

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GTP-binding nuclear protein GSP1/CNR1 (Saccharomyces cerevisiae) | BDBM50176988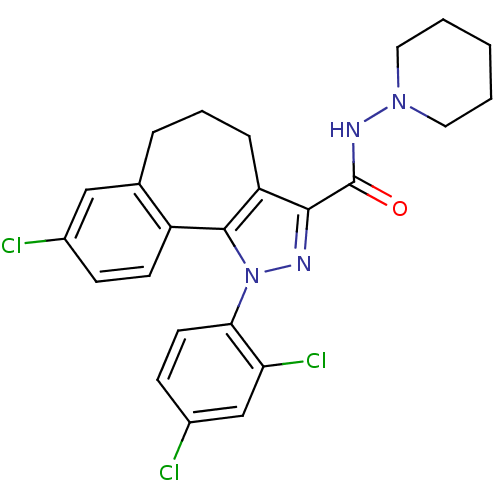 (8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research Curated by PDSP Ki Database | J Med Chem 51: 2439-46 (2008) Article DOI: 10.1021/jm701519h BindingDB Entry DOI: 10.7270/Q22R3Q7S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50300196 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JAK2 | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470113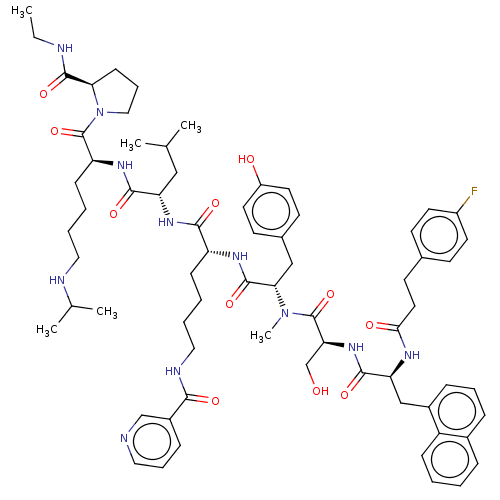 (CHEMBL439134) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50434655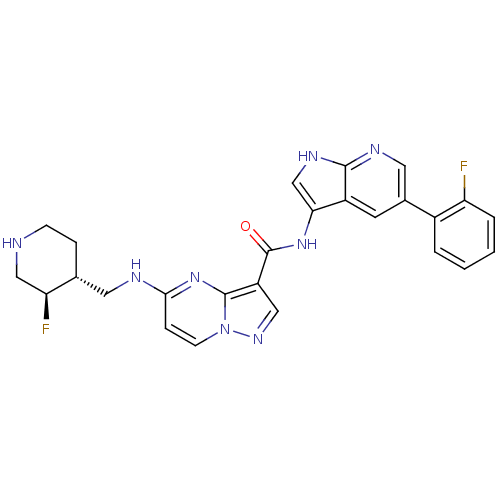 (CHEMBL2387464) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using FAM-pimtide as substrate after 90 mins by spectrophotometry in presence of ATP | Bioorg Med Chem Lett 23: 3149-53 (2013) Article DOI: 10.1016/j.bmcl.2013.04.020 BindingDB Entry DOI: 10.7270/Q2PC33S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50300196 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JAK3 | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50300196 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JAK3 | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Torpedo californica) | BDBM50039623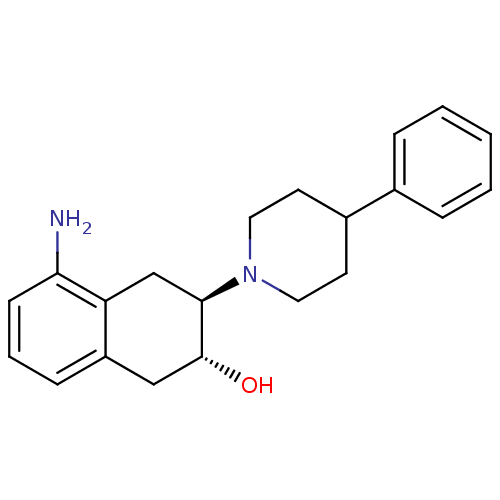 ((2R,3R)-5-Amino-3-(4-phenyl-piperidin-1-yl)-1,2,3,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description In vitro binding affinity to vesamicol receptor using [3H]-vesamicol. | J Med Chem 37: 2574-82 (1994) BindingDB Entry DOI: 10.7270/Q2HH6J44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50434656 (CHEMBL2387463) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using FAM-pimtide as substrate after 90 mins by spectrophotometry in presence of ATP | Bioorg Med Chem Lett 23: 3149-53 (2013) Article DOI: 10.1016/j.bmcl.2013.04.020 BindingDB Entry DOI: 10.7270/Q2PC33S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50434654 (CHEMBL2387465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using FAM-pimtide as substrate after 90 mins by spectrophotometry in presence of ATP | Bioorg Med Chem Lett 23: 3149-53 (2013) Article DOI: 10.1016/j.bmcl.2013.04.020 BindingDB Entry DOI: 10.7270/Q2PC33S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50434666 (CHEMBL2387471) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using FAM-pimtide as substrate after 90 mins by spectrophotometry in presence of ATP | Bioorg Med Chem Lett 23: 3149-53 (2013) Article DOI: 10.1016/j.bmcl.2013.04.020 BindingDB Entry DOI: 10.7270/Q2PC33S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50434655 (CHEMBL2387464) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using