 Found 1762 hits with Last Name = 'steinhuebel' and Initial = 'd'
Found 1762 hits with Last Name = 'steinhuebel' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50433561
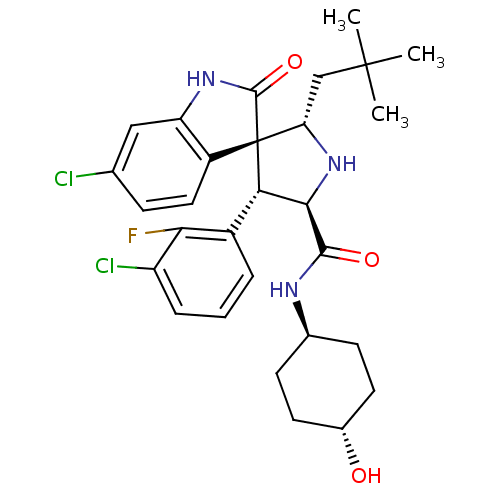
(CHEMBL2381408)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:8.8,5.4,31.34,wD:17.29,7.31,34.38,(40.88,-57.18,;39.34,-57.18,;38.57,-58.51,;40.11,-58.5,;38.57,-55.84,;37.04,-55.84,;37.52,-54.37,;36.27,-53.47,;35.03,-54.37,;33.51,-54.09,;32.52,-55.27,;31.01,-54.99,;30.49,-53.53,;31.49,-52.36,;30.98,-50.9,;33,-52.64,;34.01,-51.48,;35.49,-55.84,;36.38,-57.1,;37.93,-57.11,;35.48,-58.34,;34.01,-57.86,;32.69,-58.61,;31.36,-57.84,;30.03,-58.6,;31.37,-56.3,;32.71,-55.54,;34.03,-56.32,;36.26,-51.93,;34.93,-51.16,;37.6,-51.15,;37.6,-49.61,;36.26,-48.86,;36.26,-47.31,;37.6,-46.54,;37.6,-45,;38.93,-47.32,;38.93,-48.85,)| Show InChI InChI=1S/C29H34Cl2FN3O3/c1-28(2,3)14-22-29(19-12-7-15(30)13-21(19)34-27(29)38)23(18-5-4-6-20(31)24(18)32)25(35-22)26(37)33-16-8-10-17(36)11-9-16/h4-7,12-13,16-17,22-23,25,35-36H,8-11,14H2,1-3H3,(H,33,37)(H,34,38)/t16-,17-,22-,23-,25+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human His6-tagged HDM2 (1 to 118 residues) assessed as reduction in PMDM6-F binding incubated for 15 to 30 mins by fl... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01524
BindingDB Entry DOI: 10.7270/Q2Z03D1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2 [17-125]
(Homo sapiens (Human)) | BDBM227653
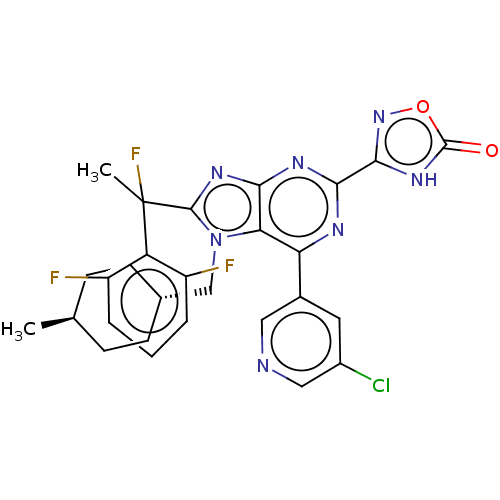
(3-{6-(5-chloropyridin-3- yl)-8-[1-(2,6- difluoroph...)Show SMILES C[C@H]1CC[C@H](Cn2c(nc3nc(nc(-c4cncc(Cl)c4)c23)-c2noc(=O)[nH]2)C(C)(F)c2c(F)cccc2F)CC1 |r,wU:4.4,wD:1.0,(7.09,-3.69,;5.6,-3.29,;5.21,-1.81,;3.72,-1.41,;2.63,-2.5,;1.14,-2.1,;.74,-.61,;1.65,.64,;.74,1.88,;-.72,1.41,;-2.05,2.18,;-3.39,1.41,;-3.39,-.13,;-2.05,-.9,;-2.05,-2.44,;-.72,-3.21,;-.72,-4.75,;-2.05,-5.52,;-3.39,-4.75,;-4.72,-5.52,;-3.39,-3.21,;-.72,-.13,;-4.72,2.18,;-6.19,1.7,;-7.09,2.95,;-6.19,4.19,;-6.96,5.52,;-4.72,3.72,;3.31,.64,;4.08,-.7,;4.85,.64,;4.08,1.97,;3.31,3.3,;1.77,3.3,;4.08,4.64,;5.62,4.64,;6.39,3.3,;5.62,1.97,;6.39,.64,;3.03,-3.98,;4.52,-4.38,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.156 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... |
US Patent US9540377 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X1F |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [17-125]
(Homo sapiens (Human)) | BDBM227651
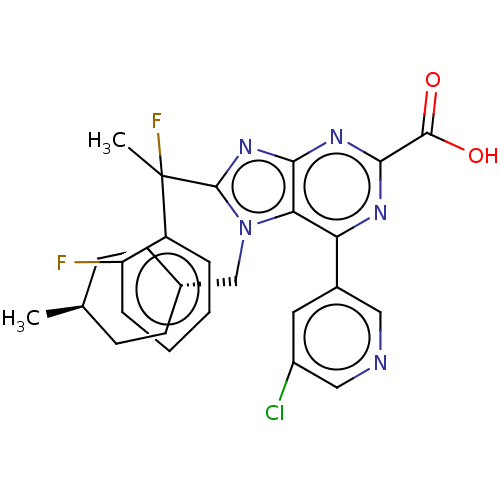
(6-(5-chloropyridin-3-yl)- 8-[1-fluoro-1-(2- fluoro...)Show SMILES C[C@H]1CC[C@H](Cn2c(nc3nc(nc(-c4cncc(Cl)c4)c23)C(O)=O)C(C)(F)c2ccccc2F)CC1 |r,wU:4.4,wD:1.0,(6.57,-3.25,;5.09,-2.85,;4.69,-1.36,;3.2,-.96,;2.11,-2.05,;.62,-1.65,;.23,-.17,;1.13,1.08,;.23,2.33,;-1.24,1.85,;-2.57,2.62,;-3.91,1.85,;-3.91,.31,;-2.57,-.46,;-2.57,-2,;-1.24,-2.77,;-1.24,-4.31,;-2.57,-5.08,;-3.91,-4.31,;-5.24,-5.08,;-3.91,-2.77,;-1.24,.31,;-5.24,2.62,;-6.57,1.85,;-5.24,4.16,;2.8,1.08,;3.57,-.25,;4.34,1.08,;3.57,2.41,;5.11,2.41,;5.88,3.75,;5.11,5.08,;3.57,5.08,;2.8,3.75,;1.26,3.75,;2.51,-3.54,;4,-3.94,)| Show InChI InChI=1S/C27H26ClF2N5O2/c1-15-7-9-16(10-8-15)14-35-22-21(17-11-18(28)13-31-12-17)32-24(25(36)37)33-23(22)34-26(35)27(2,30)19-5-3-4-6-20(19)29/h3-6,11-13,15-16H,7-10,14H2,1-2H3,(H,36,37)/t15-,16-,27? | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.176 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... |
US Patent US9540377 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X1F |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385819
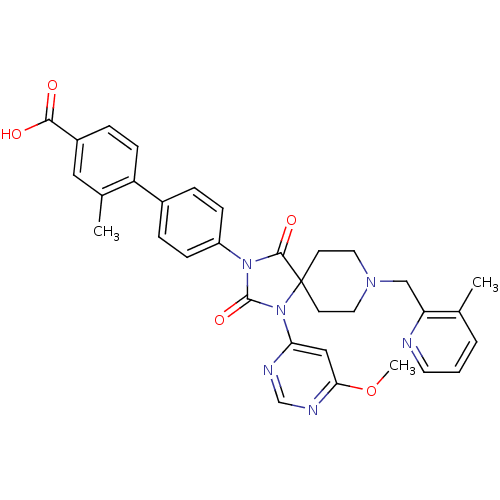
(CHEMBL2043325)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O5/c1-21-5-4-14-34-27(21)19-37-15-12-33(13-16-37)31(42)38(32(43)39(33)28-18-29(44-3)36-20-35-28)25-9-6-23(7-10-25)26-11-8-24(30(40)41)17-22(26)2/h4-11,14,17-18,20H,12-13,15-16,19H2,1-3H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385782
