 Found 441 hits with Last Name = 'song' and Initial = 'jy'
Found 441 hits with Last Name = 'song' and Initial = 'jy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86298
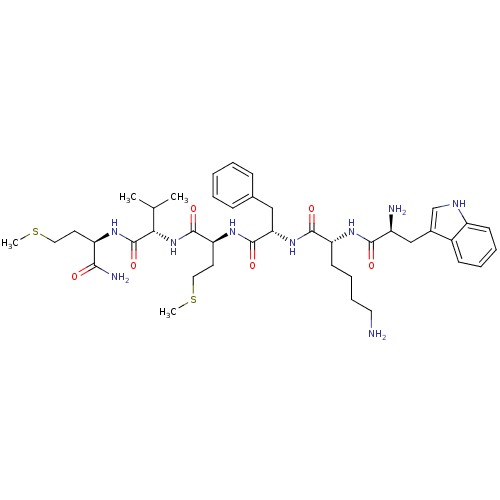
(WKFMVm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(C)C)C(N)=O Show InChI InChI=1S/C41H61N9O6S2/c1-25(2)35(41(56)46-31(36(44)51)17-20-57-3)50-39(54)33(18-21-58-4)48-40(55)34(22-26-12-6-5-7-13-26)49-38(53)32(16-10-11-19-42)47-37(52)29(43)23-27-24-45-30-15-9-8-14-28(27)30/h5-9,12-15,24-25,29,31-35,45H,10-11,16-23,42-43H2,1-4H3,(H2,44,51)(H,46,56)(H,47,52)(H,48,55)(H,49,53)(H,50,54)/t29-,31+,32+,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86301
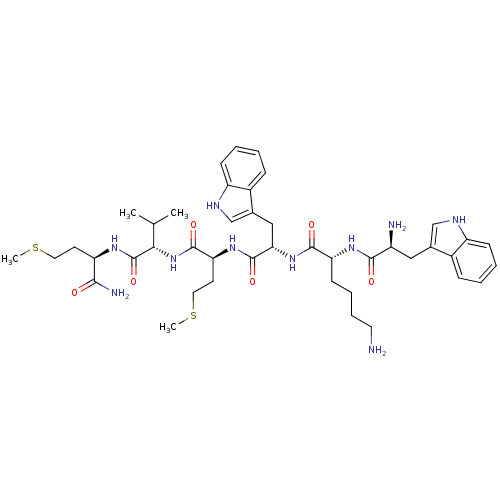
(WKWMVm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(C)C)C(N)=O Show InChI InChI=1S/C43H62N10O6S2/c1-25(2)37(43(59)49-33(38(46)54)16-19-60-3)53-41(57)35(17-20-61-4)51-42(58)36(22-27-24-48-32-14-8-6-12-29(27)32)52-40(56)34(15-9-10-18-44)50-39(55)30(45)21-26-23-47-31-13-7-5-11-28(26)31/h5-8,11-14,23-25,30,33-37,47-48H,9-10,15-22,44-45H2,1-4H3,(H2,46,54)(H,49,59)(H,50,55)(H,51,58)(H,52,56)(H,53,57)/t30-,33+,34+,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86299
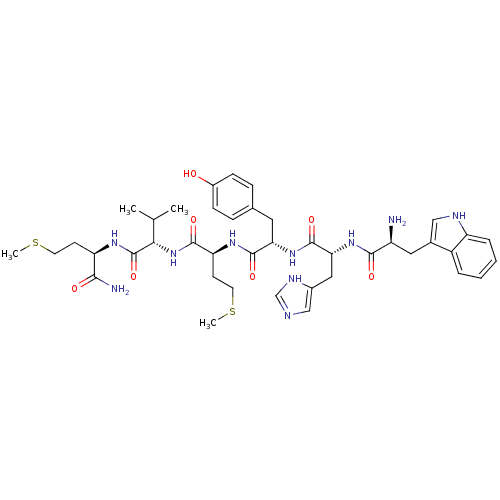
(WHYMVm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cnc[nH]1)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(C)C)C(N)=O Show InChI InChI=1S/C41H56N10O7S2/c1-23(2)35(41(58)47-31(36(43)53)13-15-59-3)51-38(55)32(14-16-60-4)48-39(56)33(17-24-9-11-27(52)12-10-24)50-40(57)34(19-26-21-44-22-46-26)49-37(54)29(42)18-25-20-45-30-8-6-5-7-28(25)30/h5-12,20-23,29,31-35,45,52H,13-19,42H2,1-4H3,(H2,43,53)(H,44,46)(H,47,58)(H,48,56)(H,49,54)(H,50,57)(H,51,55)/t29-,31+,32-,33-,34+,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86297
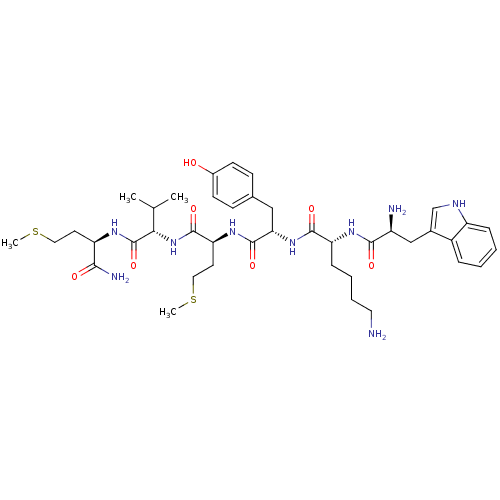
(WKYMVm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(C)C)C(N)=O Show InChI InChI=1S/C41H61N9O7S2/c1-24(2)35(41(57)46-31(36(44)52)16-19-58-3)50-39(55)33(17-20-59-4)48-40(56)34(21-25-12-14-27(51)15-13-25)49-38(54)32(11-7-8-18-42)47-37(53)29(43)22-26-23-45-30-10-6-5-9-28(26)30/h5-6,9-10,12-15,23-24,29,31-35,45,51H,7-8,11,16-22,42-43H2,1-4H3,(H2,44,52)(H,46,57)(H,47,53)(H,48,56)(H,49,54)(H,50,55)/t29-,31+,32+,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86308
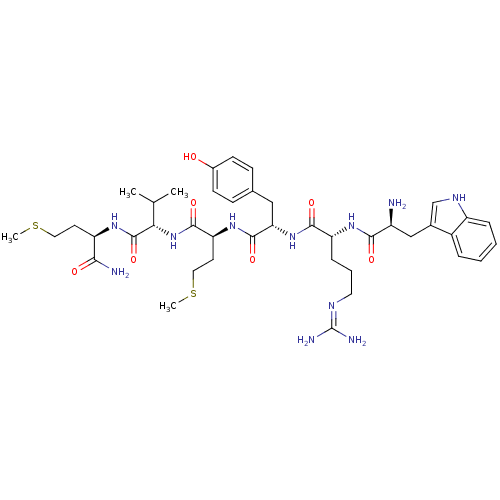
(WRYMVm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(C)C)C(N)=O |wU:32.40,12.16,wD:43.44,20.29,8.8,4.4,(5.12,.93,;6.45,1.7,;6.45,3.24,;7.79,4.01,;7.79,5.55,;9.13,6.26,;10.45,5.52,;10.45,3.98,;11.76,6.17,;13.15,5.32,;14.49,5.99,;14.49,7.53,;15.92,5.25,;15.92,3.67,;14.54,2.87,;14.63,1.34,;13.2,.65,;17.24,5.99,;18.49,5.32,;18.49,3.77,;19.9,6.1,;19.9,7.63,;18.6,8.44,;18.6,9.98,;17.27,10.75,;15.94,9.98,;14.6,10.75,;15.94,8.44,;17.27,7.67,;21.12,5.25,;22.39,6.1,;22.39,7.72,;23.71,5.32,;25.19,6.17,;25.19,7.71,;26.52,8.48,;26.52,10.02,;27.86,10.79,;27.86,12.33,;29.19,10.02,;23.71,3.79,;24.91,3.07,;26.09,3.79,;24.91,1.53,;23.76,.65,;26.25,.76,;27.58,1.53,;27.74,3.06,;29.25,3.38,;30.02,2.04,;31.52,1.72,;32.05,.25,;30.97,-.89,;29.46,-.57,;28.99,.9,;11.83,7.72,;10.58,8.51,;13.17,8.49,;6.52,6.38,;5.25,5.55,;6.52,7.93,)| Show InChI InChI=1S/C41H61N11O7S2/c1-23(2)34(40(59)48-30(35(43)54)15-18-60-3)52-38(57)32(16-19-61-4)50-39(58)33(20-24-11-13-26(53)14-12-24)51-37(56)31(10-7-17-46-41(44)45)49-36(55)28(42)21-25-22-47-29-9-6-5-8-27(25)29/h5-6,8-9,11-14,22-23,28,30-34,47,53H,7,10,15-21,42H2,1-4H3,(H2,43,54)(H,48,59)(H,49,55)(H,50,58)(H,51,56)(H,52,57)(H4,44,45,46)/t28-,30+,31+,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86300
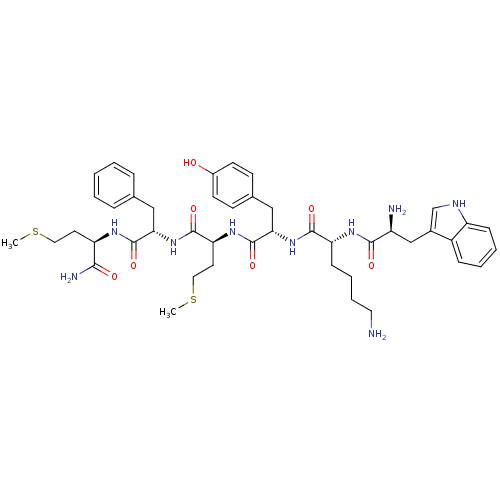
(WKYM(F/W)m-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C45H61N9O7S2/c1-62-22-19-35(40(48)56)50-44(60)38(24-28-10-4-3-5-11-28)54-43(59)37(20-23-63-2)52-45(61)39(25-29-15-17-31(55)18-16-29)53-42(58)36(14-8-9-21-46)51-41(57)33(47)26-30-27-49-34-13-7-6-12-32(30)34/h3-7,10-13,15-18,27,33,35-39,49,55H,8-9,14,19-26,46-47H2,1-2H3,(H2,48,56)(H,50,60)(H,51,57)(H,52,61)(H,53,58)(H,54,59)/t33-,35+,36+,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86302