FAM-pimtide as substrate after 90 mins by spectrophotometry in presence of ATP | Bioorg Med Chem Lett 23: 3149-53 (2013) Article DOI: 10.1016/j.bmcl.2013.04.020 BindingDB Entry DOI: 10.7270/Q2PC33S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073270 (CHEMBL333781 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36648 (3-alkylaminoindazole cyclic urea, (H)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464108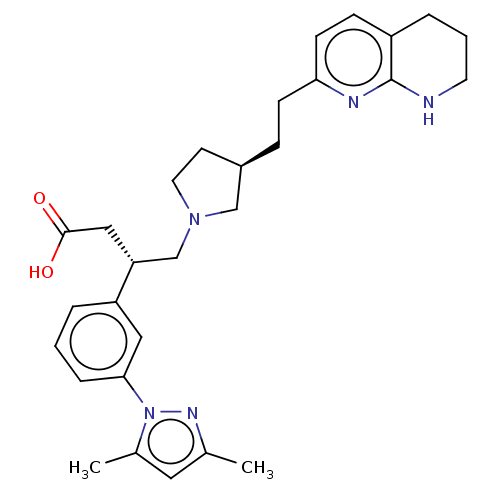 (CHEMBL4241824) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to human integrin alphaVbeta6 assessed as dissociation constant up to 48 hrs by liquid scintillation counting | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36648 (3-alkylaminoindazole cyclic urea, (H)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36647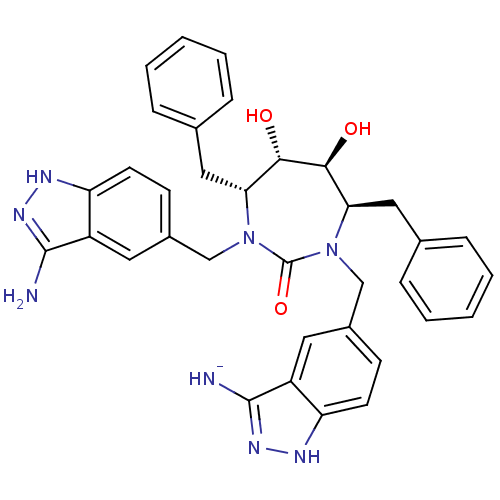 (3-Aminoindazole, 2) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50114725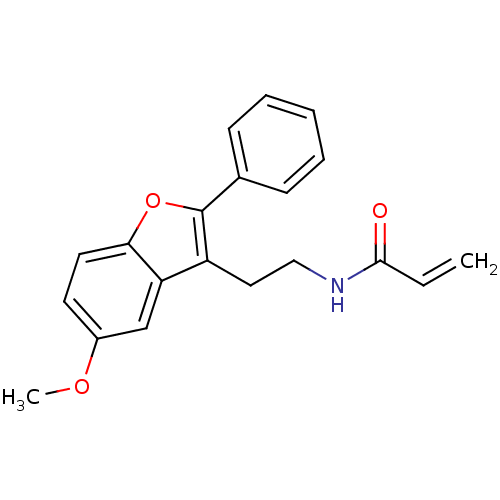 (CHEMBL314512 | N-[2-(5-Methoxy-2-phenyl-benzofuran...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie Pharmaceutique Albert Lespagnol Curated by ChEMBL | Assay Description Binding affinity on human melatonin receptor type 1B stably transfected in human embryonic kidney (HEK 293) cells using 2-[125I]-iodomelatonin as rad... | J Med Chem 45: 2788-800 (2002) BindingDB Entry