(CHEMBL2041169)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1)-c1cc[nH]n1)c1cc(=O)[nH]cn1 Show InChI InChI=1S/C33H30N8O3/c1-22-3-2-15-34-28(22)20-39-17-13-33(14-18-39)31(43)40(32(44)41(33)29-19-30(42)36-21-35-29)26-10-8-24(9-11-26)23-4-6-25(7-5-23)27-12-16-37-38-27/h2-12,15-16,19,21H,13-14,17-18,20H2,1H3,(H,37,38)(H,35,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50604556
(CHEMBL5175703)Show SMILES N.N.[H][C@@]12COP(O)(=O)O[C@@]3([H])[C@H](F)[C@@H](O[C@]3([H])COP(O)(=O)O[C@]([H])([C@@H]1O)[C@@H](O2)n1cnc2c1nc(N)[nH]c2=O)n1cnc2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385788
(CHEMBL2041175)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc2scnc2c1)c1cc(=O)[nH]cn1 Show InChI InChI=1S/C31H27N7O3S/c1-20-3-2-12-32-25(20)17-36-13-10-31(11-14-36)29(40)37(30(41)38(31)27-16-28(39)34-18-33-27)23-7-4-21(5-8-23)22-6-9-26-24(15-22)35-19-42-26/h2-9,12,15-16,18-19H,10-11,13-14,17H2,1H3,(H,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385796
(CHEMBL2041182)Show SMILES Cc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O4/c1-21-5-4-14-34-28(21)19-37-15-12-33(13-16-37)31(42)38(32(43)39(33)29-18-23(3)35-20-36-29)26-9-6-24(7-10-26)27-11-8-25(30(40)41)17-22(27)2/h4-11,14,17-18,20H,12-13,15-16,19H2,1-3H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385830
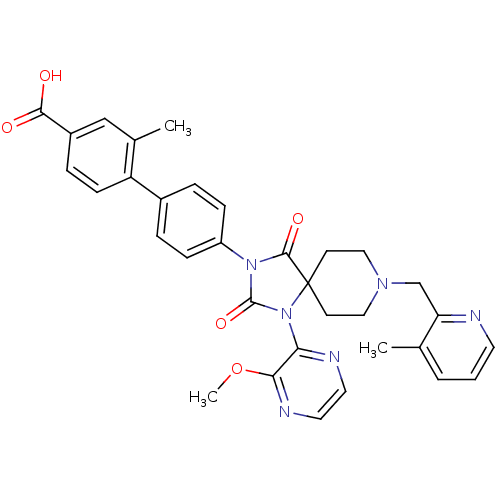
(CHEMBL2041185)Show SMILES COc1nccnc1N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O5/c1-21-5-4-14-34-27(21)20-37-17-12-33(13-18-37)31(42)38(32(43)39(33)28-29(44-3)36-16-15-35-28)25-9-6-23(7-10-25)26-11-8-24(30(40)41)19-22(26)2/h4-11,14-16,19H,12-13,17-18,20H2,1-3H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385799
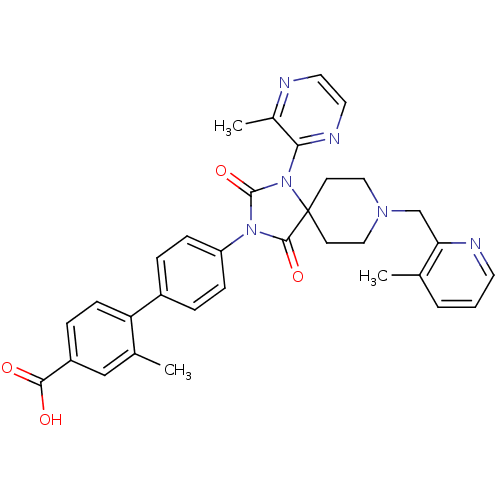
(CHEMBL2041186)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1nccnc1C Show InChI InChI=1S/C33H32N6O4/c1-21-5-4-14-35-28(21)20-37-17-12-33(13-18-37)31(42)38(32(43)39(33)29-23(3)34-15-16-36-29)26-9-6-24(7-10-26)27-11-8-25(30(40)41)19-22(27)2/h4-11,14-16,19H,12-13,17-18,20H2,1-3H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385803
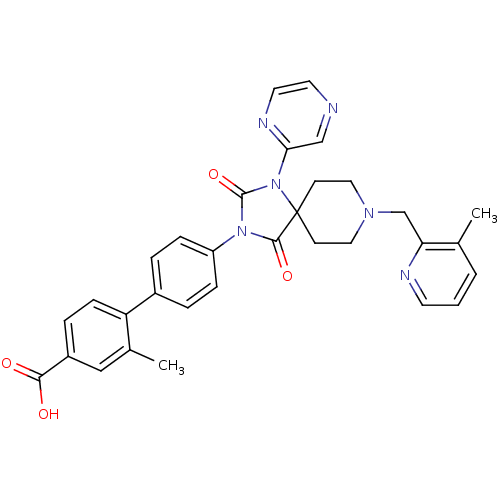
(CHEMBL2041190)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1cnccn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-13-34-27(21)20-36-16-11-32(12-17-36)30(41)37(31(42)38(32)28-19-33-14-15-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-10,13-15,18-19H,11-12,16-17,20H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385805
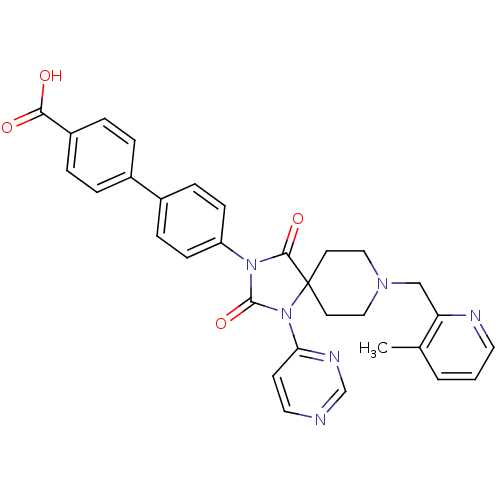
(CHEMBL2041192)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1)C(O)=O)c1ccncn1 Show InChI InChI=1S/C31H28N6O4/c1-21-3-2-15-33-26(21)19-35-17-13-31(14-18-35)29(40)36(30(41)37(31)27-12-16-32-20-34-27)25-10-8-23(9-11-25)22-4-6-24(7-5-22)28(38)39/h2-12,15-16,20H,13-14,17-19H2,1H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385806
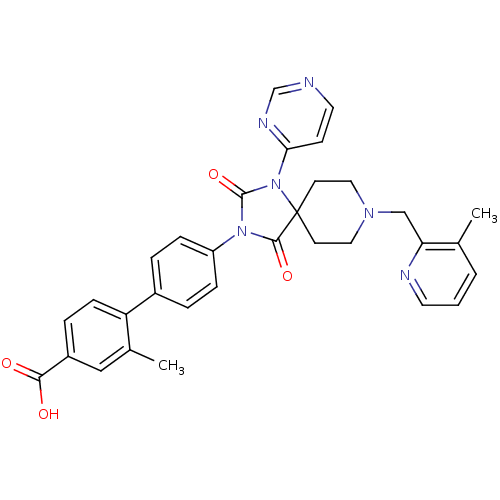
(CHEMBL2041193)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1ccncn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-14-34-27(21)19-36-16-12-32(13-17-36)30(41)37(31(42)38(32)28-11-15-33-20-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-11,14-15,18,20H,12-13,16-17,19H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385814
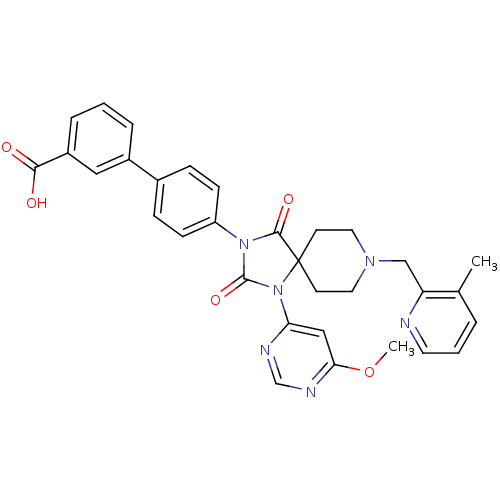
(CHEMBL2043169)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1cccc(c1)C(O)=O Show InChI InChI=1S/C32H30N6O5/c1-21-5-4-14-33-26(21)19-36-15-12-32(13-16-36)30(41)37(31(42)38(32)27-18-28(43-2)35-20-34-27)25-10-8-22(9-11-25)23-6-3-7-24(17-23)29(39)40/h3-11,14,17-18,20H,12-13,15-16,19H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385819

