 Found 47 hits with Last Name = 'staker' and Initial = 'bl'
Found 47 hits with Last Name = 'staker' and Initial = 'bl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50548088
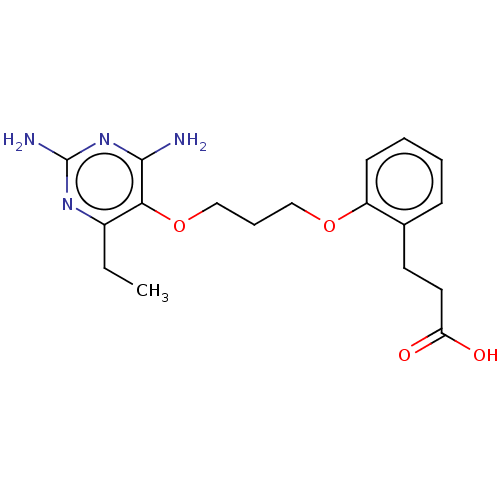
(CHEMBL3040038) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR |
Citation and Details
Article DOI: 10.1039/d0md00303d
BindingDB Entry DOI: 10.7270/Q26H4N1G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM36516
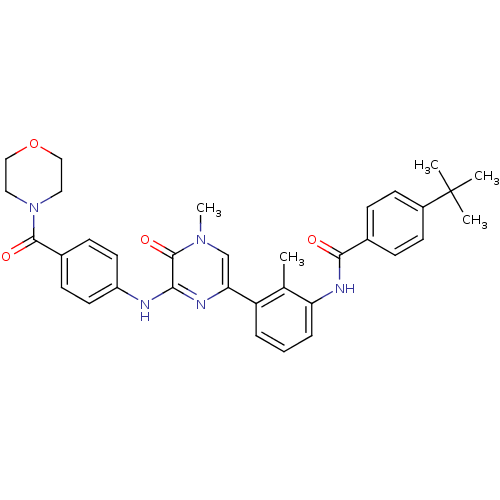
(4-(tert-Butyl)-N-(2-methyl-3-(4-methyl-6-((4-(morp...)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C34H37N5O4/c1-22-27(7-6-8-28(22)37-31(40)23-9-13-25(14-10-23)34(2,3)4)29-21-38(5)33(42)30(36-29)35-26-15-11-24(12-16-26)32(41)39-17-19-43-20-18-39/h6-16,21H,17-20H2,1-5H3,(H,35,36)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | 1.5 | n/a | n/a | n/a | 7.5 | 23 |
CGI Pharmaceuticals
| Assay Description
Biochemical assay using Lanthascreen (human, full-lenght, C-terminal v5-His6 expressed in Sf9 cell) assay from Invitrogen. |
Nat Chem Biol 7: 41-50 (2011)
Article DOI: 10.1038/nchembio.481
BindingDB Entry DOI: 10.7270/Q2B56H2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232538
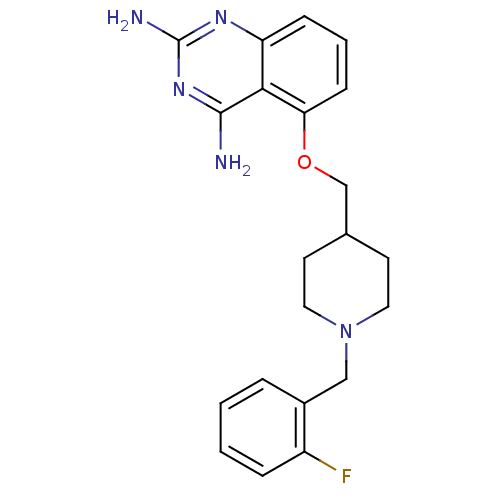
(5-((1-(2-fluorobenzyl)piperidin-4-yl)methoxy)quina...)Show InChI InChI=1S/C21H24FN5O/c22-16-5-2-1-4-15(16)12-27-10-8-14(9-11-27)13-28-18-7-3-6-17-19(18)20(23)26-21(24)25-17/h1-7,14H,8-13H2,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 7.62 | n/a | 4 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36533
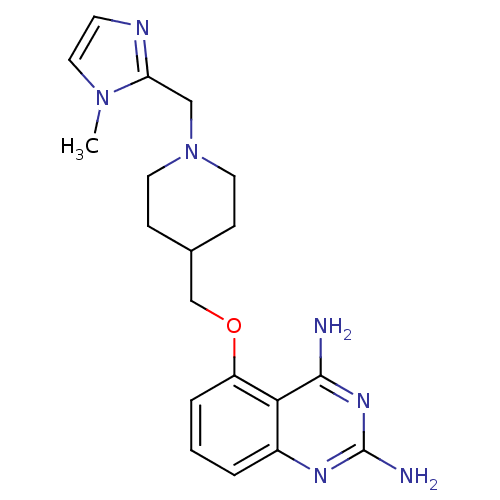
(D157555)Show InChI InChI=1S/C19H25N7O/c1-25-10-7-22-16(25)11-26-8-5-13(6-9-26)12-27-15-4-2-3-14-17(15)18(20)24-19(21)23-14/h2-4,7,10,13H,5-6,8-9,11-12H2,1H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.38 | n/a | 108 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36531