DOI: 10.7270/Q2Q52P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110121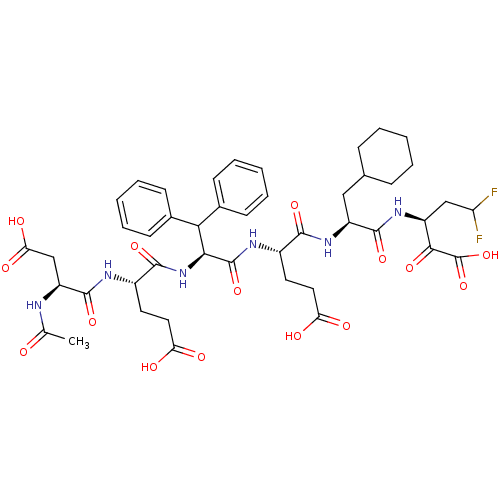 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50114718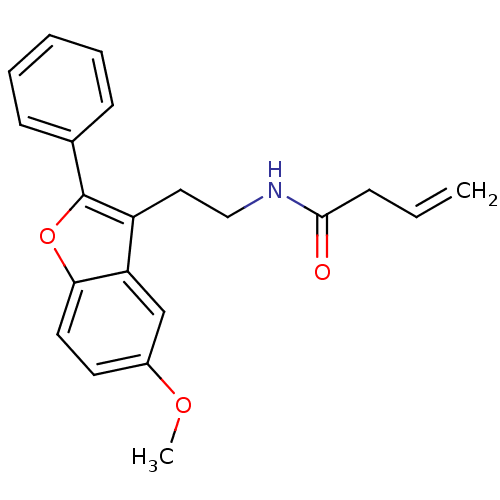 (But-3-enoic acid [2-(5-methoxy-2-phenyl-benzofuran...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie Pharmaceutique Albert Lespagnol Curated by ChEMBL | Assay Description Binding affinity on human melatonin receptor type 1A stably transfected in human embryonic kidney (HEK 293) using 2-[125I]-iodomelatonin as radioliga... | J Med Chem 45: 2788-800 (2002) BindingDB Entry DOI: 10.7270/Q2Q52P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50114703 (CHEMBL287560 | N-[2-(5-Methoxy-2-phenyl-benzofuran...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie Pharmaceutique Albert Lespagnol Curated by ChEMBL | Assay Description Binding affinity on human melatonin receptor type 1A stably transfected in human embryonic kidney (HEK 293) using 2-[125I]-iodomelatonin as radioliga... | J Med Chem 45: 2788-800 (2002) BindingDB Entry DOI: 10.7270/Q2Q52P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073223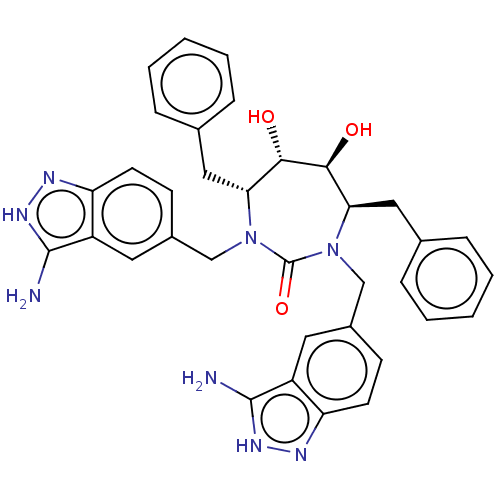 (CHEMBL73240) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV protease | Bioorg Med Chem Lett 8: 715-20 (1999) BindingDB Entry DOI: 