(CHEMBL2043325)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O5/c1-21-5-4-14-34-27(21)19-37-15-12-33(13-16-37)31(42)38(32(43)39(33)28-18-29(44-3)36-20-35-28)25-9-6-23(7-10-25)26-11-8-24(30(40)41)17-22(26)2/h4-11,14,17-18,20H,12-13,15-16,19H2,1-3H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385782

(CHEMBL2041169)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1)-c1cc[nH]n1)c1cc(=O)[nH]cn1 Show InChI InChI=1S/C33H30N8O3/c1-22-3-2-15-34-28(22)20-39-17-13-33(14-18-39)31(43)40(32(44)41(33)29-19-30(42)36-21-35-29)26-10-8-24(9-11-26)23-4-6-25(7-5-23)27-12-16-37-38-27/h2-12,15-16,19,21H,13-14,17-18,20H2,1H3,(H,37,38)(H,35,36,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385796

(CHEMBL2041182)Show SMILES Cc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O4/c1-21-5-4-14-34-28(21)19-37-15-12-33(13-16-37)31(42)38(32(43)39(33)29-18-23(3)35-20-36-29)26-9-6-24(7-10-26)27-11-8-25(30(40)41)17-22(27)2/h4-11,14,17-18,20H,12-13,15-16,19H2,1-3H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385830

(CHEMBL2041185)Show SMILES COc1nccnc1N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O5/c1-21-5-4-14-34-27(21)20-37-17-12-33(13-18-37)31(42)38(32(43)39(33)28-29(44-3)36-16-15-35-28)25-9-6-23(7-10-25)26-11-8-24(30(40)41)19-22(26)2/h4-11,14-16,19H,12-13,17-18,20H2,1-3H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385803

(CHEMBL2041190)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1cnccn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-13-34-27(21)20-36-16-11-32(12-17-36)30(41)37(31(42)38(32)28-19-33-14-15-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-10,13-15,18-19H,11-12,16-17,20H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385806

(CHEMBL2041193)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1ccncn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-14-34-27(21)19-36-16-12-32(13-17-36)30(41)37(31(42)38(32)28-11-15-33-20-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-11,14-15,18,20H,12-13,16-17,19H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095475
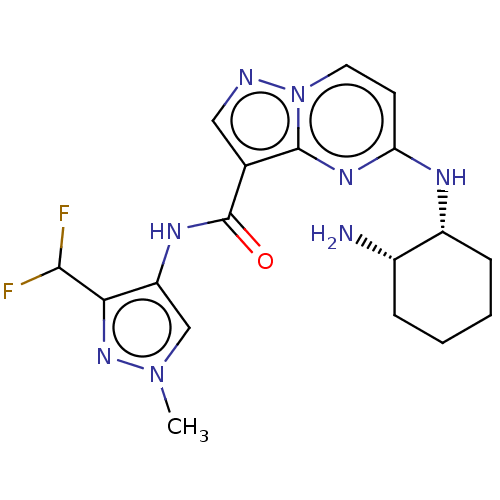
(CHEMBL3590479)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(F)F |r| Show InChI InChI=1S/C23H28N4O3/c1-26(2)14-7-15-30-22-16-21(27(25-22)17-18-8-5-4-6-9-18)23(28)24-19-10-12-20(29-3)13-11-19/h4-6,8-13,16H,7,14-15,17H2,1-3H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385788