(D158963)Show InChI InChI=1S/C16H20F3N5O/c17-16(18,19)9-24-6-4-10(5-7-24)8-25-12-3-1-2-11-13(12)14(20)23-15(21)22-11/h1-3,10H,4-9H2,(H4,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23.4 | n/a | 223 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50073854

(2,10-dihydroxy-12-(3,4,5-trihydroxy-6-hydroxymethy...)Show SMILES OCC1OC(C(O)C(O)C1O)n1c2cc(O)ccc2c2c3C(=O)NC(=O)c3c3c4ccc(O)cc4[nH]c3c12 Show InChI InChI=1S/C26H21N3O9/c30-7-14-21(33)22(34)23(35)26(38-14)29-13-6-9(32)2-4-11(13)16-18-17(24(36)28-25(18)37)15-10-3-1-8(31)5-12(10)27-19(15)20(16)29/h1-6,14,21-23,26-27,30-35H,7H2,(H,28,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE BioStructures
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase I-DNA complex in trapping assay |
J Med Chem 48: 2336-45 (2005)
Article DOI: 10.1021/jm049146p
BindingDB Entry DOI: 10.7270/Q2CF9QVT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321626
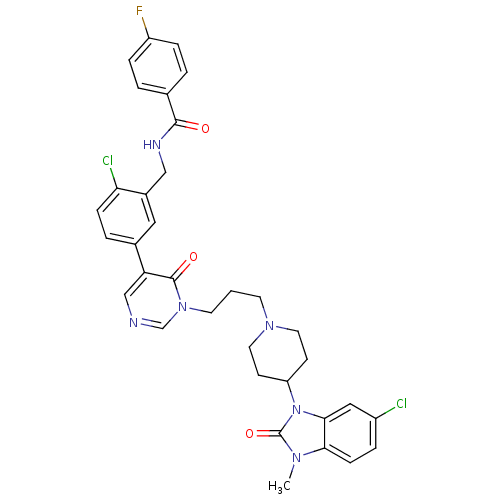
(CHEMBL1171504 | N-(2-chloro-5-(1-(3-(4-(6-chloro-3...)Show SMILES Cn1c2ccc(Cl)cc2n(C2CCN(CCCn3cncc(-c4ccc(Cl)c(CNC(=O)c5ccc(F)cc5)c4)c3=O)CC2)c1=O Show InChI InChI=1S/C34H33Cl2FN6O3/c1-40-30-10-6-25(35)18-31(30)43(34(40)46)27-11-15-41(16-12-27)13-2-14-42-21-38-20-28(33(42)45)23-5-9-29(36)24(17-23)19-39-32(44)22-3-7-26(37)8-4-22/h3-10,17-18,20-21,27H,2,11-16,19H2,1H3,(H,39,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36530
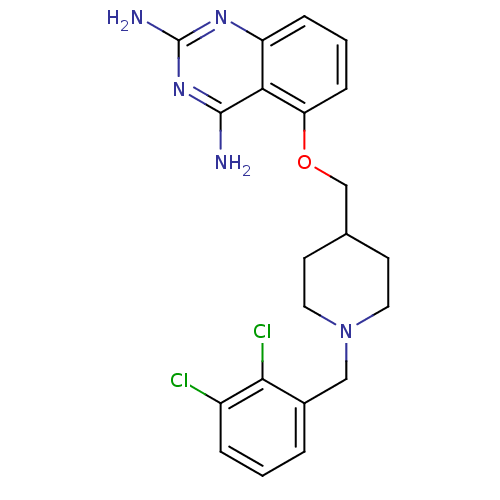
(D157493)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4cccc(Cl)c4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-4-1-3-14(19(15)23)11-28-9-7-13(8-10-28)12-29-17-6-2-5-16-18(17)20(24)27-21(25)26-16/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 43.9 | n/a | 67 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50008935
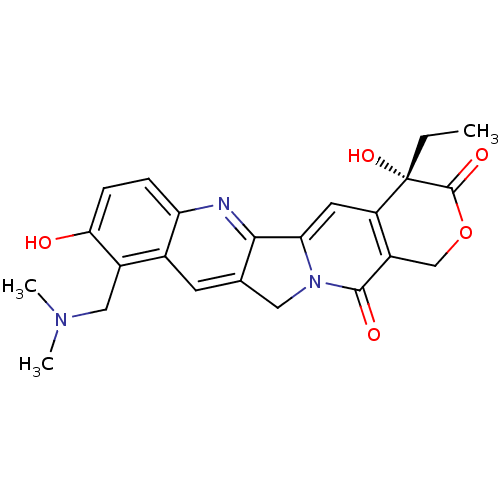
((20S)-10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4ccc(O)c(CN(C)C)c4cc3Cn1c2=O |r| Show InChI InChI=1S/C23H23N3O5/c1-4-23(30)16-8-18-20-12(9-26(18)21(28)15(16)11-31-22(23)29)7-13-14(10-25(2)3)19(27)6-5-17(13)24-20/h5-8,27,30H,4,9-11H2,1-3H3/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE BioStructures
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase I-DNA complex in trapping assay |
J Med Chem 48: 2336-45 (2005)
Article DOI: 10.1021/jm049146p
BindingDB Entry DOI: 10.7270/Q2CF9QVT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM76305
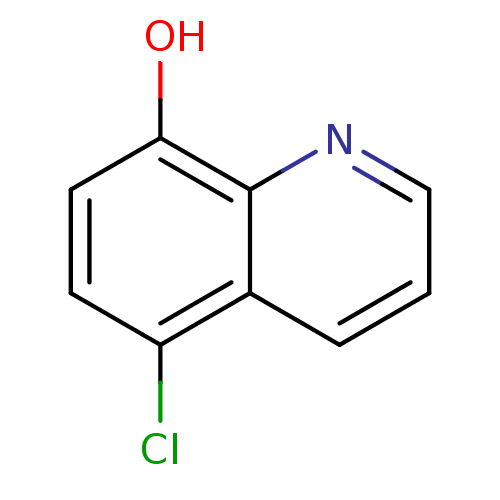
(5-chloranylquinolin-8-ol | 5-chloro-8-quinolinol |...)Show InChI InChI=1S/C9H6ClNO/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321625