10.7270/Q2FQ9VRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50214385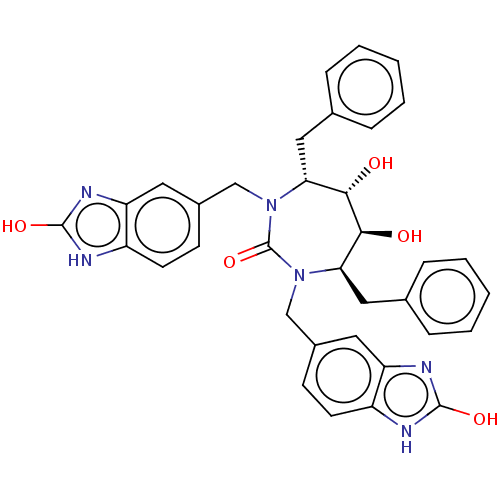 (CHEMBL316681) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I by using enzymatic assay at each of 6 substrate concentrations between 0.1 mM and... | Bioorg Med Chem Lett 8: 715-20 (1999) BindingDB Entry DOI: 10.7270/Q2FQ9VRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50214385 (CHEMBL316681) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110121 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470098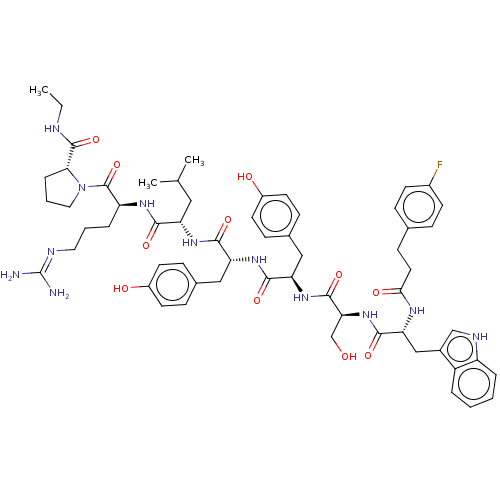 (CHEMBL2371296) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36647 (3-Aminoindazole, 2) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50434658 (CHEMBL2387479) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using FAM-pimtide as substrate after 90 mins by spectrophotometry in presence of ATP | Bioorg Med Chem Lett 23: 3149-53 (2013) Article DOI: 10.1016/j.bmcl.2013.04.020 BindingDB Entry DOI: 10.7270/Q2PC33S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470117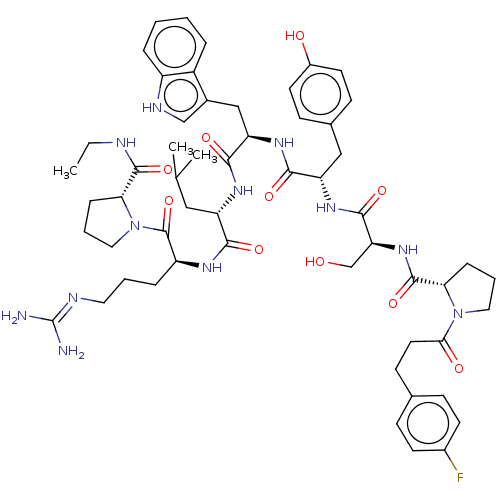 (CHEMBL264989) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110577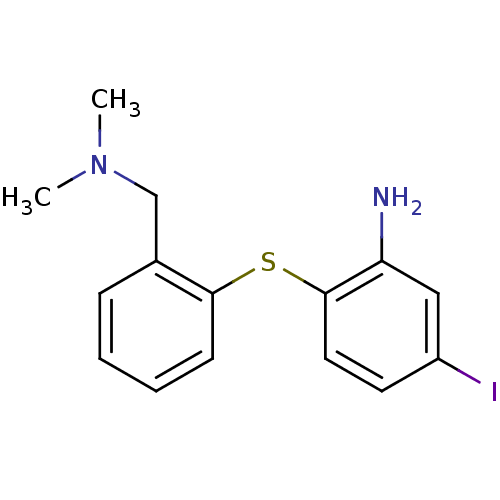 (2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM160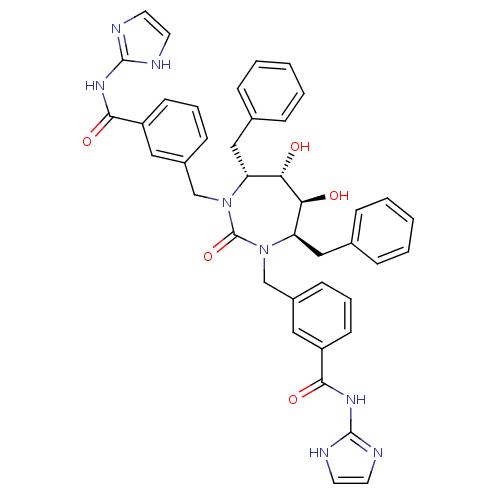 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030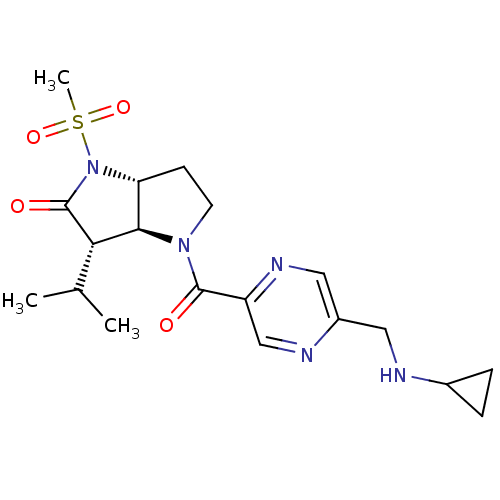 