(CHEMBL2041175)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc2scnc2c1)c1cc(=O)[nH]cn1 Show InChI InChI=1S/C31H27N7O3S/c1-20-3-2-12-32-25(20)17-36-13-10-31(11-14-36)29(40)37(30(41)38(31)27-16-28(39)34-18-33-27)23-7-4-21(5-8-23)22-6-9-26-24(15-22)35-19-42-26/h2-9,12,15-16,18-19H,10-11,13-14,17H2,1H3,(H,33,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385798
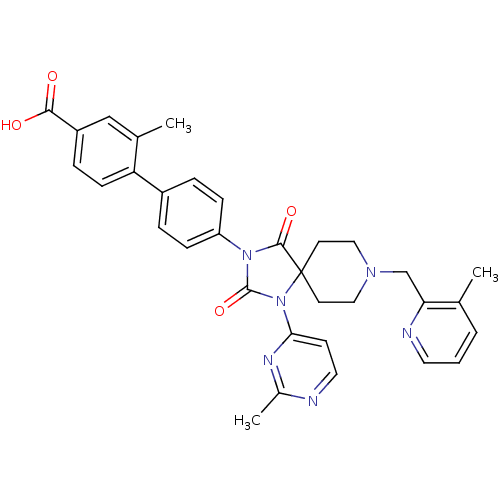
(CHEMBL2041184)Show SMILES Cc1nccc(n1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O4/c1-21-5-4-15-35-28(21)20-37-17-13-33(14-18-37)31(42)38(32(43)39(33)29-12-16-34-23(3)36-29)26-9-6-24(7-10-26)27-11-8-25(30(40)41)19-22(27)2/h4-12,15-16,19H,13-14,17-18,20H2,1-3H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [17-125]
(Homo sapiens (Human)) | BDBM223046
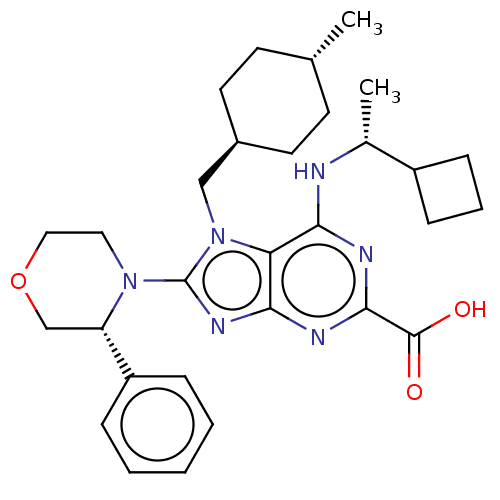
(6-{[(1r)-1- cyclobutylethyl]amino}- 7-[(trans-4- m...)Show SMILES C[C@@H](Nc1nc(nc2nc(N3CCOC[C@H]3c3ccccc3)n(C[C@H]3CC[C@H](C)CC3)c12)C(O)=O)C1CCC1 |r,wU:1.0,27.29,wD:24.25,15.16,(-5.24,-1.96,;-3.91,-2.73,;-2.57,-1.96,;-2.57,-.42,;-3.91,.35,;-3.91,1.89,;-2.57,2.66,;-1.24,1.89,;.23,2.36,;1.13,1.12,;2.67,1.12,;3.44,-.22,;4.98,-.22,;5.75,1.12,;4.98,2.45,;3.44,2.45,;2.67,3.78,;3.44,5.12,;2.67,6.45,;1.13,6.45,;.36,5.12,;1.13,3.78,;.23,-.13,;.62,-1.62,;2.11,-2.02,;2.51,-3.5,;4,-3.9,;5.09,-2.81,;6.57,-3.21,;4.69,-1.32,;3.2,-.93,;-1.24,.35,;-5.24,2.66,;-6.57,1.89,;-5.24,4.2,;-3.91,-4.27,;-5,-5.36,;-3.91,-6.45,;-2.82,-5.36,)| Show InChI InChI=1S/C30H40N6O3/c1-19-11-13-21(14-12-19)17-36-25-26(31-20(2)22-9-6-10-22)32-28(29(37)38)33-27(25)34-30(36)35-15-16-39-18-24(35)23-7-4-3-5-8-23/h3-5,7-8,19-22,24H,6,9-18H2,1-2H3,(H,37,38)(H,31,32,33)/t19-,20-,21-,24+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.202 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... |
US Patent US9540377 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X1F |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [17-125]
(Homo sapiens (Human)) | BDBM224125
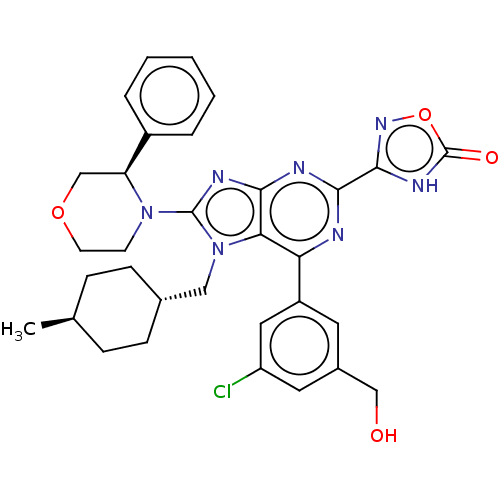
(3-{6-[3-chloro-5- (hydroxymethyl)phen- yl]-7-[(tra...)Show SMILES C[C@H]1CC[C@H](Cn2c(nc3nc(nc(-c4cc(Cl)cc(CO)c4)c23)-c2noc(=O)[nH]2)N2CCOC[C@H]2c2ccccc2)CC1 |r,wU:4.4,wD:1.0,35.40,(7.86,-3.92,;6.37,-3.52,;5.98,-2.03,;4.49,-1.63,;3.4,-2.72,;1.91,-2.32,;1.51,-.83,;2.42,.41,;1.51,1.66,;.05,1.18,;-1.28,1.95,;-2.62,1.18,;-2.62,-.36,;-1.28,-1.13,;-1.28,-2.67,;-2.62,-3.44,;-2.62,-4.98,;-3.95,-5.75,;-1.28,-5.75,;.05,-4.98,;1.38,-5.75,;2.72,-4.98,;.05,-3.44,;.05,-.36,;-3.95,1.95,;-3.95,3.49,;-5.42,3.97,;-6.32,2.72,;-7.86,2.72,;-5.42,1.48,;3.96,.41,;4.73,-.92,;6.27,-.92,;7.04,.41,;6.27,1.75,;4.73,1.75,;3.96,3.08,;4.73,4.41,;3.96,5.75,;2.42,5.75,;1.65,4.41,;2.42,3.08,;3.8,-4.21,;5.29,-4.61,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.205 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... |