(CHEMBL1171503 | CatS_4 | N-(2-chloro-5-(2-(3-(4-(6...)Show SMILES Cn1c2ccc(Cl)cc2n(C2CCN(CCCn3nccc(-c4ccc(Cl)c(CNC(=O)c5ccc(F)cc5)c4)c3=O)CC2)c1=O Show InChI InChI=1S/C34H33Cl2FN6O3/c1-40-30-10-6-25(35)20-31(30)43(34(40)46)27-12-17-41(18-13-27)15-2-16-42-33(45)28(11-14-39-42)23-5-9-29(36)24(19-23)21-38-32(44)22-3-7-26(37)8-4-22/h3-11,14,19-20,27H,2,12-13,15-18,21H2,1H3,(H,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36532

(D158885)Show InChI InChI=1S/C20H22ClN5O/c21-14-4-1-2-6-16(14)26-10-8-13(9-11-26)12-27-17-7-3-5-15-18(17)19(22)25-20(23)24-15/h1-7,13H,8-12H2,(H4,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 101 | n/a | 795 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321627
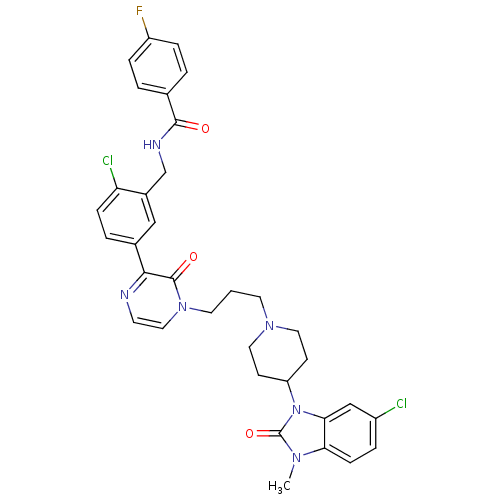
(CHEMBL1171505 | CatS_6 | N-(2-chloro-5-(4-(3-(4-(6...)Show SMILES Cn1c2ccc(Cl)cc2n(C2CCN(CCCn3ccnc(-c4ccc(Cl)c(CNC(=O)c5ccc(F)cc5)c4)c3=O)CC2)c1=O Show InChI InChI=1S/C34H33Cl2FN6O3/c1-40-29-10-6-25(35)20-30(29)43(34(40)46)27-11-16-41(17-12-27)14-2-15-42-18-13-38-31(33(42)45)23-5-9-28(36)24(19-23)21-39-32(44)22-3-7-26(37)8-4-22/h3-10,13,18-20,27H,2,11-12,14-17,21H2,1H3,(H,39,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50008923

((S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4ccccc4cc3Cn1c2=O |r| Show InChI InChI=1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE BioStructures
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase I-DNA complex in trapping assay |
J Med Chem 48: 2336-45 (2005)
Article DOI: 10.1021/jm049146p
BindingDB Entry DOI: 10.7270/Q2CF9QVT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321624

(CHEMBL1171502 | N-(2-chloro-5-(1-(3-(4-(6-chloro-3...)Show SMILES Cn1c2ccc(Cl)cc2n(C2CCN(CCCn3nc(ccc3=O)-c3ccc(Cl)c(CNC(=O)c4ccc(F)cc4)c3)CC2)c1=O Show InChI InChI=1S/C34H33Cl2FN6O3/c1-40-30-11-6-25(35)20-31(30)43(34(40)46)27-13-17-41(18-14-27)15-2-16-42-32(44)12-10-29(39-42)23-5-9-28(36)24(19-23)21-38-33(45)22-3-7-26(37)8-4-22/h3-12,19-20,27H,2,13-18,21H2,1H3,(H,38,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36529

(D156676)Show SMILES CC(=O)Nc1nc(CN2CCC(COc3cccc4nc(N)nc(N)c34)CC2)cs1 Show InChI InChI=1S/C20H25N7O2S/c1-12(28)23-20-24-14(11-30-20)9-27-7-5-13(6-8-27)10-29-16-4-2-3-15-17(16)18(21)26-19(22)25-15/h2-4,11,13H,5-10H2,1H3,(H,23,24,28)(H4,21,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 339 | n/a | 2.54E+3 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321623
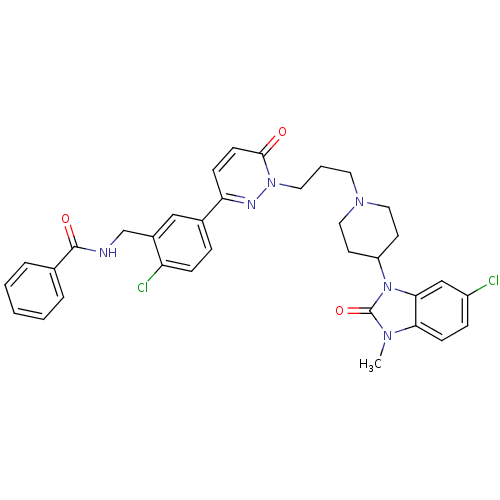
(CHEMBL1171314 | N-(2-chloro-5-(1-(3-(4-(6-chloro-3...)Show SMILES Cn1c2ccc(Cl)cc2n(C2CCN(CCCn3nc(ccc3=O)-c3ccc(Cl)c(CNC(=O)c4ccccc4)c3)CC2)c1=O Show InChI InChI=1S/C34H34Cl2N6O3/c1-39-30-12-9-26(35)21-31(30)42(34(39)45)27-14-18-40(19-15-27)16-5-17-41-32(43)13-11-29(38-41)24-8-10-28(36)25(20-24)22-37-33(44)23-6-3-2-4-7-23/h2-4,6-13,20-21,27H,5,14-19,22H2,1H3,(H,37,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM36521
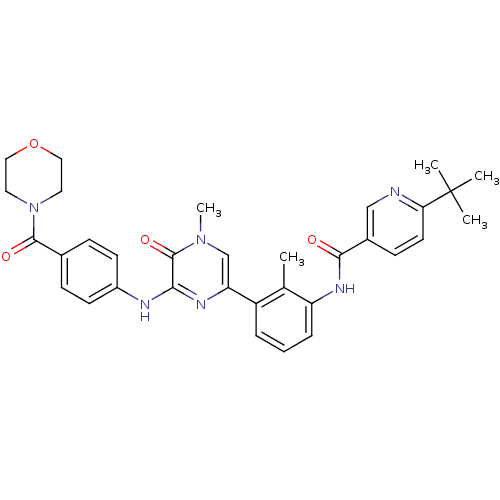
(6-(tert-Butyl)-N-(2-methyl-3-(4-methyl-6-((4-(morp...)Show SMILES Cc1c(NC(=O)c2ccc(nc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C33H36N6O4/c1-21-25(7-6-8-26(21)37-30(40)23-11-14-28(34-19-23)33(2,3)4)27-20-38(5)32(42)29(36-27)35-24-12-9-22(10-13-24)31(41)39-15-17-43-18-16-39/h6-14,19-20H,15-18H2,1-5H3,(H,35,36)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.5 | 23 |
CGI Pharmaceuticals
| Assay Description
Biochemical assay using Lanthascreen (human, full-lenght, C-terminal v5-His6 expressed in Sf9 cell) assay from Invitrogen. |
Nat Chem Biol 7: 41-50 (2011)
Article DOI: 10.1038/nchembio.481
BindingDB Entry DOI: 10.7270/Q2B56H2T |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM32203

(8-quinolinol | CHEMBL310555 | US10005735, Table 1....)Show InChI InChI=1S/C9H7NO/c11-8-5-1-3-7-4-2-6-10-9(7)8/h1-6,11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321622
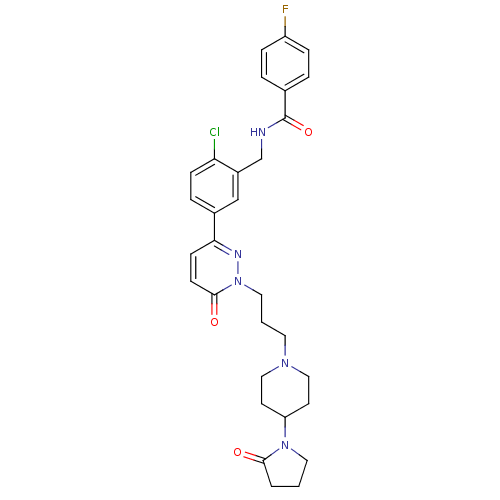
(CHEMBL1170609 | N-(2-chloro-5-(6-oxo-1-(3-(4-(2-ox...)Show SMILES Fc1ccc(cc1)C(=O)NCc1cc(ccc1Cl)-c1ccc(=O)n(CCCN2CCC(CC2)N2CCCC2=O)n1 Show InChI InChI=1S/C30H33ClFN5O3/c31-26-9-6-22(19-23(26)20-33-30(40)21-4-7-24(32)8-5-21)27-10-11-29(39)37(34-27)16-2-14-35-17-12-25(13-18-35)36-15-1-3-28(36)38/h4-11,19,25H,1-3,12-18,20H2,(H,33,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321620
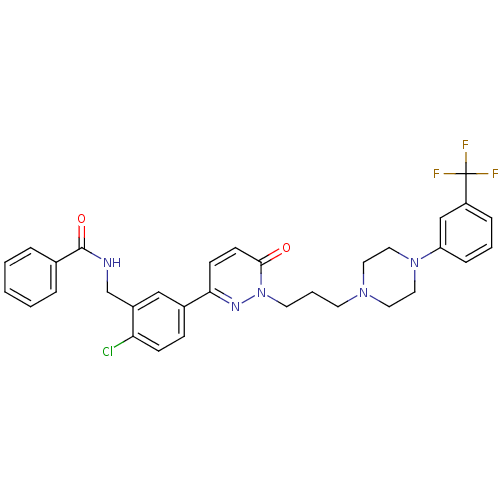
(CHEMBL1171313 | N-(2-chloro-5-(6-oxo-1-(3-(4-(3-(t...)Show SMILES FC(F)(F)c1cccc(c1)N1CCN(CCCn2nc(ccc2=O)-c2ccc(Cl)c(CNC(=O)c3ccccc3)c2)CC1 Show InChI InChI=1S/C32H31ClF3N5O2/c33-28-11-10-24(20-25(28)22-37-31(43)23-6-2-1-3-7-23)29-12-13-30(42)41(38-29)15-5-14-39-16-18-40(19-17-39)27-9-4-8-26(21-27)32(34,35)36/h1-4,6-13,20-21H,5,14-19,22H2,(H,37,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321625