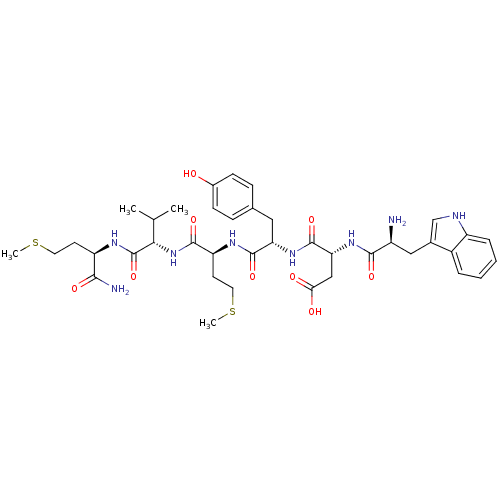
(WDYMVm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(C)C)C(N)=O Show InChI InChI=1S/C39H54N8O9S2/c1-21(2)33(39(56)43-28(34(41)51)13-15-57-3)47-36(53)29(14-16-58-4)44-37(54)30(17-22-9-11-24(48)12-10-22)46-38(55)31(19-32(49)50)45-35(52)26(40)18-23-20-42-27-8-6-5-7-25(23)27/h5-12,20-21,26,28-31,33,42,48H,13-19,40H2,1-4H3,(H2,41,51)(H,43,56)(H,44,54)(H,45,52)(H,46,55)(H,47,53)(H,49,50)/t26-,28+,29-,30-,31+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86303
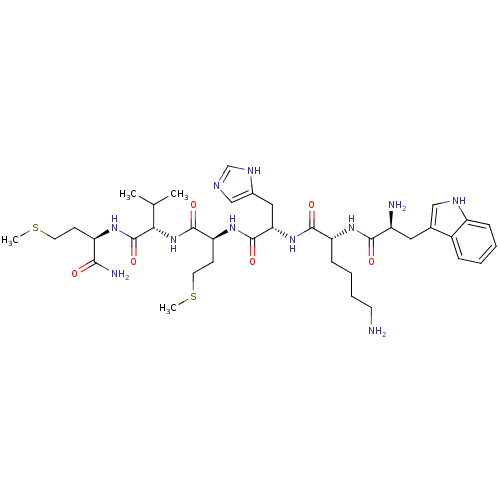
(WKHMVm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(C)C)C(N)=O Show InChI InChI=1S/C38H59N11O6S2/c1-22(2)32(38(55)45-28(33(41)50)12-15-56-3)49-36(53)30(13-16-57-4)47-37(54)31(18-24-20-42-21-44-24)48-35(52)29(11-7-8-14-39)46-34(51)26(40)17-23-19-43-27-10-6-5-9-25(23)27/h5-6,9-10,19-22,26,28-32,43H,7-8,11-18,39-40H2,1-4H3,(H2,41,50)(H,42,44)(H,45,55)(H,46,51)(H,47,54)(H,48,52)(H,49,53)/t26-,28+,29+,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86306
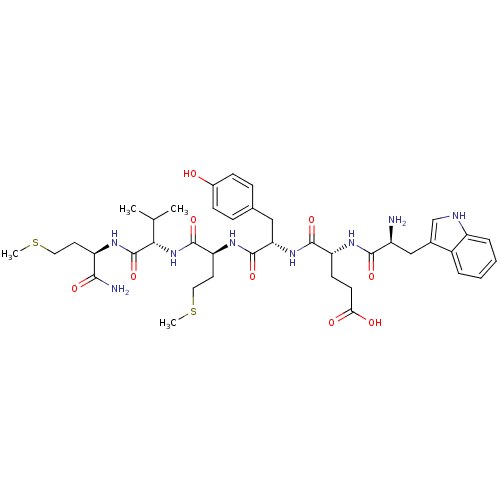
(WEYMVm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(C)C)C(N)=O Show InChI InChI=1S/C40H56N8O9S2/c1-22(2)34(40(57)44-29(35(42)52)15-17-58-3)48-38(55)31(16-18-59-4)46-39(56)32(19-23-9-11-25(49)12-10-23)47-37(54)30(13-14-33(50)51)45-36(53)27(41)20-24-21-43-28-8-6-5-7-26(24)28/h5-12,21-22,27,29-32,34,43,49H,13-20,41H2,1-4H3,(H2,42,52)(H,44,57)(H,45,53)(H,46,56)(H,47,54)(H,48,55)(H,50,51)/t27-,29+,30+,31-,32-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86305
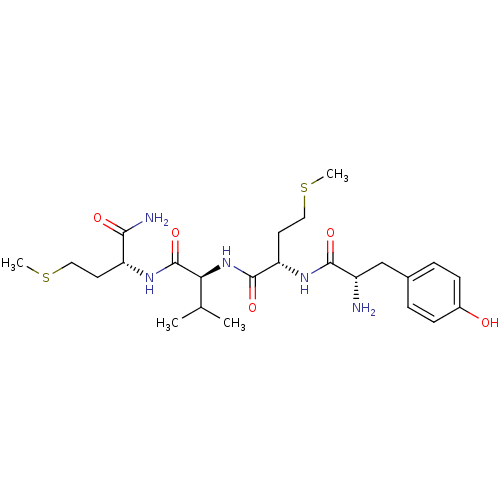
(YMVm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(N)=O Show InChI InChI=1S/C24H39N5O5S2/c1-14(2)20(24(34)27-18(21(26)31)9-11-35-3)29-23(33)19(10-12-36-4)28-22(32)17(25)13-15-5-7-16(30)8-6-15/h5-8,14,17-20,30H,9-13,25H2,1-4H3,(H2,26,31)(H,27,34)(H,28,32)(H,29,33)/t17-,18+,19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 567 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86307
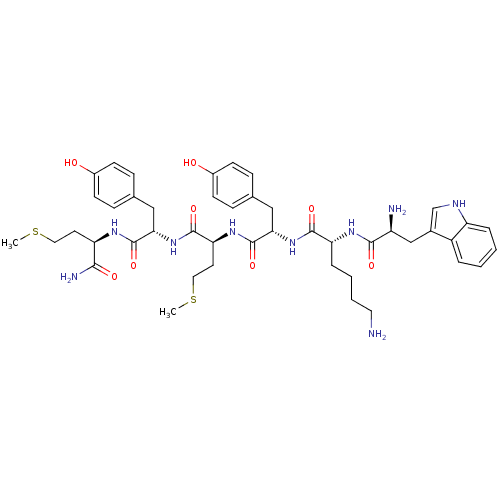
(WKYMYm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C45H61N9O8S2/c1-63-21-18-35(40(48)57)50-44(61)38(23-27-10-14-30(55)15-11-27)54-43(60)37(19-22-64-2)52-45(62)39(24-28-12-16-31(56)17-13-28)53-42(59)36(9-5-6-20-46)51-41(58)33(47)25-29-26-49-34-8-4-3-7-32(29)34/h3-4,7-8,10-17,26,33,35-39,49,55-56H,5-6,9,18-25,46-47H2,1-2H3,(H2,48,57)(H,50,61)(H,51,58)(H,52,62)(H,53,59)(H,54,60)/t33-,35+,36+,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 671 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86304
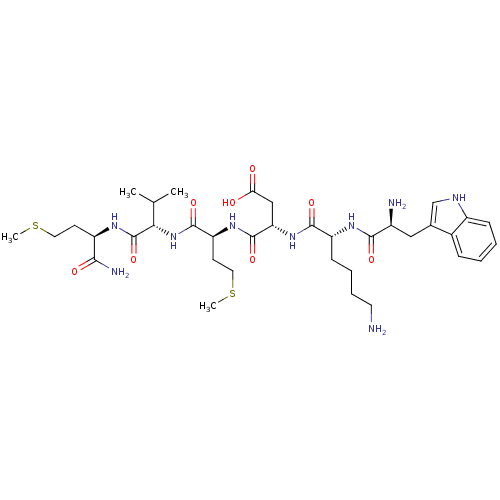
(WKDMVm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(C)C)C(N)=O Show InChI InChI=1S/C36H57N9O8S2/c1-20(2)30(36(53)41-25(31(39)48)12-15-54-3)45-34(51)27(13-16-55-4)43-35(52)28(18-29(46)47)44-33(50)26(11-7-8-14-37)42-32(49)23(38)17-21-19-40-24-10-6-5-9-22(21)24/h5-6,9-10,19-20,23,25-28,30,40H,7-8,11-18,37-38H2,1-4H3,(H2,39,48)(H,41,53)(H,42,49)(H,43,52)(H,44,50)(H,45,51)(H,46,47)/t23-,25+,26+,27-,28-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM86296
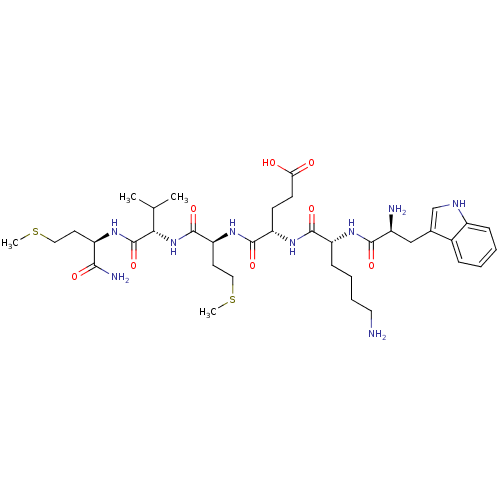
(WKEMVm-NH2)Show SMILES CSCC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(C)C)C(N)=O Show InChI InChI=1S/C37H59N9O8S2/c1-21(2)31(37(54)42-26(32(40)49)14-17-55-3)46-36(53)29(15-18-56-4)45-35(52)28(12-13-30(47)48)44-34(51)27(11-7-8-16-38)43-33(50)24(39)19-22-20-41-25-10-6-5-9-23(22)25/h5-6,9-10,20-21,24,26-29,31,41H,7-8,11-19,38-39H2,1-4H3,(H2,40,49)(H,42,54)(H,43,50)(H,44,51)(H,45,52)(H,46,53)(H,47,48)/t24-,26+,27+,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 841-7 (2003)
Article DOI: 10.1124/mol.64.4.841
BindingDB Entry DOI: 10.7270/Q2WQ02D4 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581458

(CHEMBL5092802)Show SMILES COc1cc(ccc1OCCN1CCOCC1)N(CC1CCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50331095
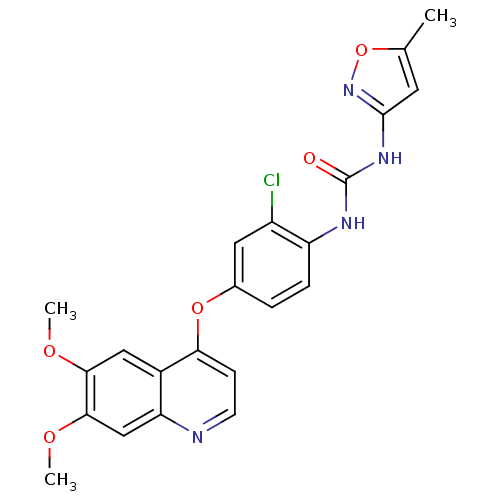
(CHEMBL1289494 | Tivozanib | US10464902, Tivozanib)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)Nc4cc(C)on4)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C22H19ClN4O5/c1-12-8-21(27-32-12)26-22(28)25-16-5-4-13(9-15(16)23)31-18-6-7-24-17-11-20(30-3)19(29-2)10-14(17)18/h4-11H,1-3H3,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assay |
Eur J Med Chem 45: 5420-7 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.002
BindingDB Entry DOI: 10.7270/Q2TX3FMZ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM25117

(AG-013736 | AXITINIB | N-methyl-2-({3-[(E)-2-(pyri...)Show SMILES CNC(=O)c1ccccc1Sc1ccc2c(\C=C\c3ccccn3)n[nH]c2c1 Show InChI InChI=1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assay |
Eur J Med Chem 45: 5420-7 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.002
BindingDB Entry DOI: 10.7270/Q2TX3FMZ |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581397
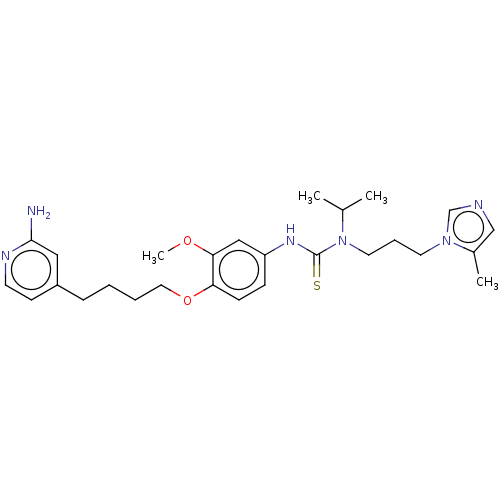
(CHEMBL5085353)Show SMILES COc1cc(NC(=S)N(CCCn2cncc2C)C(C)C)ccc1OCCCCc1ccnc(N)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50331091
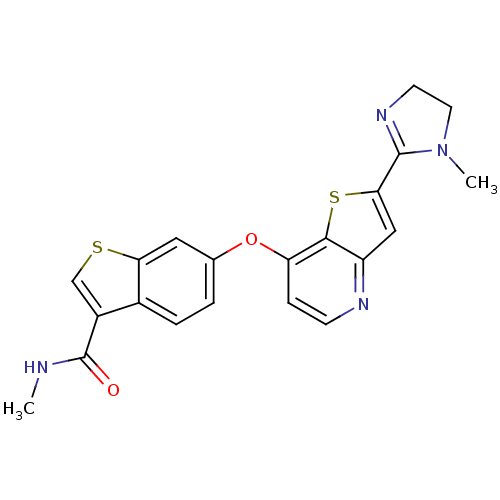
(AG-028262 | CHEMBL1289925 | N-methyl-6-(2-(1-methy...)Show SMILES CNC(=O)c1csc2cc(Oc3ccnc4cc(sc34)C3=NCCN3C)ccc12 |t:22| Show InChI InChI=1S/C21H18N4O2S2/c1-22-21(26)14-11-28-17-9-12(3-4-13(14)17)27-16-5-6-23-15-10-18(29-19(15)16)20-24-7-8-25(20)2/h3-6,9-11H,7-8H2,1-2H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assay |
Eur J Med Chem 45: 5420-7 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.002
BindingDB Entry DOI: 10.7270/Q2TX3FMZ |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581442

(CHEMBL5086776)Show SMILES COc1cc(ccc1OCCN1CCNCC1)N(CC1CCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581399

(CHEMBL5085226)Show SMILES COc1cc(ccc1OCCCCc1ccnc(N)c1)N(C1CCCC1)C(=S)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581400
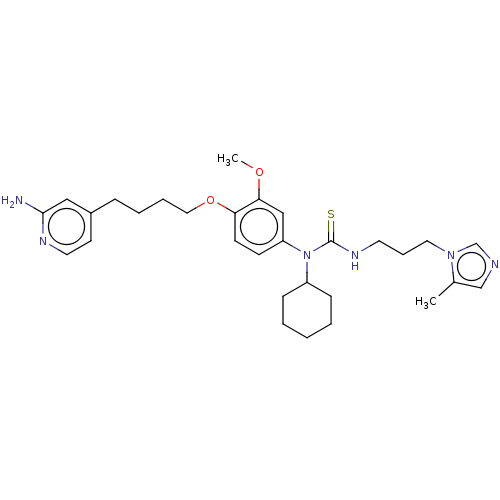
(CHEMBL5075575)Show SMILES COc1cc(ccc1OCCCCc1ccnc(N)c1)N(C1CCCCC1)C(=S)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581426

(CHEMBL5089771)Show SMILES COc1cc(ccc1OCCCCN(C)C)N(C1CCN(C)CC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581398
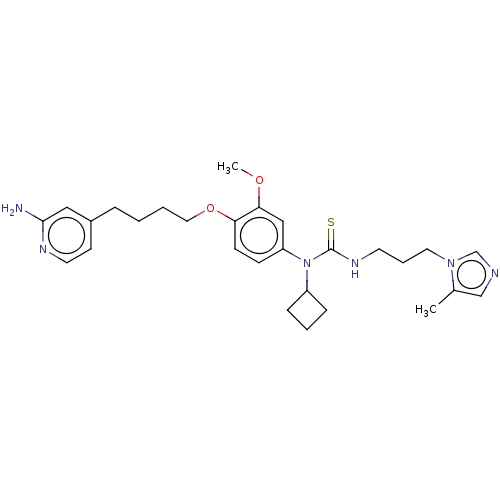
(CHEMBL5083519)Show SMILES COc1cc(ccc1OCCCCc1ccnc(N)c1)N(C1CCC1)C(=S)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581412

(CHEMBL5088732)Show SMILES COc1cc(ccc1OCCCCc1ccnc(N)c1)N(Cc1ccccc1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581450

(CHEMBL5085670)Show SMILES COc1cc(ccc1OCCN1CCN(C)CC1)N(CC1CCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM185584
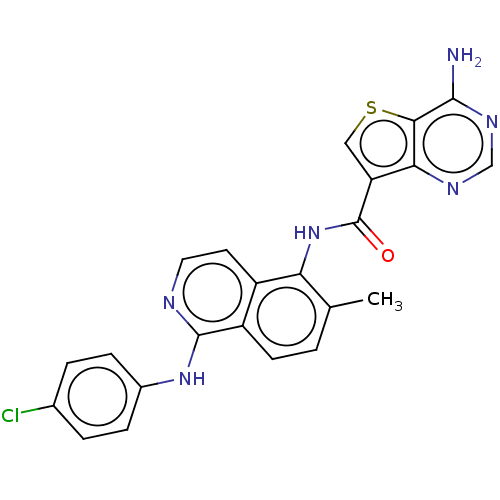
(US9156852, 1 | USRE47451, Example 1)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1NC(=O)c1csc2c(N)ncnc12 Show InChI InChI=1S/C23H17ClN6OS/c1-12-2-7-16-15(8-9-26-22(16)29-14-5-3-13(24)4-6-14)18(12)30-23(31)17-10-32-20-19(17)27-11-28-21(20)25/h2-11H,1H3,(H,26,29)(H,30,31)(H2,25,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD
US Patent
| Assay Description
The compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profiling Servic... |
US Patent US9156852 (2015)
BindingDB Entry DOI: 10.7270/Q24T6H4D |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM185584

(US9156852, 1 | USRE47451, Example 1)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1NC(=O)c1csc2c(N)ncnc12 Show InChI InChI=1S/C23H17ClN6OS/c1-12-2-7-16-15(8-9-26-22(16)29-14-5-3-13(24)4-6-14)18(12)30-23(31)17-10-32-20-19(17)27-11-28-21(20)25/h2-11H,1H3,(H,26,29)(H,30,31)(H2,25,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC
Curated by PubChem BioAssay
| Assay Description
As such, the compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profili... |
PubChem Bioassay (2006)
BindingDB Entry DOI: 10.7270/Q2XW4N61 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Inhibition of Src kinase (unknown origin) |
Bioorg Med Chem 17: 3152-61 (2009)
Article DOI: 10.1016/j.bmc.2009.02.054
BindingDB Entry DOI: 10.7270/Q2V9880T |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581445

(CHEMBL5089285)Show SMILES COc1cc(ccc1OCCN1CCNCC1)N(Cc1ccc(F)cc1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581441
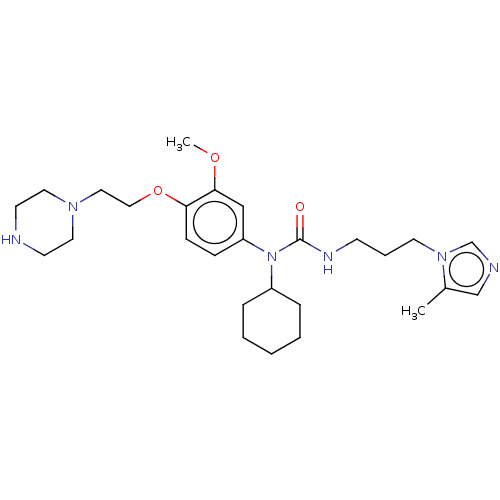
(CHEMBL5081653)Show SMILES COc1cc(ccc1OCCN1CCNCC1)N(C1CCCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50331090
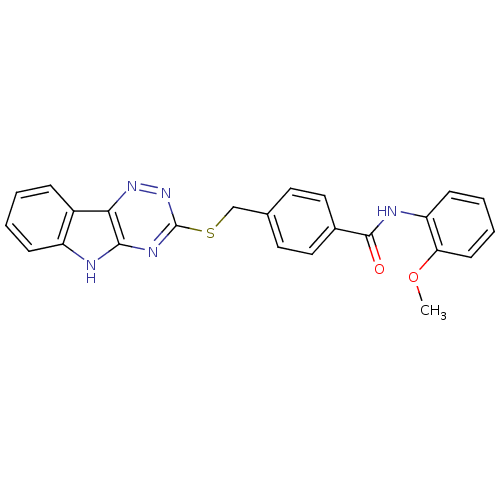
(4-((5H-[1,2,4]triazino[5,6-b]indol-3-ylthio)methyl...)Show SMILES COc1ccccc1NC(=O)c1ccc(CSc2nnc3c(n2)[nH]c2ccccc32)cc1 Show InChI InChI=1S/C24H19N5O2S/c1-31-20-9-5-4-8-19(20)26-23(30)16-12-10-15(11-13-16)14-32-24-27-22-21(28-29-24)17-6-2-3-7-18(17)25-22/h2-13H,14H2,1H3,(H,26,30)(H,25,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assay |
Eur J Med Chem 45: 5420-7 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.002
BindingDB Entry DOI: 10.7270/Q2TX3FMZ |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581436

(CHEMBL5081891)Show SMILES COc1cc(ccc1OCCc1ccnc(N)c1)N(Cc1ccc(F)cc1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581443
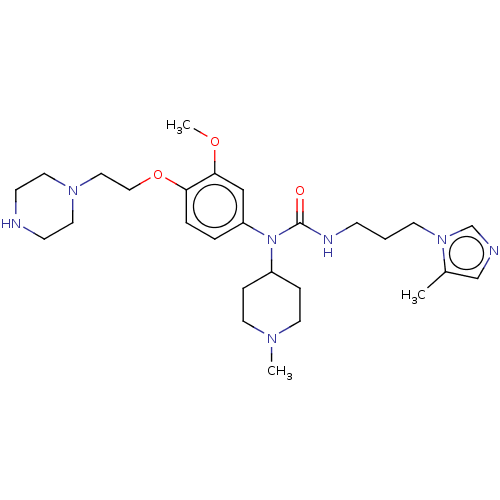
(CHEMBL5083687)Show SMILES COc1cc(ccc1OCCN1CCNCC1)N(C1CCN(C)CC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581434

(CHEMBL5081537)Show SMILES COc1cc(ccc1OCCc1ccnc(N)c1)N(C1CCCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581451
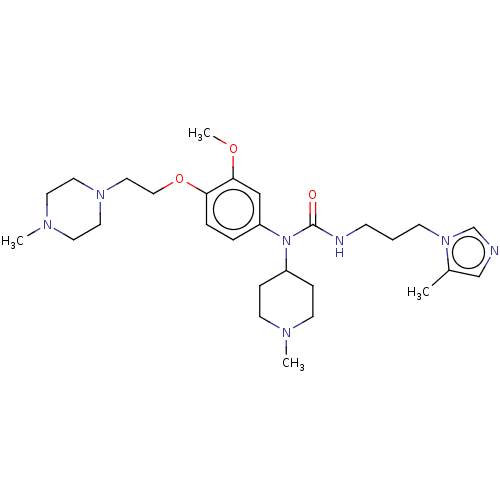
(CHEMBL5092925)Show SMILES COc1cc(ccc1OCCN1CCN(C)CC1)N(C1CCN(C)CC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM185584

(US9156852, 1 | USRE47451, Example 1)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1NC(=O)c1csc2c(N)ncnc12 Show InChI InChI=1S/C23H17ClN6OS/c1-12-2-7-16-15(8-9-26-22(16)29-14-5-3-13(24)4-6-14)18(12)30-23(31)17-10-32-20-19(17)27-11-28-21(20)25/h2-11H,1H3,(H,26,29)(H,30,31)(H2,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC
Curated by PubChem BioAssay
| Assay Description
As such, the compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profili... |
PubChem Bioassay (2006)
BindingDB Entry DOI: 10.7270/Q2XW4N61 |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM185584

(US9156852, 1 | USRE47451, Example 1)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1NC(=O)c1csc2c(N)ncnc12 Show InChI InChI=1S/C23H17ClN6OS/c1-12-2-7-16-15(8-9-26-22(16)29-14-5-3-13(24)4-6-14)18(12)30-23(31)17-10-32-20-19(17)27-11-28-21(20)25/h2-11H,1H3,(H,26,29)(H,30,31)(H2,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD
US Patent
| Assay Description
The compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profiling Servic... |
US Patent US9156852 (2015)
BindingDB Entry DOI: 10.7270/Q24T6H4D |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581460

(CHEMBL5084485)Show SMILES COc1cc(ccc1OCCN1CCOCC1)N(Cc1ccc(F)cc1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581448

(CHEMBL5094369)Show SMILES COc1cc(ccc1OCCN1CCN(C)CC1)N(C(C)C)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581422
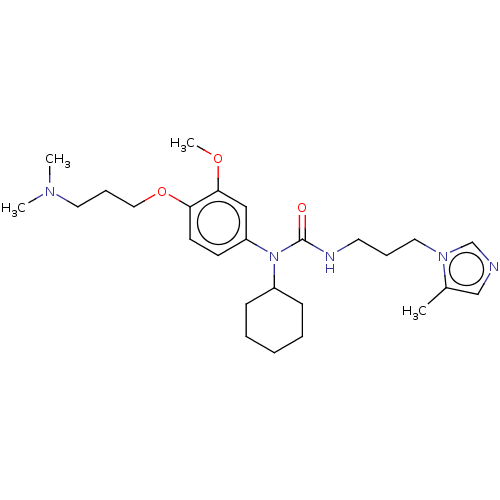
(CHEMBL5079181)Show SMILES COc1cc(ccc1OCCCN(C)C)N(C1CCCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581410
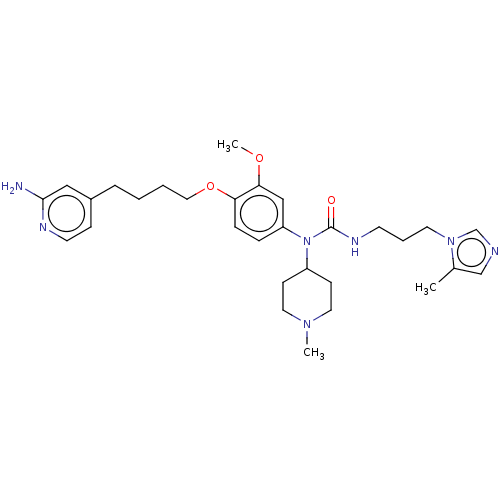
(CHEMBL5081843)Show SMILES COc1cc(ccc1OCCCCc1ccnc(N)c1)N(C1CCN(C)CC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50331083

(CHEMBL1290362 | ethyl 2-(3-methyl-7-(3-methylbenzy...)Show SMILES CCOC(=O)CSc1nc2n(C)c(=O)[nH]c(=O)c2n1Cc1cccc(C)c1 Show InChI InChI=1S/C18H20N4O4S/c1-4-26-13(23)10-27-18-19-15-14(16(24)20-17(25)21(15)3)22(18)9-12-7-5-6-11(2)8-12/h5-8H,4,9-10H2,1-3H3,(H,20,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assay |
Eur J Med Chem 45: 5420-7 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.002
BindingDB Entry DOI: 10.7270/Q2TX3FMZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Inhibition of Src kinase (unknown origin) |
Bioorg Med Chem 17: 3152-61 (2009)
Article DOI: 10.1016/j.bmc.2009.02.054
BindingDB Entry DOI: 10.7270/Q2V9880T |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Inhibition of Src kinase (unknown origin) |
Bioorg Med Chem 17: 3152-61 (2009)
Article DOI: 10.1016/j.bmc.2009.02.054
BindingDB Entry DOI: 10.7270/Q2V9880T |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM24773
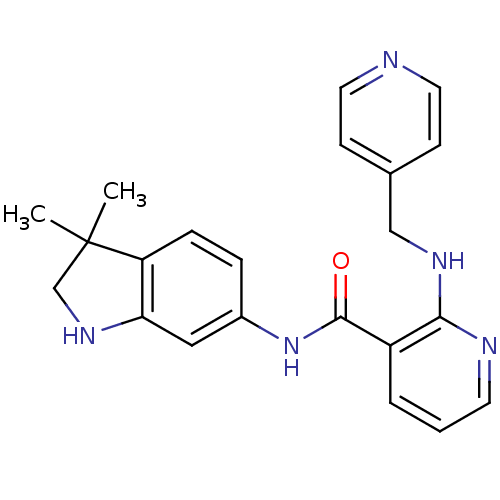
(AMG 706 | AMG-706 | Motesanib | N-(3,3-dimethyl-1,...)Show SMILES CC1(C)CNc2cc(NC(=O)c3cccnc3NCc3ccncc3)ccc12 Show InChI InChI=1S/C22H23N5O/c1-22(2)14-26-19-12-16(5-6-18(19)22)27-21(28)17-4-3-9-24-20(17)25-13-15-7-10-23-11-8-15/h3-12,26H,13-14H2,1-2H3,(H,24,25)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assay |
Eur J Med Chem 45: 5420-7 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.002
BindingDB Entry DOI: 10.7270/Q2TX3FMZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581453
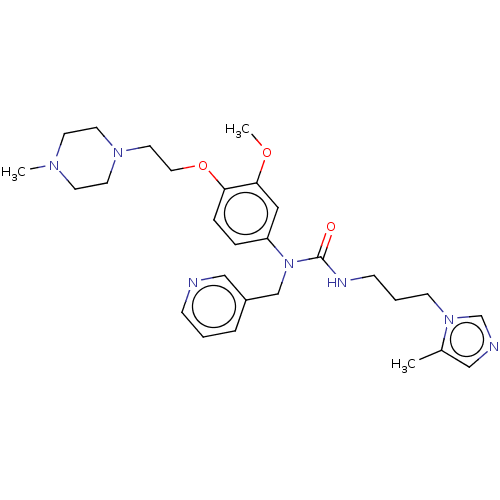
(CHEMBL5076836)Show SMILES COc1cc(ccc1OCCN1CCN(C)CC1)N(Cc1cccnc1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50384888
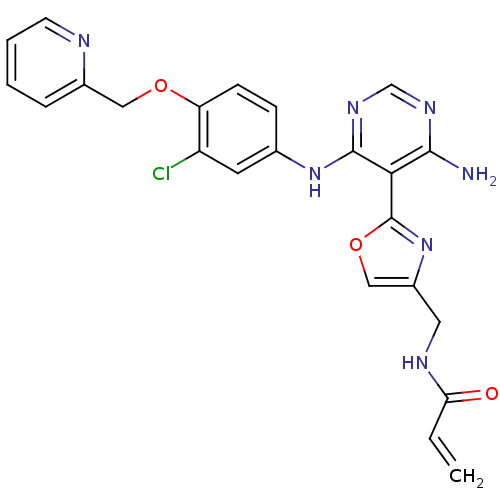
(CHEMBL2035810)Show SMILES Nc1ncnc(Nc2ccc(OCc3ccccn3)c(Cl)c2)c1-c1nc(CNC(=O)C=C)co1 Show InChI InChI=1S/C23H20ClN7O3/c1-2-19(32)27-10-16-12-34-23(31-16)20-21(25)28-13-29-22(20)30-14-6-7-18(17(24)9-14)33-11-15-5-3-4-8-26-15/h2-9,12-13H,1,10-11H2,(H,27,32)(H3,25,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR preincubated for 10 mins followed by incubation for 30 mins by fluorescence polarization assay |
J Med Chem 55: 2846-57 (2012)
Article DOI: 10.1021/jm201758g
BindingDB Entry DOI: 10.7270/Q23T9J8F |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581409
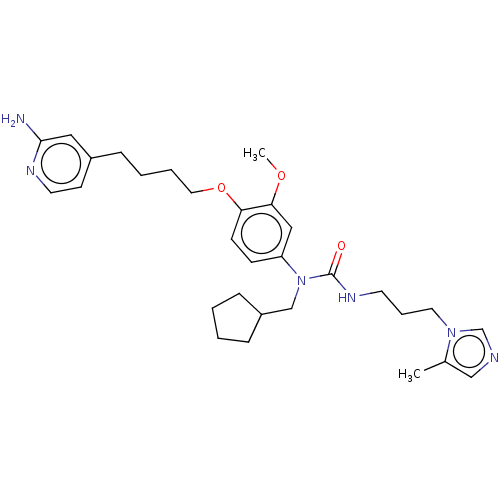
(CHEMBL5088413)Show SMILES COc1cc(ccc1OCCCCc1ccnc(N)c1)N(CC1CCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50331089
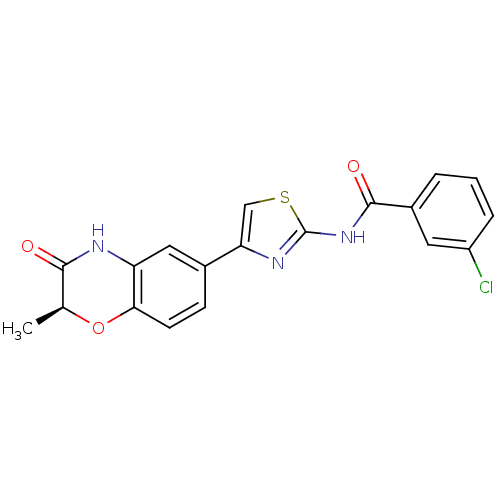
((S)-3-chloro-N-(4-(2-methyl-3-oxo-3,4-dihydro-2H-b...)Show SMILES C[C@@H]1Oc2ccc(cc2NC1=O)-c1csc(NC(=O)c2cccc(Cl)c2)n1 |r| Show InChI InChI=1S/C19H14ClN3O3S/c1-10-17(24)21-14-8-11(5-6-16(14)26-10)15-9-27-19(22-15)23-18(25)12-3-2-4-13(20)7-12/h2-10H,1H3,(H,21,24)(H,22,23,25)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assay |
Eur J Med Chem 45: 5420-7 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.002
BindingDB Entry DOI: 10.7270/Q2TX3FMZ |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581473
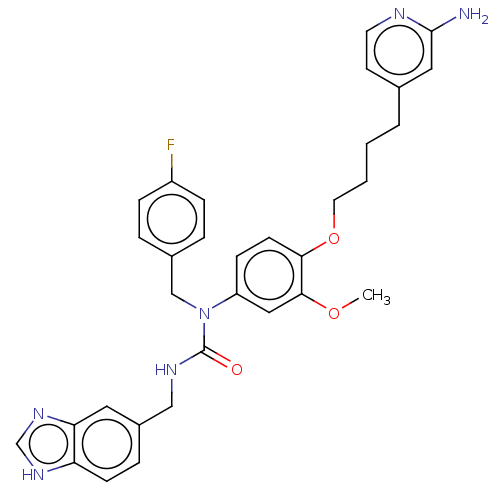
(CHEMBL5088100)Show SMILES COc1cc(ccc1OCCCCc1ccnc(N)c1)N(Cc1ccc(F)cc1)C(=O)NCc1ccc2[nH]cnc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data