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50434663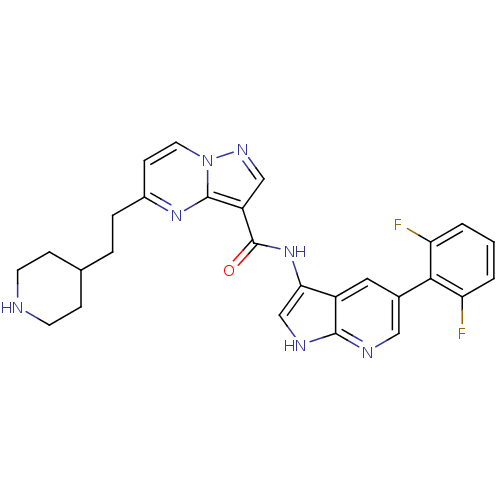 (CHEMBL2387474) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using FAM-pimtide as substrate after 90 mins by spectrophotometry in presence of ATP | Bioorg Med Chem Lett 23: 3149-53 (2013) Article DOI: 10.1016/j.bmcl.2013.04.020 BindingDB Entry DOI: 10.7270/Q2PC33S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7084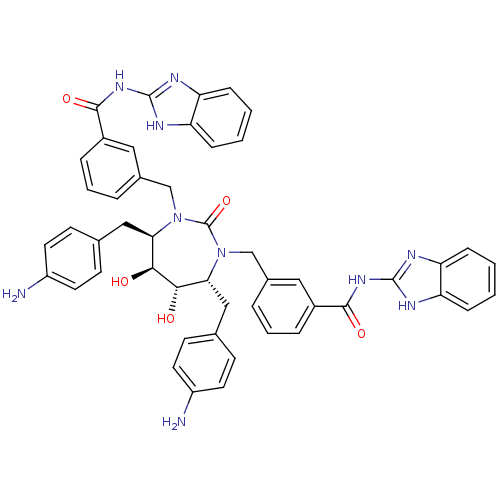 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-4,7-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | -64.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069201 ((4R,5S,6S,7R)-5,6-Dihydroxy-4,7-bis-(4-hydroxy-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065075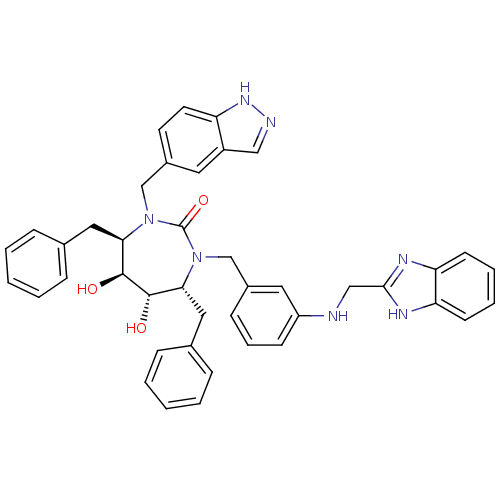 ((4R,5S,6S,7R)-1-{3-[(1H-Benzoimidazol-2-ylmethyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50434657 (CHEMBL2387462) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using FAM-pimtide as substrate after 90 mins by spectrophotometry in presence of ATP | Bioorg Med Chem Lett 23: 3149-53 (2013) Article DOI: 10.1016/j.bmcl.2013.04.020 BindingDB Entry DOI: 10.7270/Q2PC33S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470118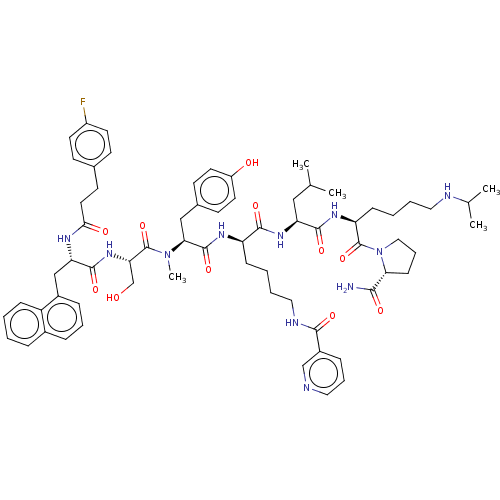 (CHEMBL412751) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065080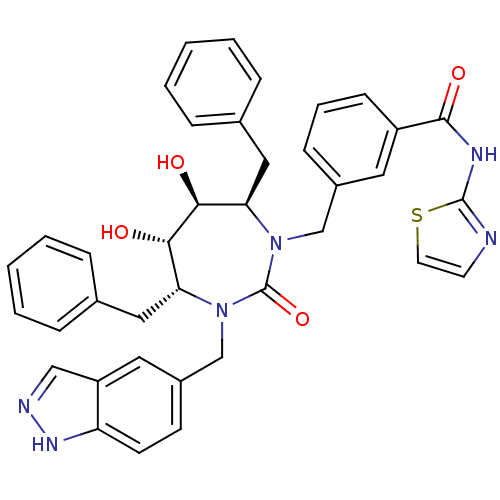 (3-[(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36656 (Cyclobutylmethyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36656 (Cyclobutylmethyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470097 (CHEMBL262235) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470080 (CHEMBL266475) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470082 (CHEMBL408861) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470099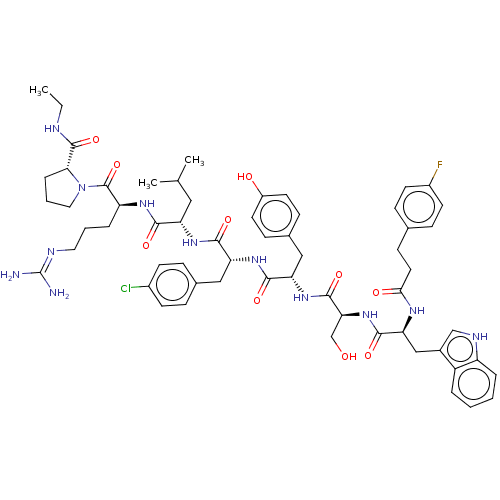 (CHEMBL2371295) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM161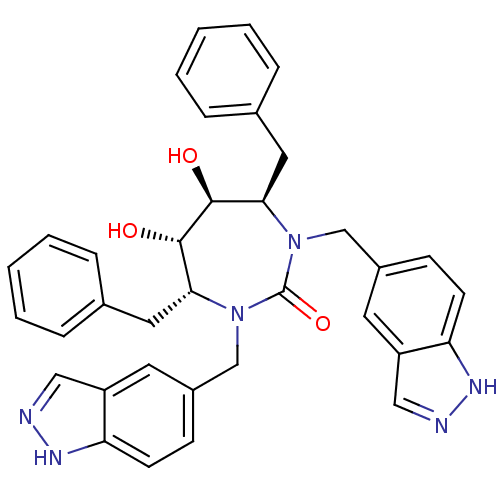 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288430 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065089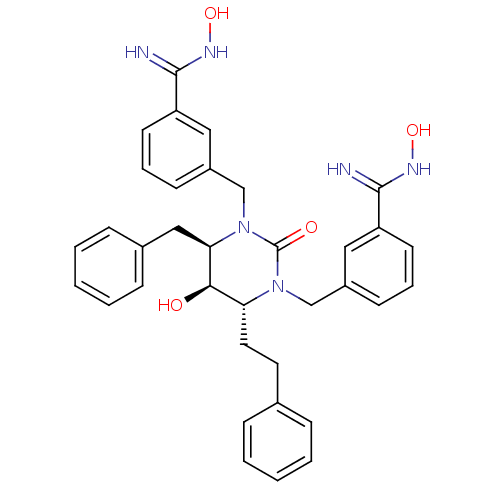 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-benzamide oxime)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065086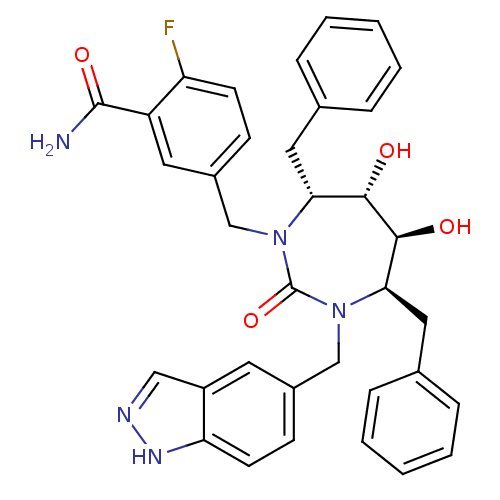 (5-[(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM161 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069033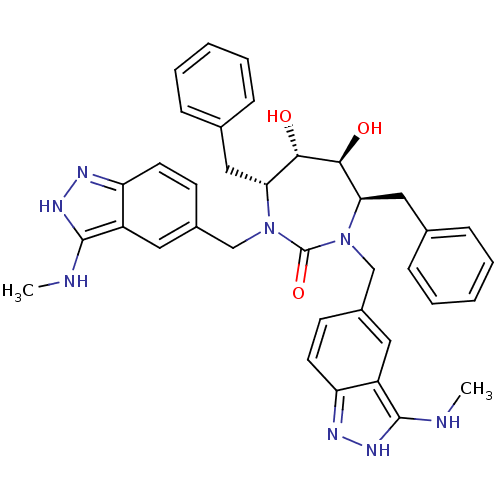 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV protease | Bioorg Med Chem Lett 8: 715-20 (1999) BindingDB Entry DOI: 10.7270/Q2FQ9VRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 119256 total ) | Next | Last >> |