US Patent US9540377 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X1F |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [17-125]
(Homo sapiens (Human)) | BDBM224261
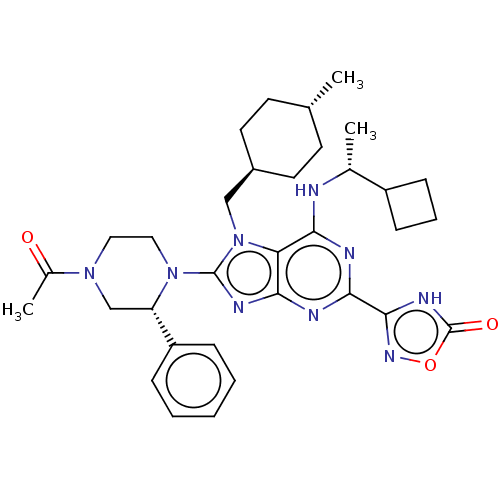
(3-{8-[(2r)-4-acetyl- 2-phenylpiperazin-1- yl]-6-{[...)Show SMILES C[C@@H](Nc1nc(nc2nc(N3CCN(C[C@H]3c3ccccc3)C(C)=O)n(C[C@H]3CC[C@H](C)CC3)c12)-c1noc(=O)[nH]1)C1CCC1 |r,wU:1.0,27.28,wD:30.32,15.16,(-5.47,-1.96,;-4.13,-2.73,;-2.8,-1.96,;-2.8,-.42,;-4.13,.35,;-4.13,1.89,;-2.8,2.66,;-1.46,1.89,;0,2.36,;.91,1.12,;2.45,1.12,;3.22,-.22,;4.76,-.22,;5.53,1.12,;4.76,2.45,;3.22,2.45,;2.45,3.78,;3.22,5.12,;2.45,6.45,;.91,6.45,;.14,5.12,;.91,3.78,;7.07,1.12,;7.84,2.45,;7.84,-.22,;0,-.13,;.4,-1.62,;1.89,-2.02,;2.98,-.93,;4.46,-1.32,;4.86,-2.81,;6.35,-3.21,;3.77,-3.9,;2.28,-3.5,;-1.46,.35,;-5.47,2.66,;-6.93,2.18,;-7.84,3.43,;-6.93,4.67,;-7.7,6.01,;-5.47,4.2,;-4.13,-4.27,;-5.22,-5.36,;-4.13,-6.45,;-3.04,-5.36,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.215 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... |
US Patent US9540377 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X1F |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [17-125]
(Homo sapiens (Human)) | BDBM227665
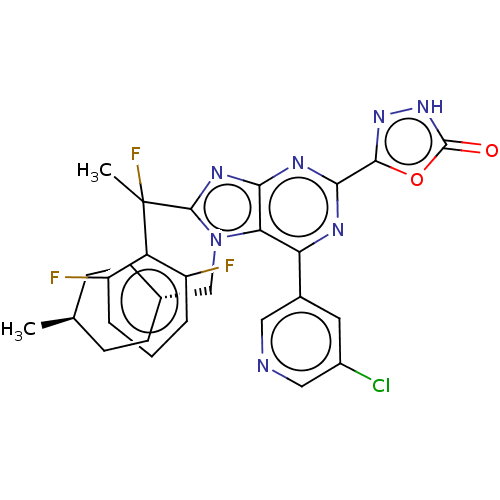
(5-{6-(5-chloropyridin-3- yl)-8-[1-(2,6- difluoroph...)Show SMILES C[C@H]1CC[C@H](Cn2c(nc3nc(nc(-c4cncc(Cl)c4)c23)-c2n[nH]c(=O)o2)C(C)(F)c2c(F)cccc2F)CC1 |r,wU:4.4,wD:1.0,(7.09,-3.69,;5.6,-3.29,;5.21,-1.81,;3.72,-1.41,;2.63,-2.5,;1.14,-2.1,;.74,-.61,;1.65,.64,;.74,1.88,;-.72,1.41,;-2.05,2.18,;-3.39,1.41,;-3.39,-.13,;-2.05,-.9,;-2.05,-2.44,;-.72,-3.21,;-.72,-4.75,;-2.05,-5.52,;-3.39,-4.75,;-4.72,-5.52,;-3.39,-3.21,;-.72,-.13,;-4.72,2.18,;-6.19,1.7,;-7.09,2.95,;-6.19,4.19,;-6.96,5.52,;-4.72,3.72,;3.19,.64,;3.96,-.7,;4.73,.64,;3.96,1.97,;3.19,3.3,;1.65,3.3,;3.96,4.64,;5.5,4.64,;6.27,3.3,;5.5,1.97,;6.27,.64,;3.03,-3.98,;4.52,-4.38,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.289 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... |
US Patent US9540377 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X1F |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385813
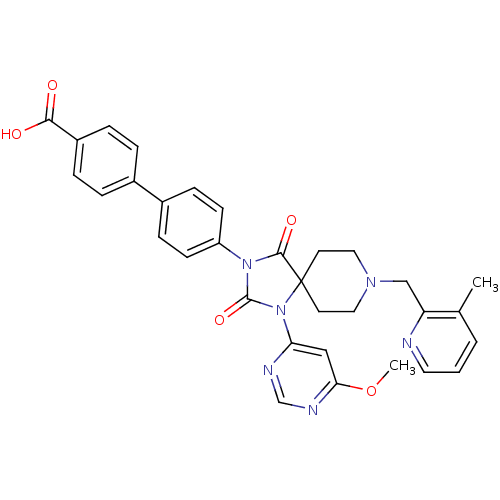
(CHEMBL2043168)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C32H30N6O5/c1-21-4-3-15-33-26(21)19-36-16-13-32(14-17-36)30(41)37(31(42)38(32)27-18-28(43-2)35-20-34-27)25-11-9-23(10-12-25)22-5-7-24(8-6-22)29(39)40/h3-12,15,18,20H,13-14,16-17,19H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385826
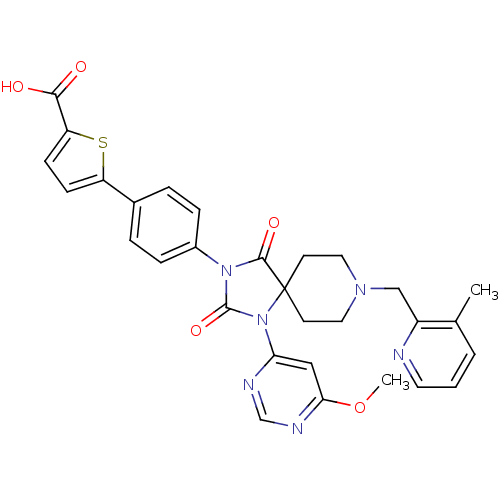
(CHEMBL2041010)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(s1)C(O)=O Show InChI InChI=1S/C30H28N6O5S/c1-19-4-3-13-31-22(19)17-34-14-11-30(12-15-34)28(39)35(29(40)36(30)25-16-26(41-2)33-18-32-25)21-7-5-20(6-8-21)23-9-10-24(42-23)27(37)38/h3-10,13,16,18H,11-12,14-15,17H2,1-2H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385798

(CHEMBL2041184)Show SMILES Cc1nccc(n1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O4/c1-21-5-4-15-35-28(21)20-37-17-13-33(14-18-37)31(42)38(32(43)39(33)29-12-16-34-23(3)36-29)26-9-6-24(7-10-26)27-11-8-25(30(40)41)19-22(27)2/h4-12,15-16,19H,13-14,17-18,20H2,1-3H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385799

(CHEMBL2041186)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1nccnc1C Show InChI InChI=1S/C33H32N6O4/c1-21-5-4-14-35-28(21)20-37-17-12-33(13-18-37)31(42)38(32(43)39(33)29-23(3)34-15-16-36-29)26-9-6-24(7-10-26)27-11-8-25(30(40)41)19-22(27)2/h4-11,14-16,19H,12-13,17-18,20H2,1-3H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385801
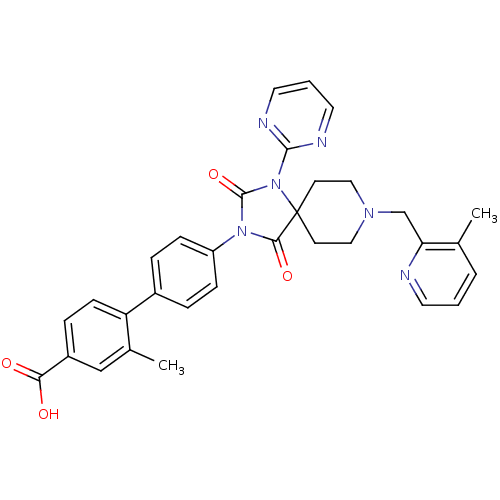
(CHEMBL2041188)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1ncccn1 Show InChI InChI=1S/C32H30N6O4/c1-21-5-3-14-33-27(21)20-36-17-12-32(13-18-36)29(41)37(31(42)38(32)30-34-15-4-16-35-30)25-9-6-23(7-10-25)26-11-8-24(28(39)40)19-22(26)2/h3-11,14-16,19H,12-13,17-18,20H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50509399
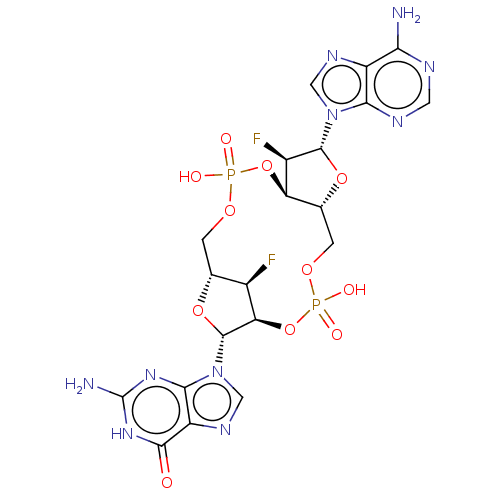
(CHEMBL4468010)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2COP(O)(=O)O[C@@H]3[C@@H](COP(O)(=O)O[C@@H]1[C@@H]2F)O[C@H]([C@@H]3F)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H22F2N10O11P2/c21-8-6-1-38-44(34,35)42-12-7(41-18(9(12)22)31-4-27-10-14(23)25-3-26-15(10)31)2-39-45(36,37)43-13(8)19(40-6)32-5-28-11-16(32)29-20(24)30-17(11)33/h3-9,12-13,18-19H,1-2H2,(H,34,35)(H,36,37)(H2,23,25,26)(H3,24,29,30,33)/t6-,7-,8-,9-,12-,13-,18-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095470
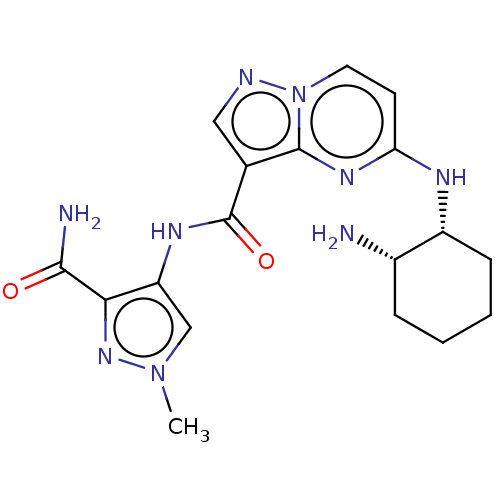
(CHEMBL3590474 | US10155765, Example 9)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(N)=O |r| Show InChI InChI=1S/C21H25N3O/c1-23(2)14-9-15-25-21-16-20(19-12-7-4-8-13-19)24(22-21)17-18-10-5-3-6-11-18/h3-8,10-13,16H,9,14-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095474
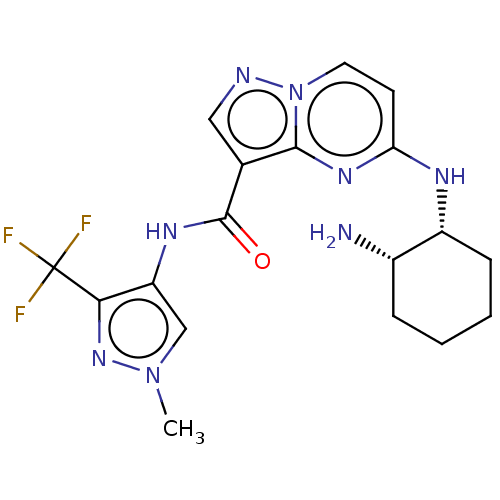
(CHEMBL3590478 | US10329294, Example 2)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H23N3O/c1-22(2)14-9-15-24-20-16-19(17-10-5-3-6-11-17)23(21-20)18-12-7-4-8-13-18/h3-8,10-13,16H,9,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385820
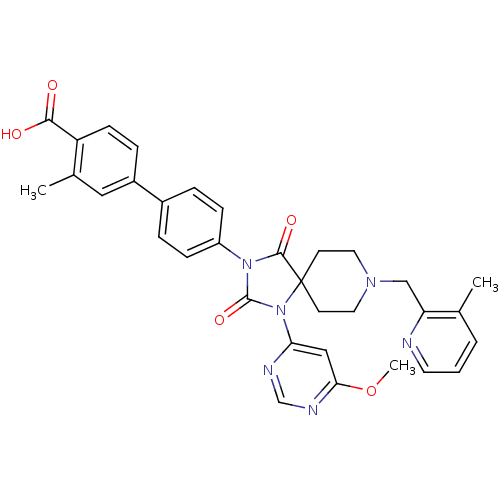
(CHEMBL2043326)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(C(O)=O)c(C)c1 Show InChI InChI=1S/C33H32N6O5/c1-21-5-4-14-34-27(21)19-37-15-12-33(13-16-37)31(42)38(32(43)39(33)28-18-29(44-3)36-20-35-28)25-9-6-23(7-10-25)24-8-11-26(30(40)41)22(2)17-24/h4-11,14,17-18,20H,12-13,15-16,19H2,1-3H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385791
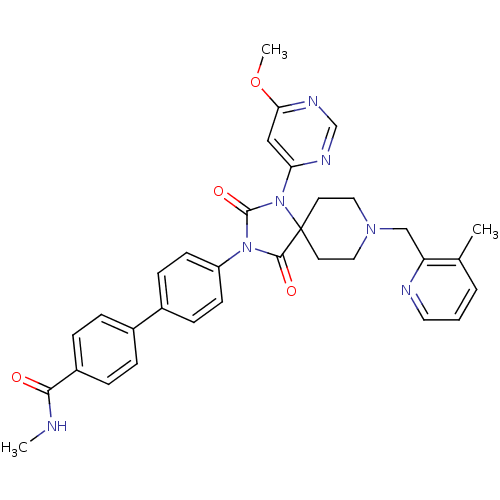
(CHEMBL2041178)Show SMILES CNC(=O)c1ccc(cc1)-c1ccc(cc1)N1C(=O)N(c2cc(OC)ncn2)C2(CCN(Cc3ncccc3C)CC2)C1=O Show InChI InChI=1S/C33H33N7O4/c1-22-5-4-16-35-27(22)20-38-17-14-33(15-18-38)31(42)39(32(43)40(33)28-19-29(44-3)37-21-36-28)26-12-10-24(11-13-26)23-6-8-25(9-7-23)30(41)34-2/h4-13,16,19,21H,14-15,17-18,20H2,1-3H3,(H,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385805