(CHEMBL1171503 | CatS_4 | N-(2-chloro-5-(2-(3-(4-(6...)Show SMILES Cn1c2ccc(Cl)cc2n(C2CCN(CCCn3nccc(-c4ccc(Cl)c(CNC(=O)c5ccc(F)cc5)c4)c3=O)CC2)c1=O Show InChI InChI=1S/C34H33Cl2FN6O3/c1-40-30-10-6-25(35)20-31(30)43(34(40)46)27-12-17-41(18-13-27)15-2-16-42-33(45)28(11-14-39-42)23-5-9-29(36)24(19-23)21-38-32(44)22-3-7-26(37)8-4-22/h3-11,14,19-20,27H,2,12-13,15-18,21H2,1H3,(H,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S-mediated invariant chain degradation in human JY B-cells assessed as accumulation of p10 fragment by Western blot analysis |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50065785

(2-Methyl-quinolin-8-ol | CHEMBL316892)Show InChI InChI=1S/C10H9NO/c1-7-5-6-8-3-2-4-9(12)10(8)11-7/h2-6,12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321621

(CHEMBL1171608 | N-(2-chloro-5-(6-oxo-1-(3-(4-(2-ox...)Show SMILES Clc1ccc(cc1CNC(=O)c1ccccc1)-c1ccc(=O)n(CCCN2CCC(CC2)N2CCCC2=O)n1 Show InChI InChI=1S/C30H34ClN5O3/c31-26-10-9-23(20-24(26)21-32-30(39)22-6-2-1-3-7-22)27-11-12-29(38)36(33-27)17-5-15-34-18-13-25(14-19-34)35-16-4-8-28(35)37/h1-3,6-7,9-12,20,25H,4-5,8,13-19,21H2,(H,32,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM36518

(CID46942382 | N-(2-Methyl-3-(4-methyl-6-((4-(morph...)Show SMILES CC(=O)Nc1cccc(c1C)-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C25H27N5O4/c1-16-20(5-4-6-21(16)26-17(2)31)22-15-29(3)25(33)23(28-22)27-19-9-7-18(8-10-19)24(32)30-11-13-34-14-12-30/h4-10,15H,11-14H2,1-3H3,(H,26,31)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
CGI Pharmaceuticals
| Assay Description
Biochemical assay using Lanthascreen (human, full-lenght, C-terminal v5-His6 expressed in Sf9 cell) assay from Invitrogen. |
Nat Chem Biol 7: 41-50 (2011)
Article DOI: 10.1038/nchembio.481
BindingDB Entry DOI: 10.7270/Q2B56H2T |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM36520
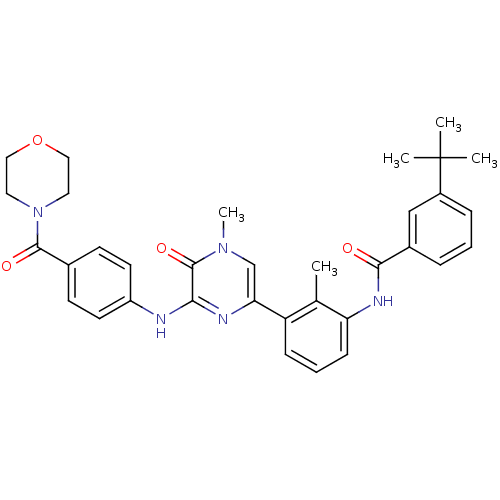
(3-(tert-Butyl)-N-(2-methyl-3-(4-methyl-6-((4-(morp...)Show SMILES Cc1c(NC(=O)c2cccc(c2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C34H37N5O4/c1-22-27(10-7-11-28(22)37-31(40)24-8-6-9-25(20-24)34(2,3)4)29-21-38(5)33(42)30(36-29)35-26-14-12-23(13-15-26)32(41)39-16-18-43-19-17-39/h6-15,20-21H,16-19H2,1-5H3,(H,35,36)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
CGI Pharmaceuticals
| Assay Description
Biochemical assay using Lanthascreen (human, full-lenght, C-terminal v5-His6 expressed in Sf9 cell) assay from Invitrogen. |
Nat Chem Biol 7: 41-50 (2011)
Article DOI: 10.1038/nchembio.481
BindingDB Entry DOI: 10.7270/Q2B56H2T |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164286

(4-(5,11-Dioxo-5,6a,11,11a-tetrahydro-indeno[1,2-c]...)Show InChI InChI=1S/C20H17NO4/c22-16(23)10-5-11-21-18-13-7-2-3-8-14(13)19(24)17(18)12-6-1-4-9-15(12)20(21)25/h1-4,6-9,17-18H,5,10-11H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE BioStructures
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase I-DNA complex in trapping assay |
J Med Chem 48: 2336-45 (2005)
Article DOI: 10.1021/jm049146p
BindingDB Entry DOI: 10.7270/Q2CF9QVT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM36517
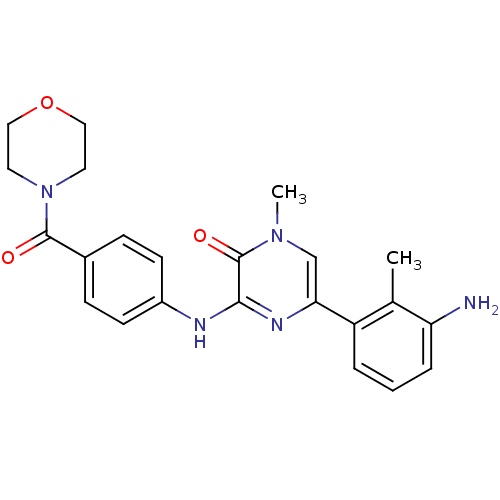
(5-(3-Amino-2-methylphenyl)-1-methyl-3-((4-(morphol...)Show SMILES Cc1c(N)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C23H25N5O3/c1-15-18(4-3-5-19(15)24)20-14-27(2)23(30)21(26-20)25-17-8-6-16(7-9-17)22(29)28-10-12-31-13-11-28/h3-9,14H,10-13,24H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.44E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
CGI Pharmaceuticals
| Assay Description
Biochemical assay using Lanthascreen (human, full-lenght, C-terminal v5-His6 expressed in Sf9 cell) assay from Invitrogen. |
Nat Chem Biol 7: 41-50 (2011)
Article DOI: 10.1038/nchembio.481
BindingDB Entry DOI: 10.7270/Q2B56H2T |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321626

(CHEMBL1171504 | N-(2-chloro-5-(1-(3-(4-(6-chloro-3...)Show SMILES Cn1c2ccc(Cl)cc2n(C2CCN(CCCn3cncc(-c4ccc(Cl)c(CNC(=O)c5ccc(F)cc5)c4)c3=O)CC2)c1=O Show InChI InChI=1S/C34H33Cl2FN6O3/c1-40-30-10-6-25(35)18-31(30)43(34(40)46)27-11-15-41(16-12-27)13-2-14-42-21-38-20-28(33(42)45)23-5-9-29(36)24(17-23)19-39-32(44)22-3-7-26(37)8-4-22/h3-10,17-18,20-21,27H,2,11-16,19H2,1H3,(H,39,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S-mediated invariant chain degradation in human JY B-cells assessed as accumulation of p10 fragment by Western blot analysis |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321627

(CHEMBL1171505 | CatS_6 | N-(2-chloro-5-(4-(3-(4-(6...)Show SMILES Cn1c2ccc(Cl)cc2n(C2CCN(CCCn3ccnc(-c4ccc(Cl)c(CNC(=O)c5ccc(F)cc5)c4)c3=O)CC2)c1=O Show InChI InChI=1S/C34H33Cl2FN6O3/c1-40-29-10-6-25(35)20-30(29)43(34(40)46)27-11-16-41(17-12-27)14-2-15-42-18-13-38-31(33(42)45)23-5-9-28(36)24(19-23)21-39-32(44)22-3-7-26(37)8-4-22/h3-10,13,18-20,27H,2,11-12,14-17,21H2,1H3,(H,39,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S-mediated invariant chain degradation in human JY B-cells assessed as accumulation of p10 fragment by Western blot analysis |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM36519
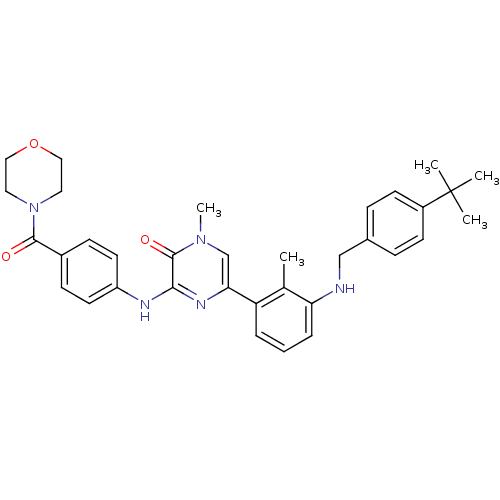
(5-(3-((4-(tert-Butyl)benzyl)amino)-2-methylphenyl)...)Show SMILES Cc1c(NCc2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C34H39N5O3/c1-23-28(7-6-8-29(23)35-21-24-9-13-26(14-10-24)34(2,3)4)30-22-38(5)33(41)31(37-30)36-27-15-11-25(12-16-27)32(40)39-17-19-42-20-18-39/h6-16,22,35H,17-21H2,1-5H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.06E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
CGI Pharmaceuticals
| Assay Description
Biochemical assay using Lanthascreen (human, full-lenght, C-terminal v5-His6 expressed in Sf9 cell) assay from Invitrogen. |
Nat Chem Biol 7: 41-50 (2011)
Article DOI: 10.1038/nchembio.481
BindingDB Entry DOI: 10.7270/Q2B56H2T |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM32203

(8-quinolinol | CHEMBL310555 | US10005735, Table 1....)Show InChI InChI=1S/C9H7NO/c11-8-5-1-3-7-4-2-6-10-9(7)8/h1-6,11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50321619
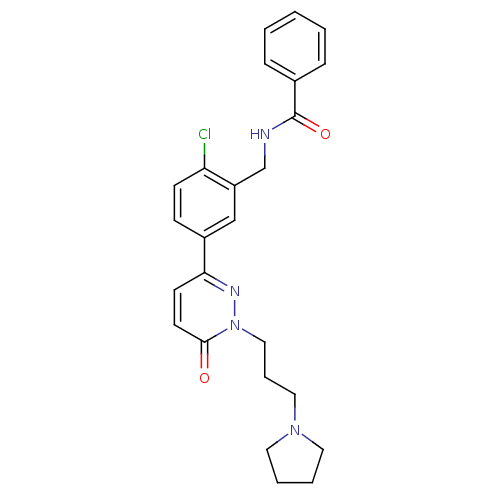
(CHEMBL1171421 | N-(2-chloro-5-(6-oxo-1-(3-(pyrroli...)Show SMILES Clc1ccc(cc1CNC(=O)c1ccccc1)-c1ccc(=O)n(CCCN2CCCC2)n1 Show InChI InChI=1S/C25H27ClN4O2/c26-22-10-9-20(17-21(22)18-27-25(32)19-7-2-1-3-8-19)23-11-12-24(31)30(28-23)16-6-15-29-13-4-5-14-29/h1-3,7-12,17H,4-6,13-16,18H2,(H,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 4060-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.086
BindingDB Entry DOI: 10.7270/Q20Z747Q |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50065785

(2-Methyl-quinolin-8-ol | CHEMBL316892)Show InChI InChI=1S/C10H9NO/c1-7-5-6-8-3-2-4-9(12)10(8)11-7/h2-6,12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50211290
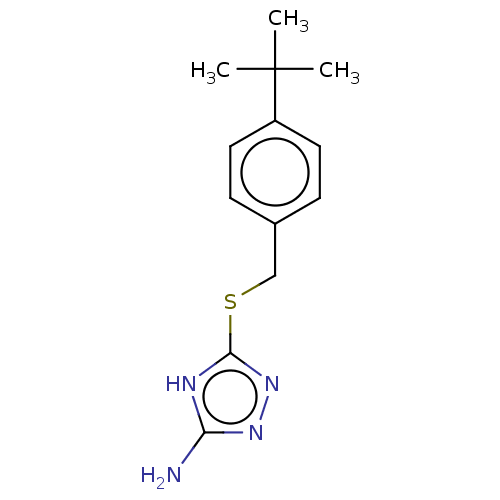
(CHEMBL2407096)Show InChI InChI=1S/C13H18N4S/c1-13(2,3)10-6-4-9(5-7-10)8-18-12-15-11(14)16-17-12/h4-7H,8H2,1-3H3,(H3,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50211290

(CHEMBL2407096)Show InChI InChI=1S/C13H18N4S/c1-13(2,3)10-6-4-9(5-7-10)8-18-12-15-11(14)16-17-12/h4-7H,8H2,1-3H3,(H3,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM76305

(5-chloranylquinolin-8-ol | 5-chloro-8-quinolinol |...)Show InChI InChI=1S/C9H6ClNO/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443775
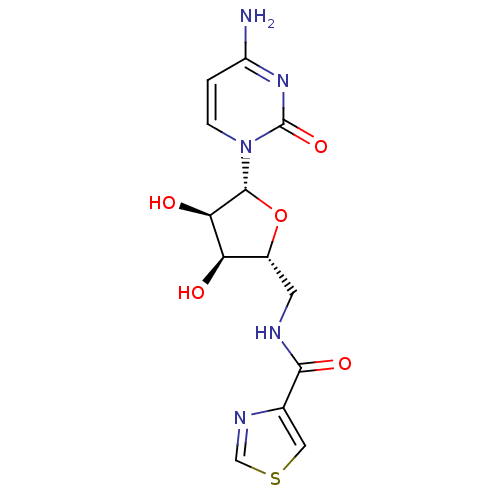
(CHEMBL3094105)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3cscn3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C13H15N5O5S/c14-8-1-2-18(13(22)17-8)12-10(20)9(19)7(23-12)3-15-11(21)6-4-24-5-16-6/h1-2,4-5,7,9-10,12,19-20H,3H2,(H,15,21)(H2,14,17,22)/t7-,9-,10-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36534
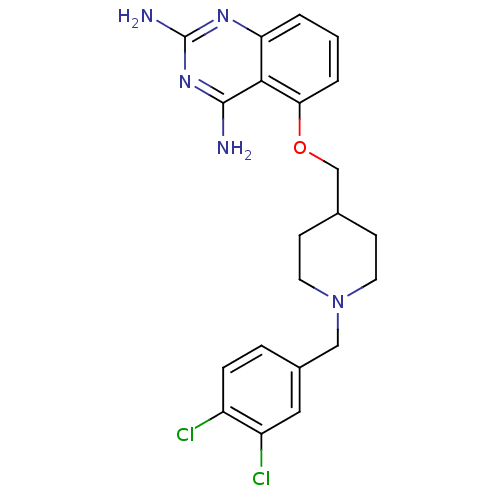
(D156095)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4ccc(Cl)c(Cl)c4)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-5-4-14(10-16(15)23)11-28-8-6-13(7-9-28)12-29-18-3-1-2-17-19(18)20(24)27-21(25)26-17/h1-5,10,13H,6-9,11-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 104 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443771
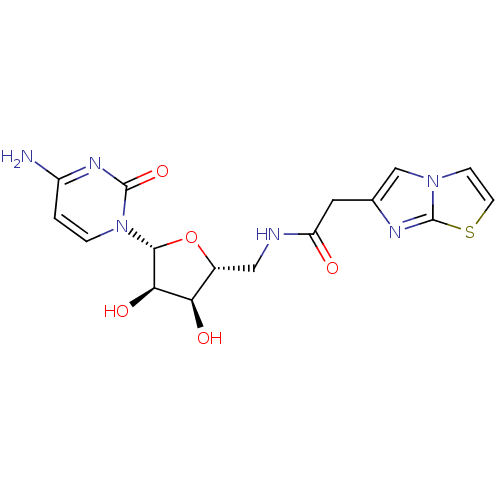
(CHEMBL3094103)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)Cc3cn4ccsc4n3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C16H18N6O5S/c17-10-1-2-22(15(26)20-10)14-13(25)12(24)9(27-14)6-18-11(23)5-8-7-21-3-4-28-16(21)19-8/h1-4,7,9,12-14,24-25H,5-6H2,(H,18,23)(H2,17,20,26)/t9-,12-,13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.48E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443770

