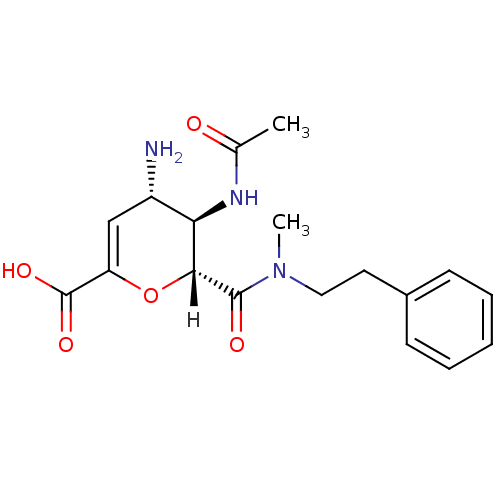 ((2R,3R,4S)-4-amino-3-acetamido-2-[methyl(2-phenyle...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4951 ((2R,3R,4S)-4-amino-3-acetamido-2-[methyl(2-phenyle...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50063303 ((2R,3R,4S)-3-acetamido-4-amino-2-((1R,2R)-1,2,3-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4937 ((2R,3R,4S)-4-amino-2-(diethylcarbamoyl)-3-acetamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza B sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50063303 ((2R,3R,4S)-3-acetamido-4-amino-2-((1R,2R)-1,2,3-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza B sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4941 ((2R,3R,4S)-4-amino-3-acetamido-2-[ethyl(propyl)car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza B sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50063303 ((2R,3R,4S)-3-acetamido-4-amino-2-((1R,2R)-1,2,3-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type B | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4936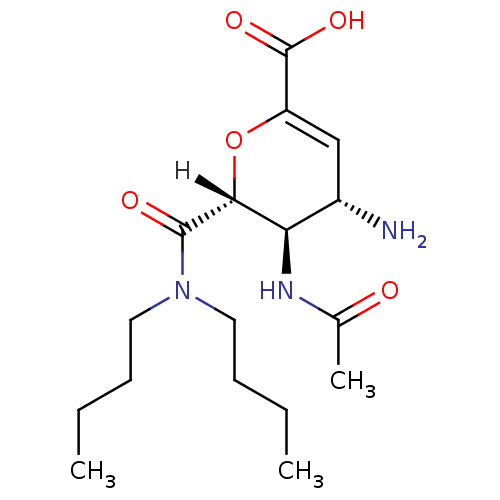 ((2R,3R,4S)-4-amino-2-(dibutylcarbamoyl)-3-acetamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4940 ((2R,3R,4S)-4-carbamimidamido-2-(dipropylcarbamoyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza B sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |