

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50362892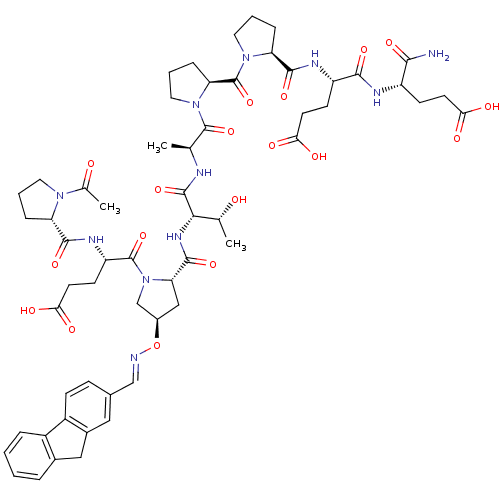 (CHEMBL1946564) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... | ACS Med Chem Lett 2: 337-341 (2011) Article DOI: 10.1021/ml1002579 BindingDB Entry DOI: 10.7270/Q22B8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50362891 (CHEMBL1946260) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... | ACS Med Chem Lett 2: 337-341 (2011) Article DOI: 10.1021/ml1002579 BindingDB Entry DOI: 10.7270/Q22B8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50388546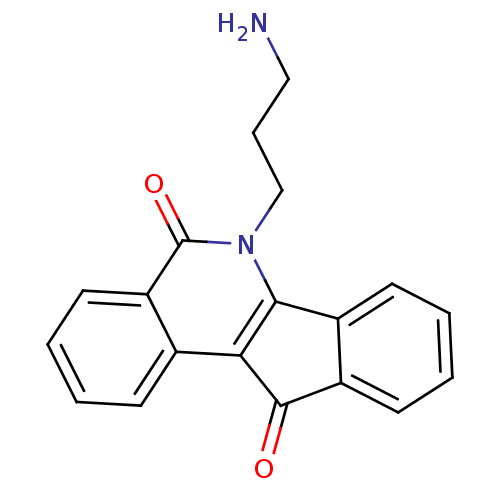 (CHEMBL213072 | CHEMBL333363) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant Tdp1 after 1 hr by FRET assay | J Med Chem 55: 4457-78 (2012) Article DOI: 10.1021/jm300335n BindingDB Entry DOI: 10.7270/Q2SB46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50362890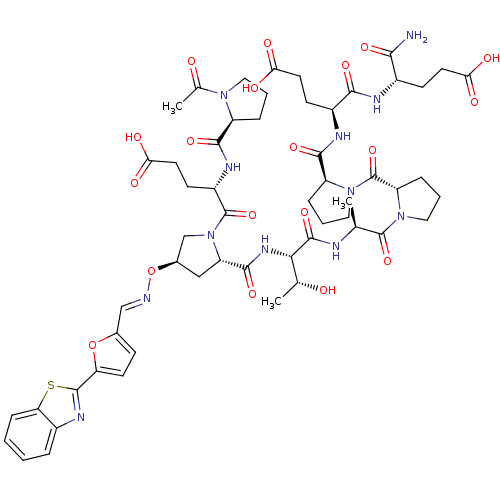 (CHEMBL1946259) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... | ACS Med Chem Lett 2: 337-341 (2011) Article DOI: 10.1021/ml1002579 BindingDB Entry DOI: 10.7270/Q22B8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50362889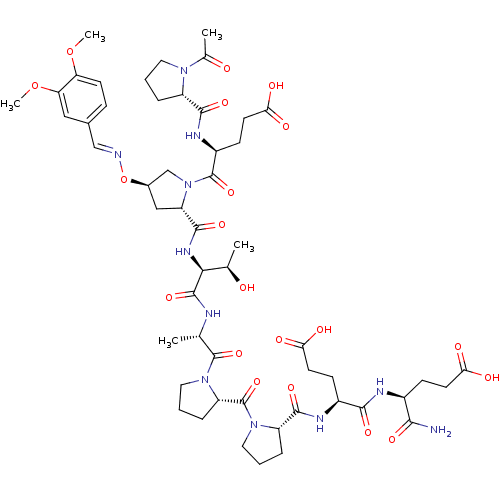 (CHEMBL1946129) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... | ACS Med Chem Lett 2: 337-341 (2011) Article DOI: 10.1021/ml1002579 BindingDB Entry DOI: 