(CHEMBL3094104)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3cn4ccsc4n3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C15H16N6O5S/c16-9-1-2-21(14(25)19-9)13-11(23)10(22)8(26-13)5-17-12(24)7-6-20-3-4-27-15(20)18-7/h1-4,6,8,10-11,13,22-23H,5H2,(H,17,24)(H2,16,19,25)/t8-,10-,11-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443772

(CHEMBL1230597)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3ccncc3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C15H17N5O5/c16-10-3-6-20(15(24)19-10)14-12(22)11(21)9(25-14)7-18-13(23)8-1-4-17-5-2-8/h1-6,9,11-12,14,21-22H,7H2,(H,18,23)(H2,16,19,24)/t9-,11-,12-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36535

(D157554)Show InChI InChI=1S/C20H30N6O/c21-19-18-16(24-20(22)25-19)2-1-3-17(18)27-13-15-6-10-26(11-7-15)12-14-4-8-23-9-5-14/h1-3,14-15,23H,4-13H2,(H4,21,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 921 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443774
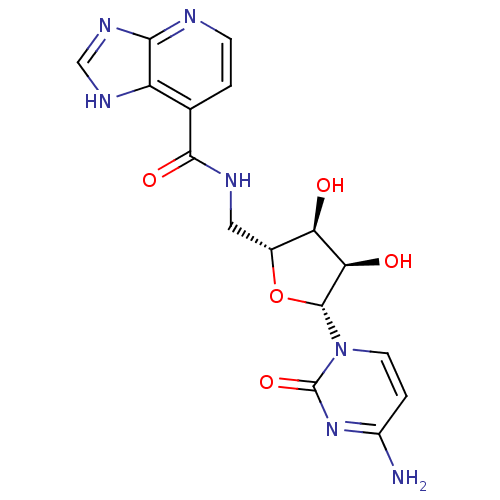
(CHEMBL3094106)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3ccnc4nc[nH]c34)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C16H17N7O5/c17-9-2-4-23(16(27)22-9)15-12(25)11(24)8(28-15)5-19-14(26)7-1-3-18-13-10(7)20-6-21-13/h1-4,6,8,11-12,15,24-25H,5H2,(H,19,26)(H2,17,22,27)(H,18,20,21)/t8-,11-,12-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443773
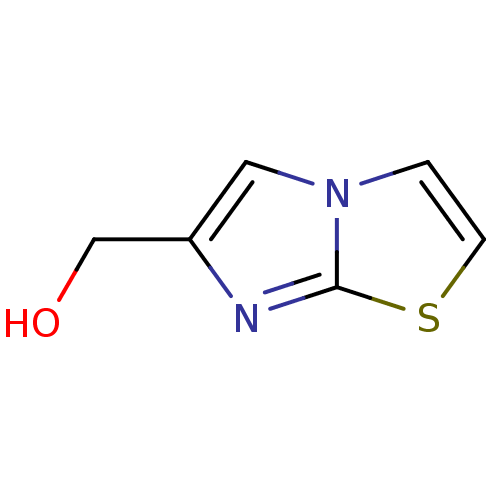
(CHEMBL1230528)Show InChI InChI=1S/C6H6N2OS/c9-4-5-3-8-1-2-10-6(8)7-5/h1-3,9H,4H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50194153

(5'-CDP | CDP | CHEMBL425252 | Cytidine | Cytidine ...)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 Show InChI InChI=1S/C9H15N3O11P2/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(22-8)3-21-25(19,20)23-24(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36536

(D153215)Show InChI InChI=1S/C15H13IN4O/c16-10-5-1-2-7-12(10)21-8-9-4-3-6-11-13(9)14(17)20-15(18)19-11/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 243 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data