(CHEMBL2041192)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1)C(O)=O)c1ccncn1 Show InChI InChI=1S/C31H28N6O4/c1-21-3-2-15-33-26(21)19-35-17-13-31(14-18-35)29(40)36(30(41)37(31)27-12-16-32-20-34-27)25-10-8-23(9-11-25)22-4-6-24(7-5-22)28(38)39/h2-12,15-16,20H,13-14,17-19H2,1H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [17-125]
(Homo sapiens (Human)) | BDBM227683
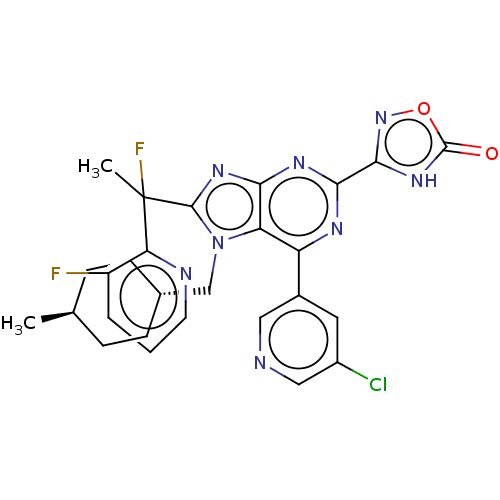
(3-{6-(5-chloropyridin-3- yl)-8-[1-fluoro-1-(3- flu...)Show SMILES C[C@H]1CC[C@H](Cn2c(nc3nc(nc(-c4cncc(Cl)c4)c23)-c2noc(=O)[nH]2)C(C)(F)c2ncccc2F)CC1 |r,wU:4.4,wD:1.0,(7.09,-3.69,;5.6,-3.29,;5.21,-1.81,;3.72,-1.41,;2.63,-2.5,;1.14,-2.1,;.74,-.61,;1.65,.64,;.74,1.88,;-.72,1.41,;-2.05,2.18,;-3.39,1.41,;-3.39,-.13,;-2.05,-.9,;-2.05,-2.44,;-3.39,-3.21,;-3.39,-4.75,;-2.05,-5.52,;-.72,-4.75,;.61,-5.52,;-.72,-3.21,;-.72,-.13,;-4.72,2.18,;-6.19,1.7,;-7.09,2.95,;-6.19,4.19,;-6.96,5.52,;-4.72,3.72,;3.19,.64,;4.73,.64,;3.96,-.7,;3.96,1.97,;5.5,1.97,;6.27,3.3,;5.5,4.64,;3.96,4.64,;3.19,3.3,;1.65,3.3,;3.03,-3.98,;4.52,-4.38,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.315 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... |
US Patent US9540377 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X1F |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [17-125]
(Homo sapiens (Human)) | BDBM223705
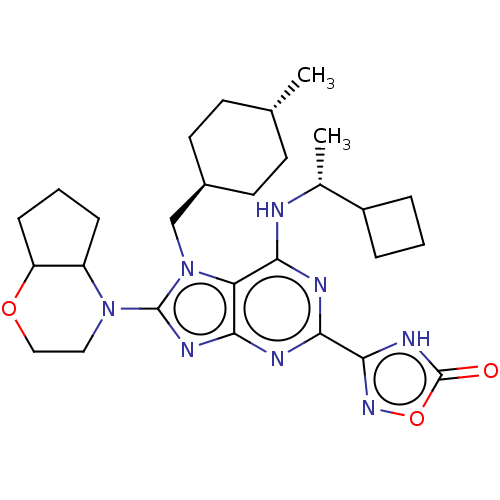
(3-(6-{[(1r)-1- cyclobutylethyl]amino}- 8- (hexahyd...)Show SMILES C[C@@H](Nc1nc(nc2nc(N3CCOC4CCCC34)n(C[C@H]3CC[C@H](C)CC3)c12)-c1noc(=O)[nH]1)C1CCC1 |r,wU:1.0,21.22,wD:24.26,(-4.51,-1.47,;-3.18,-2.24,;-1.85,-1.47,;-1.85,.07,;-3.18,.84,;-3.18,2.38,;-1.85,3.15,;-.51,2.38,;.95,2.86,;1.86,1.61,;3.4,1.61,;4.17,.28,;5.71,.28,;6.48,1.61,;5.71,2.95,;6.18,4.41,;4.94,5.32,;3.69,4.41,;4.17,2.95,;.95,.37,;1.35,-1.12,;2.84,-1.52,;3.93,-.43,;5.41,-.83,;5.81,-2.31,;7.3,-2.71,;4.72,-3.4,;3.24,-3,;-.51,.84,;-4.51,3.15,;-5.76,2.25,;-7.01,3.15,;-6.53,4.62,;-7.3,5.95,;-4.99,4.62,;-3.18,-3.78,;-4.27,-4.86,;-3.18,-5.95,;-2.09,-4.86,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.344 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... |
US Patent US9540377 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X1F |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [17-125]
(Homo sapiens (Human)) | BDBM227669
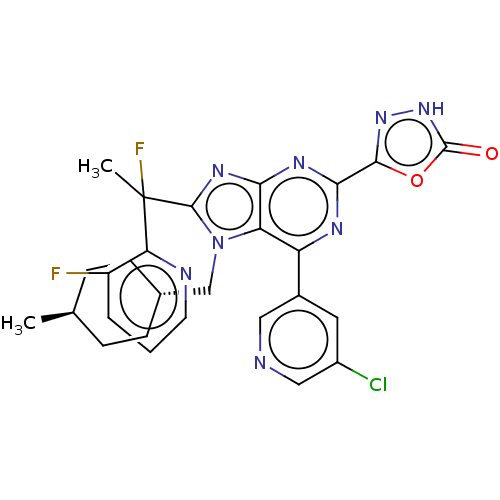
(5-{6-(5-chloropyridin-3- yl)-8-[1-fluoro-1-(3- flu...)Show SMILES C[C@H]1CC[C@H](Cn2c(nc3nc(nc(-c4cncc(Cl)c4)c23)-c2n[nH]c(=O)o2)C(C)(F)c2ncccc2F)CC1 |r,wU:4.4,wD:1.0,(7.09,-3.69,;5.6,-3.29,;5.21,-1.81,;3.72,-1.41,;2.63,-2.5,;1.14,-2.1,;.74,-.61,;1.65,.64,;.74,1.88,;-.72,1.41,;-2.05,2.18,;-3.39,1.41,;-3.39,-.13,;-2.05,-.9,;-2.05,-2.44,;-.72,-3.21,;-.72,-4.75,;-2.05,-5.52,;-3.39,-4.75,;-4.72,-5.52,;-3.39,-3.21,;-.72,-.13,;-4.72,2.18,;-6.19,1.7,;-7.09,2.95,;-6.19,4.19,;-6.96,5.52,;-4.72,3.72,;3.19,.64,;4.73,.64,;3.96,-.7,;3.96,1.97,;5.5,1.97,;6.27,3.3,;5.5,4.64,;3.96,4.64,;3.19,3.3,;1.65,3.3,;3.03,-3.98,;4.52,-4.38,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.359 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... |
US Patent US9540377 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X1F |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [17-125]
(Homo sapiens (Human)) | BDBM223402
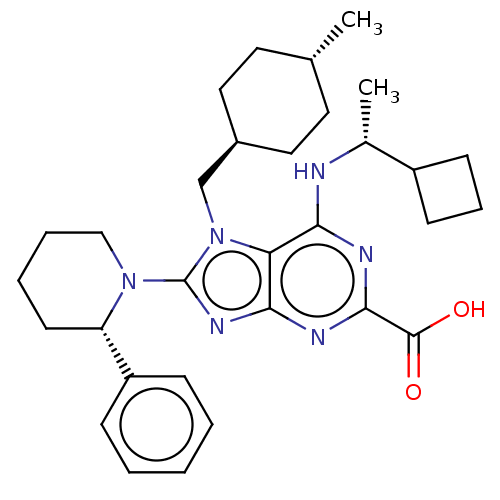
(6-{[(1r)-1- cyclobutylethyl]amino}- 7-[(trans-4- m...)Show SMILES C[C@@H](Nc1nc(nc2nc(N3CCCC[C@H]3c3ccccc3)n(C[C@H]3CC[C@H](C)CC3)c12)C(O)=O)C1CCC1 |r,wU:1.0,27.29,wD:24.25,15.16,(-5.24,-1.96,;-3.91,-2.73,;-2.57,-1.96,;-2.57,-.42,;-3.91,.35,;-3.91,1.89,;-2.57,2.66,;-1.24,1.89,;.23,2.36,;1.13,1.12,;2.67,1.12,;3.44,-.22,;4.98,-.22,;5.75,1.12,;4.98,2.45,;3.44,2.45,;2.67,3.78,;3.44,5.12,;2.67,6.45,;1.13,6.45,;.36,5.12,;1.13,3.78,;.23,-.13,;.62,-1.62,;2.11,-2.02,;2.51,-3.5,;4,-3.9,;5.09,-2.81,;6.57,-3.21,;4.69,-1.32,;3.2,-.93,;-1.24,.35,;-5.24,2.66,;-6.57,1.89,;-5.24,4.2,;-3.91,-4.27,;-5,-5.36,;-3.91,-6.45,;-2.82,-5.36,)| Show InChI InChI=1S/C31H42N6O2/c1-20-14-16-22(17-15-20)19-37-26-27(32-21(2)23-11-8-12-23)33-29(30(38)39)34-28(26)35-31(37)36-18-7-6-13-25(36)24-9-4-3-5-10-24/h3-5,9-10,20-23,25H,6-8,11-19H2,1-2H3,(H,38,39)(H,32,33,34)/t20-,21-,22-,25+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.381 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... |
US Patent US9540377 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X1F |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385818
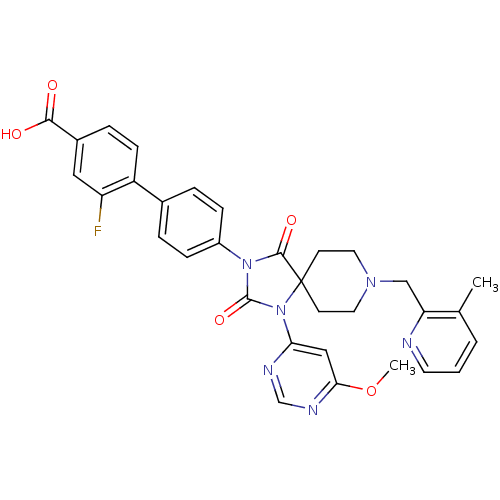
(CHEMBL2043324)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1F)C(O)=O Show InChI InChI=1S/C32H29FN6O5/c1-20-4-3-13-34-26(20)18-37-14-11-32(12-15-37)30(42)38(31(43)39(32)27-17-28(44-2)36-19-35-27)23-8-5-21(6-9-23)24-10-7-22(29(40)41)16-25(24)33/h3-10,13,16-17,19H,11-12,14-15,18H2,1-2H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385801

(CHEMBL2041188)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1ncccn1 Show InChI InChI=1S/C32H30N6O4/c1-21-5-3-14-33-27(21)20-36-17-12-32(13-18-36)29(41)37(31(42)38(32)30-34-15-4-16-35-30)25-9-6-23(7-10-25)26-11-8-24(28(39)40)19-22(26)2/h3-11,14-16,19H,12-13,17-18,20H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385802
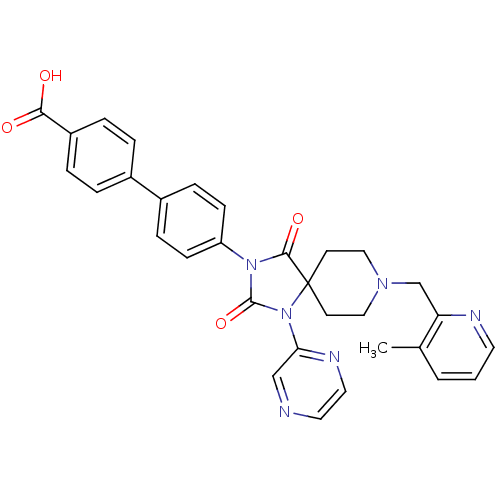
(CHEMBL2041189)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1)C(O)=O)c1cnccn1 Show InChI InChI=1S/C31H28N6O4/c1-21-3-2-14-33-26(21)20-35-17-12-31(13-18-35)29(40)36(30(41)37(31)27-19-32-15-16-34-27)25-10-8-23(9-11-25)22-4-6-24(7-5-22)28(38)39/h2-11,14-16,19H,12-13,17-18,20H2,1H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385825
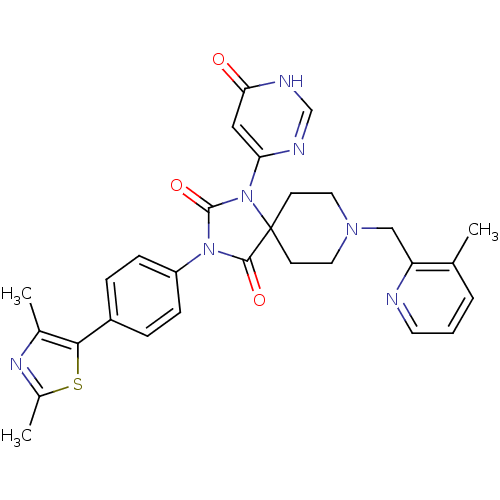
(CHEMBL2040897)Show SMILES Cc1nc(C)c(s1)-c1ccc(cc1)N1C(=O)N(c2cc(=O)[nH]cn2)C2(CCN(Cc3ncccc3C)CC2)C1=O Show InChI InChI=1S/C29H29N7O3S/c1-18-5-4-12-30-23(18)16-34-13-10-29(11-14-34)27(38)35(28(39)36(29)24-15-25(37)32-17-31-24)22-8-6-21(7-9-22)26-19(2)33-20(3)40-26/h4-9,12,15,17H,10-11,13-14,16H2,1-3H3,(H,31,32,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385818

(CHEMBL2043324)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1F)C(O)=O Show InChI InChI=1S/C32H29FN6O5/c1-20-4-3-13-34-26(20)18-37-14-11-32(12-15-37)30(42)38(31(43)39(32)27-17-28(44-2)36-19-35-27)23-8-5-21(6-9-23)24-10-7-22(29(40)41)16-25(24)33/h3-10,13,16-17,19H,11-12,14-15,18H2,1-2H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385802

(CHEMBL2041189)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1)C(O)=O)c1cnccn1 Show InChI InChI=1S/C31H28N6O4/c1-21-3-2-14-33-26(21)20-35-17-12-31(13-18-35)29(40)36(30(41)37(31)27-19-32-15-16-34-27)25-10-8-23(9-11-25)22-4-6-24(7-5-22)28(38)39/h2-11,14-16,19H,12-13,17-18,20H2,1H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385813

(CHEMBL2043168)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C32H30N6O5/c1-21-4-3-15-33-26(21)19-36-16-13-32(14-17-36)30(41)37(31(42)38(32)27-18-28(43-2)35-20-34-27)25-11-9-23(10-12-25)22-5-7-24(8-6-22)29(39)40/h3-12,15,18,20H,13-14,16-17,19H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385814

(CHEMBL2043169)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1cccc(c1)C(O)=O Show InChI InChI=1S/C32H30N6O5/c1-21-5-4-14-33-26(21)19-36-15-12-32(13-16-36)30(41)37(31(42)38(32)27-18-28(43-2)35-20-34-27)25-10-8-22(9-11-25)23-6-3-7-24(17-23)29(39)40/h3-11,14,17-18,20H,12-13,15-16,19H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data