10.7270/Q22B8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397314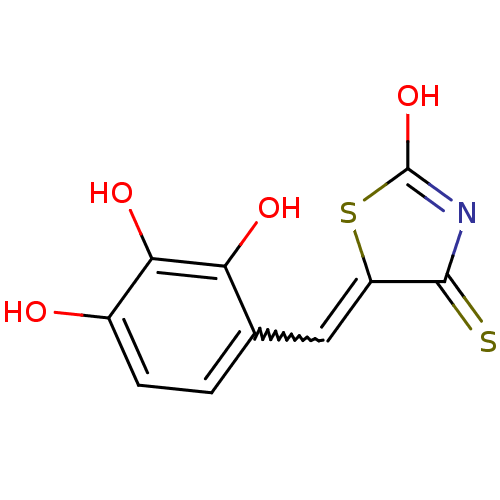 (CHEMBL2170127) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50158383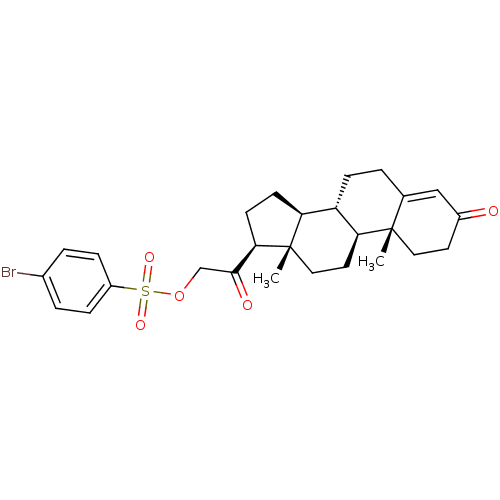 (2-((8S,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50388544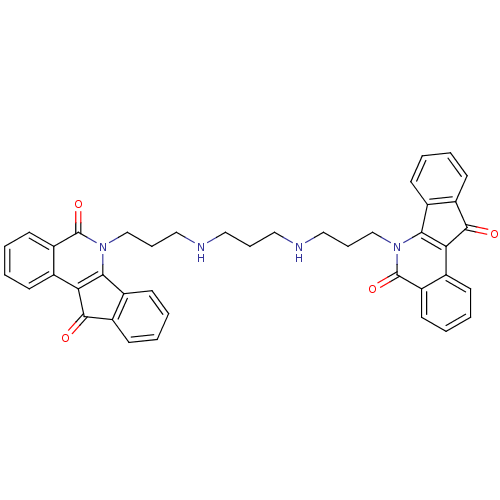 (CHEMBL2057323) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate assessed a... | J Med Chem 55: 4457-78 (2012) Article DOI: 10.1021/jm300335n BindingDB Entry DOI: 10.7270/Q2SB46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50388544 (CHEMBL2057323) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human Tdp1 in Tdp1-deficient chicken DT40 whole cell extract using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-p... | J Med Chem 55: 4457-78 (2012) Article DOI: 10.1021/jm300335n BindingDB Entry DOI: 10.7270/Q2SB46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397340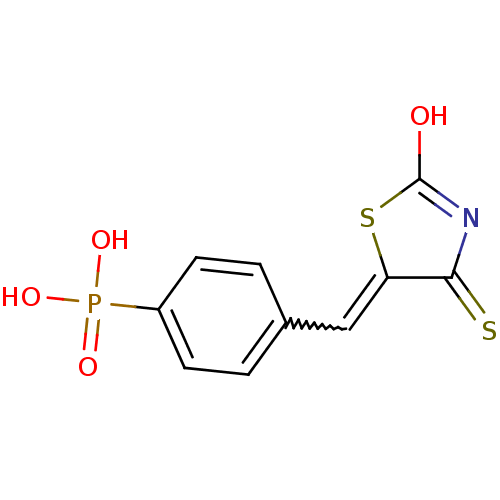 (CHEMBL2170149) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397321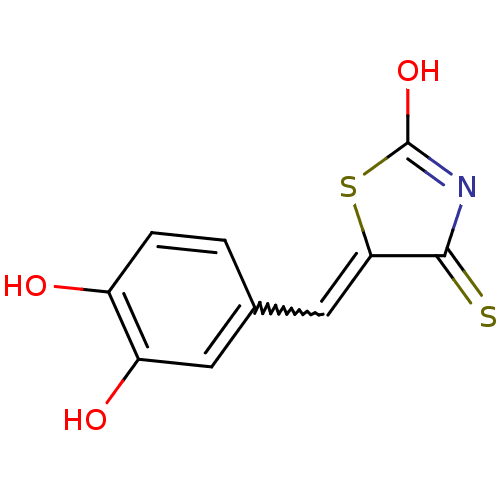 (CHEMBL2170121) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397314 (CHEMBL2170127) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397316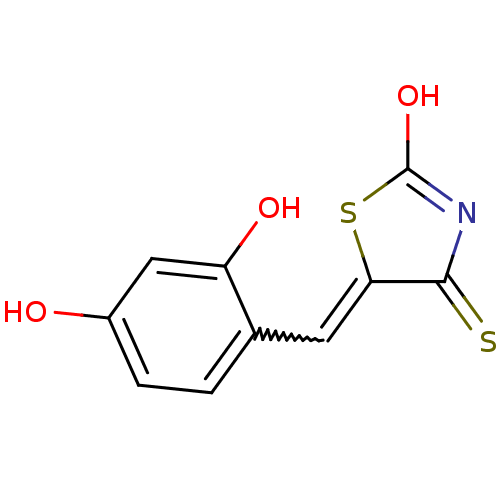 (CHEMBL2170125) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425045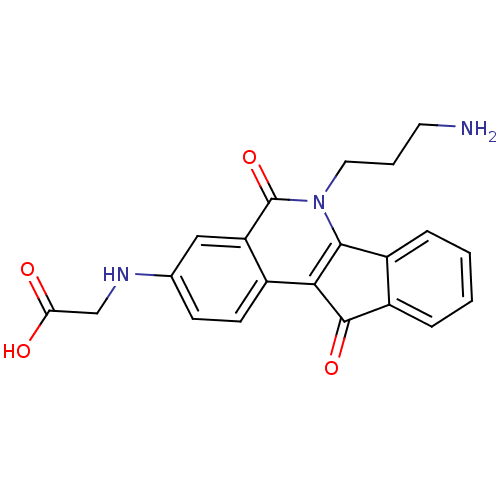 (CHEMBL2312896 | CHEMBL2322964 | US9402842, 43) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425086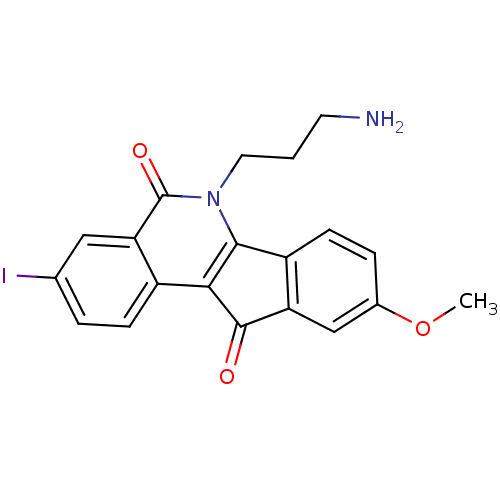 (CHEMBL2312906 | US9402842, 84) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397317 (CHEMBL2170124) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425087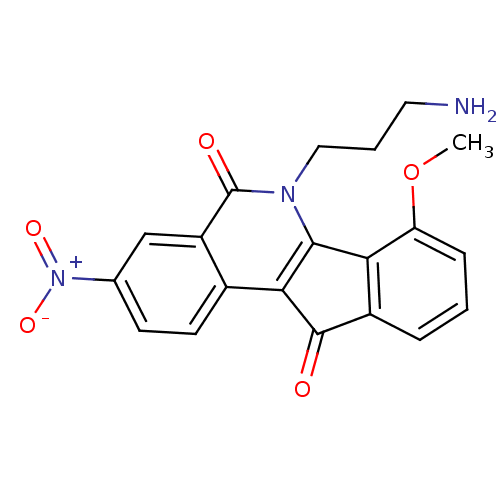 (CHEMBL218884 | US9402842, 55) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SHC-transforming protein 1 (Homo sapiens (Human)) | BDBM50276388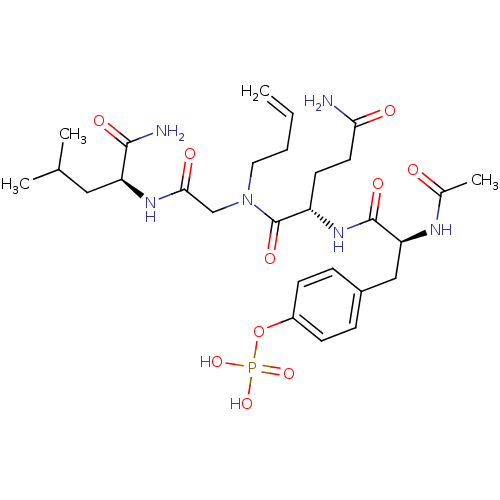 (4-((S)-2-acetamido-3-((S)-5-amino-1-((2-((S)-1-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... | J Med Chem 52: 1612-8 (2009) Article DOI: 10.1021/jm800789h BindingDB Entry DOI: 10.7270/Q2XP74TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425085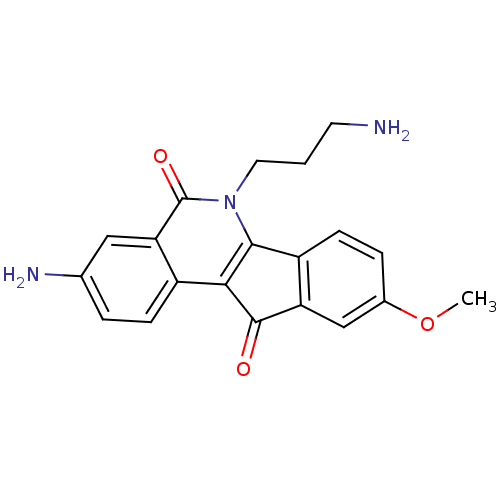 (CHEMBL2312908 | US9402842, 89) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397341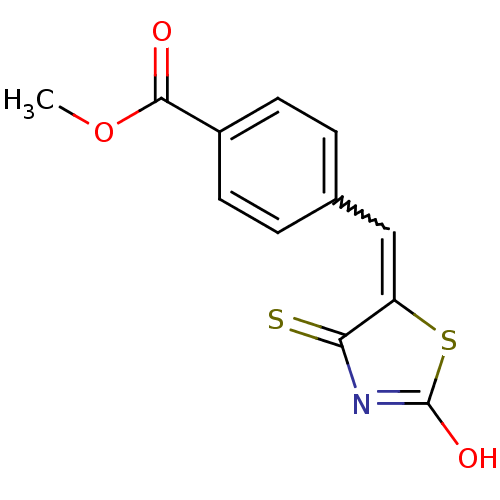 (CHEMBL2170137) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397343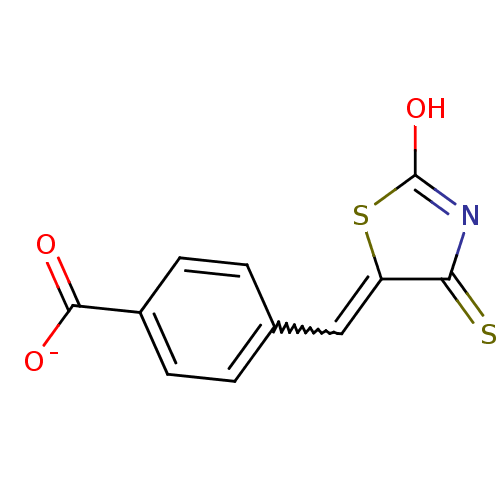 (CHEMBL2170135) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 expressed in Tdp1 knockout chicken DT40 cells using 5'-32P-labeled 5'-GATCTAAAAGACTT-pY-3' as substrate after 15... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397315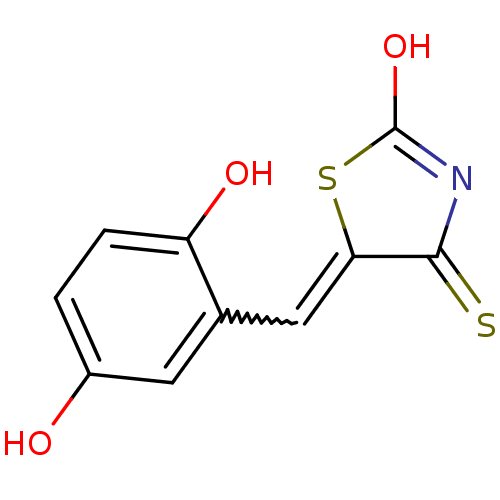 (CHEMBL2170126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50158383 (2-((8S,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate assessed a... | J Med Chem 55: 4457-78 (2012) Article DOI: 10.1021/jm300335n BindingDB Entry DOI: 10.7270/Q2SB46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50158383 (2-((8S,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human Tdp1 expressed in Escherichia coli assessed as blockade of enzyme-mediated hydrolysis of phosphodiester linkage between tyrosine ... | J Med Chem 52: 7122-31 (2009) Article DOI: 10.1021/jm901061s BindingDB Entry DOI: 10.7270/Q2H1325T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397343 (CHEMBL2170135) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SHC-transforming protein 1 (Homo sapiens (Human)) | BDBM50276339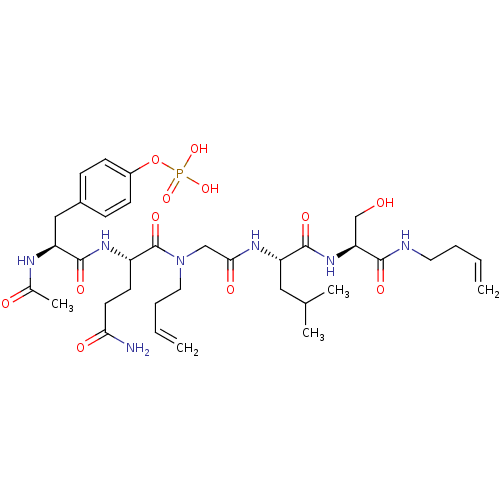 (4-((2S,5S,11S,14S)-2-acetamido-5-(3-amino-3-oxopro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... | J Med Chem 52: 1612-8 (2009) Article DOI: 10.1021/jm800789h BindingDB Entry DOI: 10.7270/Q2XP74TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397342 (CHEMBL2170136) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SHC-transforming protein 1 (Homo sapiens (Human)) | BDBM50276387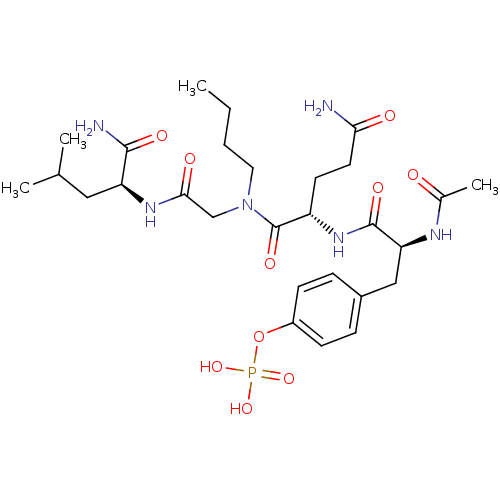 (4-((S)-2-acetamido-3-((S)-5-amino-1-((2-((S)-1-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... | J Med Chem 52: 1612-8 (2009) Article DOI: 10.1021/jm800789h BindingDB Entry DOI: 10.7270/Q2XP74TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SHC-transforming protein 1 (Homo sapiens (Human)) | BDBM50276396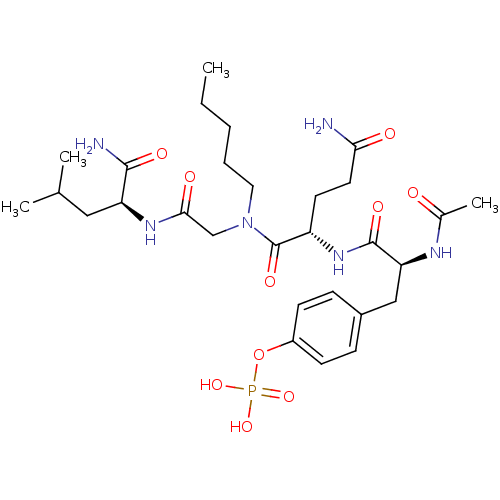 (4-((S)-2-acetamido-3-((S)-5-amino-1-((2-((S)-1-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... | J Med Chem 52: 1612-8 (2009) Article DOI: 10.1021/jm800789h BindingDB Entry DOI: 10.7270/Q2XP74TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397310 (CHEMBL2170131) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397320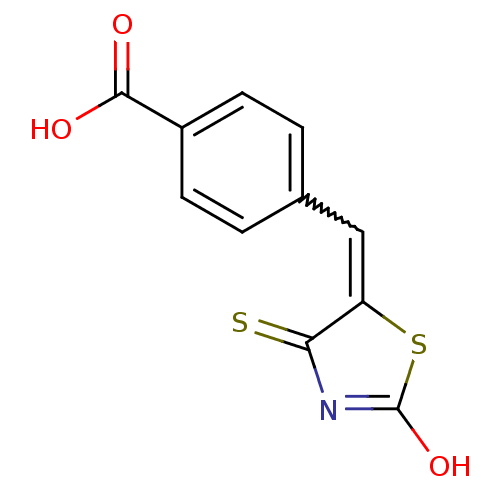 (CHEMBL2170122) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 expressed in Tdp1 knockout chicken DT40 cells using 5'-32P-labeled 5'-GATCTAAAAGACTT-pY-3' as substrate after 15... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425088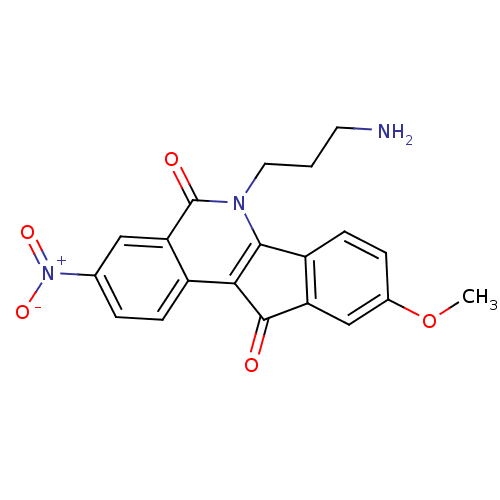 (CHEMBL375623 | US9402842, 54) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425084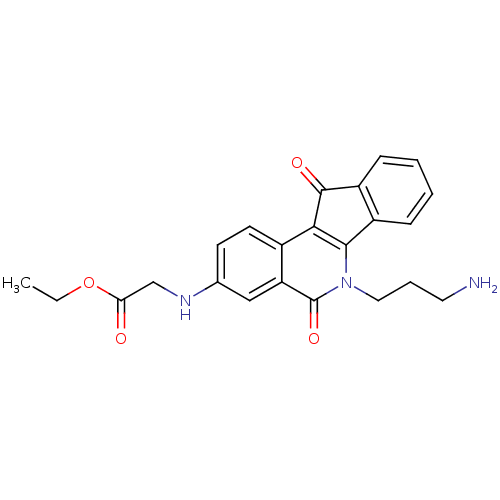 (CHEMBL2312893 | US9402842, 40) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50230460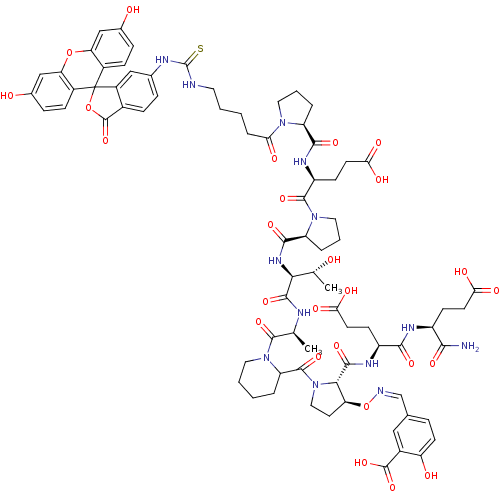 (5-[(1Z)-({[(2S,3S)-2-{[(1S)-1-{[(1S)-1-carbamoyl-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by ChEMBL | Assay Description Binding affinity to Tsg101 | Bioorg Med Chem Lett 18: 1096-101 (2008) Article DOI: 10.1016/j.bmcl.2007.12.003 BindingDB Entry DOI: 10.7270/Q2930V0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50230449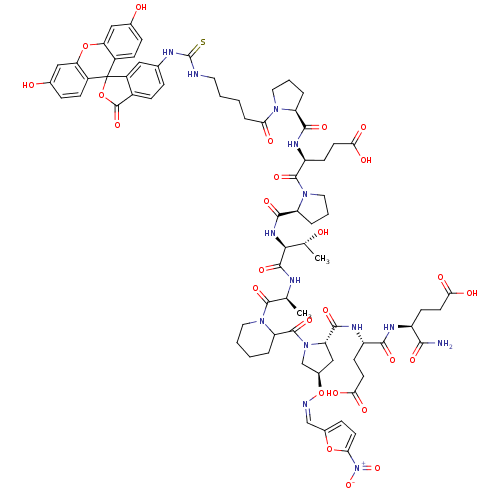 ((4S)-4-{[(1S)-1-carbamoyl-3-carboxypropyl]carbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by ChEMBL | Assay Description Binding affinity to Tsg101 | Bioorg Med Chem Lett 18: 1096-101 (2008) Article DOI: 10.1016/j.bmcl.2007.12.003 BindingDB Entry DOI: 10.7270/Q2930V0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50362891 (CHEMBL1946260) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Inhibition of human Tsg101 binding to GST-tagged P6 protein by surface plasmon resonance method | ACS Med Chem Lett 2: 337-341 (2011) Article DOI: 10.1021/ml1002579 BindingDB Entry DOI: 10.7270/Q22B8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397335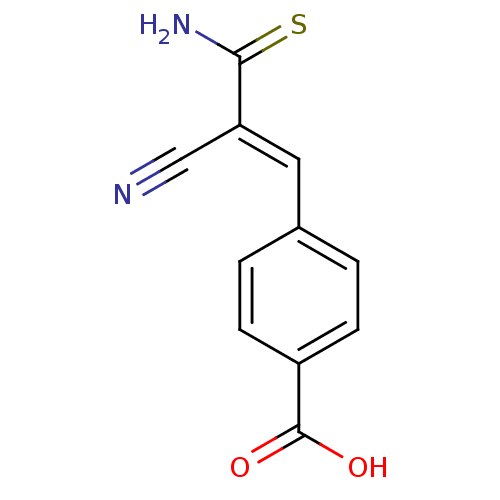 (CHEMBL2170154) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50230470 ((4S)-4-{[(1S)-1-carbamoyl-3-carboxypropyl]carbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by ChEMBL | Assay Description Binding affinity to Tsg101 | Bioorg Med Chem Lett 18: 1096-101 (2008) Article DOI: 10.1016/j.bmcl.2007.12.003 BindingDB Entry DOI: 10.7270/Q2930V0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397318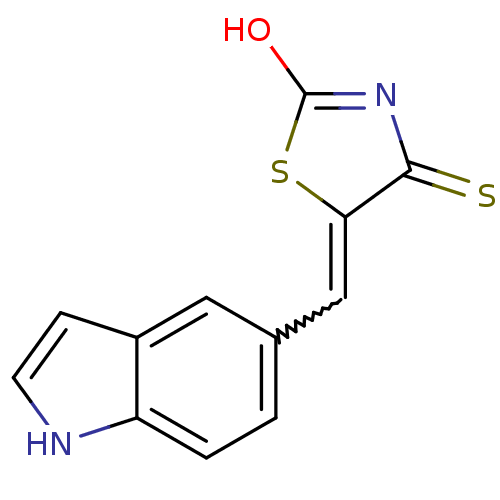 (CHEMBL2170123) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50230450 (4-carbamoyl-4-(4-carboxy-2-{[(2S,3S)-1-({1-[(2S)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by ChEMBL | Assay Description Binding affinity to Tsg101 | Bioorg Med Chem Lett 18: 1096-101 (2008) Article DOI: 10.1016/j.bmcl.2007.12.003 BindingDB Entry DOI: 10.7270/Q2930V0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM4363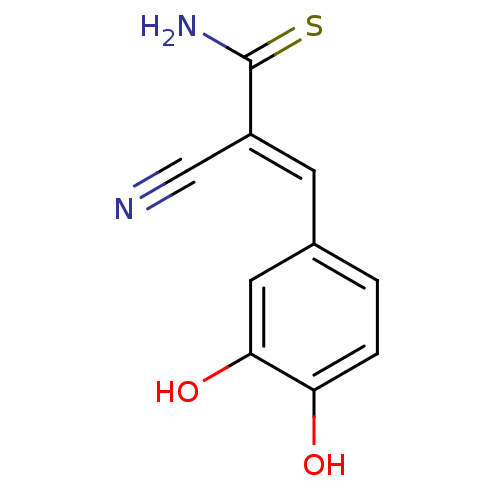 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enethio...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50396238 (CHEMBL2172212 | US9402842, 62) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50388546 (CHEMBL213072 | CHEMBL333363) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50425054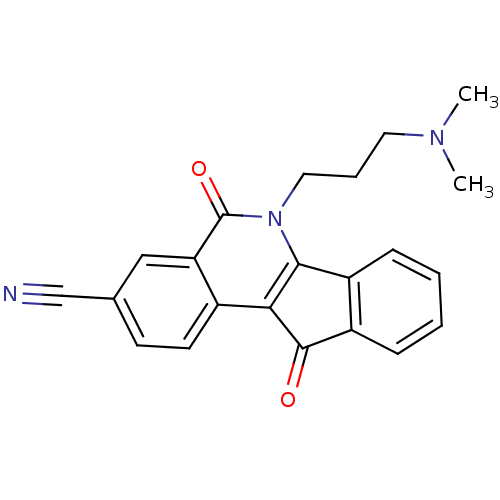 (CHEMBL2312904) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50388554 (CHEMBL223545) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate assessed a... | J Med Chem 55: 4457-78 (2012) Article DOI: 10.1021/jm300335n BindingDB Entry DOI: 10.7270/Q2SB46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50371256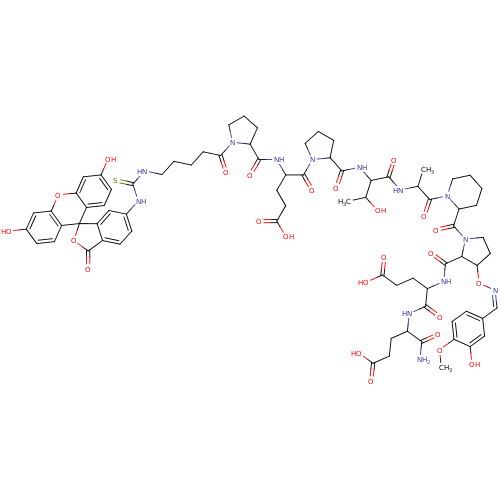 (CHEMBL1162967) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by ChEMBL | Assay Description Binding affinity to Tsg101 | Bioorg Med Chem Lett 18: 1096-101 (2008) Article DOI: 10.1016/j.bmcl.2007.12.003 BindingDB Entry DOI: 10.7270/Q2930V0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50397327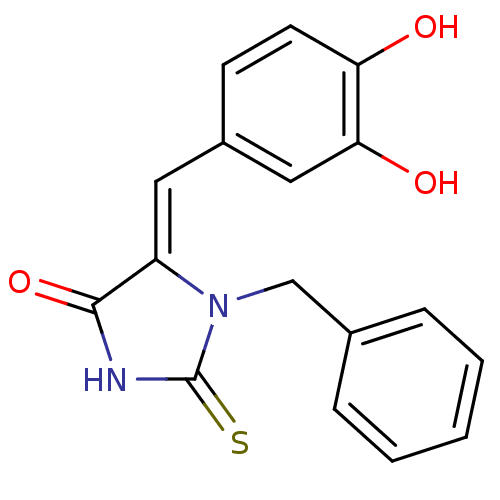 (CHEMBL2170162) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... | J Med Chem 55: 8671-84 (2012) Article DOI: 10.1021/jm3008773 BindingDB Entry DOI: 10.7270/Q2MP54D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50230469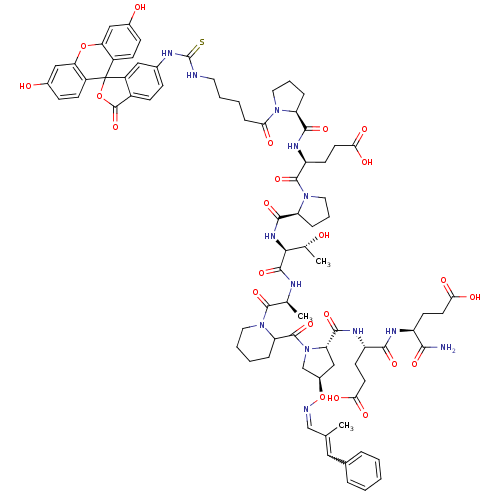 ((4S)-4-{[(1S)-1-carbamoyl-3-carboxypropyl]carbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by ChEMBL | Assay Description Binding affinity to Tsg101 | Bioorg Med Chem Lett 18: 1096-101 (2008) Article DOI: 10.1016/j.bmcl.2007.12.003 BindingDB Entry DOI: 10.7270/Q2930V0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SHC-transforming protein 1 (Homo sapiens (Human)) | BDBM50276402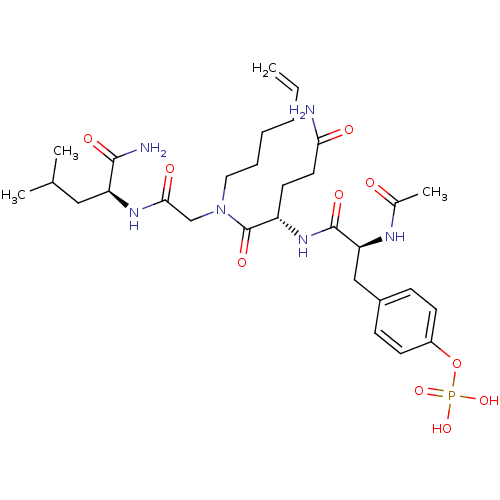 (4-((S)-2-acetamido-3-((S)-5-amino-1-((2-((S)-1-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... | J Med Chem 52: 1612-8 (2009) Article DOI: 10.1021/jm800789h BindingDB Entry DOI: 10.7270/Q2XP74TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SHC-transforming protein 1 (Homo sapiens (Human)) | BDBM50276346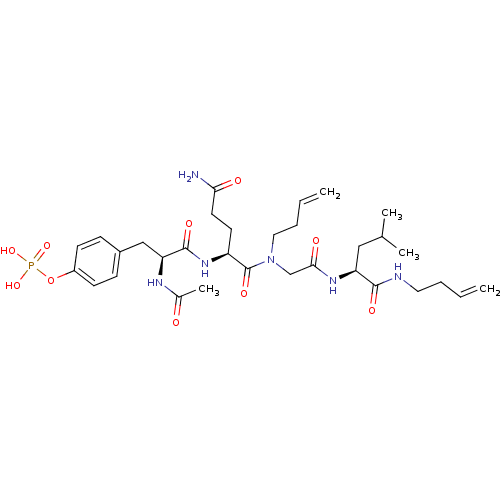 (4-((2S,5S,11S)-2-acetamido-5-(3-amino-3-oxopropyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... | J Med Chem 52: 1612-8 (2009) Article DOI: 10.1021/jm800789h BindingDB Entry DOI: 10.7270/Q2XP74TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 381 total ) | Next | Last >> |