

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146832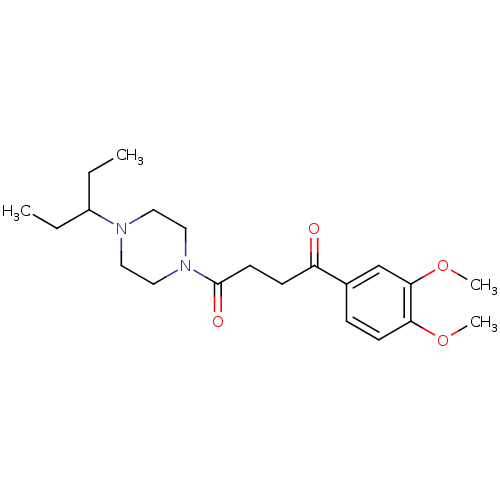 (1-(3,4-Dimethoxy-phenyl)-4-[4-(1-ethyl-propyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146832 (1-(3,4-Dimethoxy-phenyl)-4-[4-(1-ethyl-propyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370330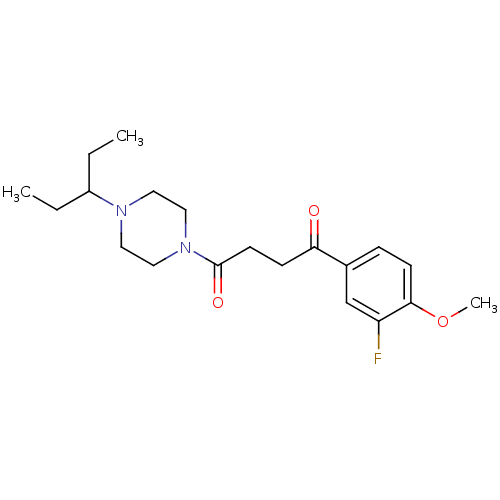 (CHEMBL1202893) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146836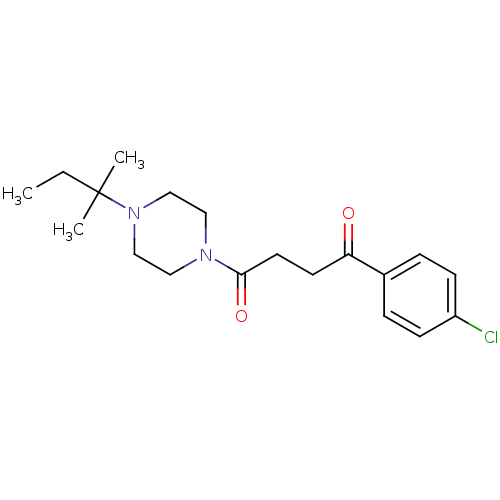 (1-(4-Chloro-phenyl)-4-[4-(1,1-dimethyl-propyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370328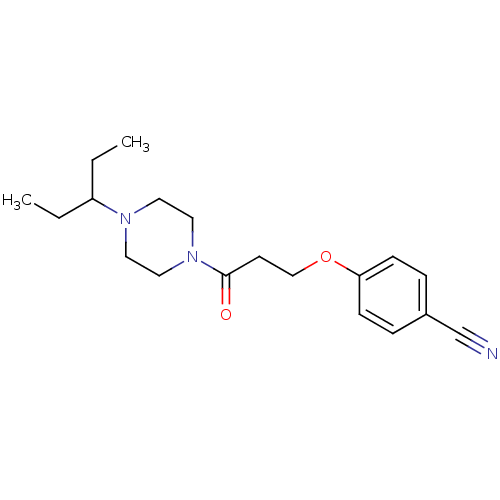 (CHEMBL1202892) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146838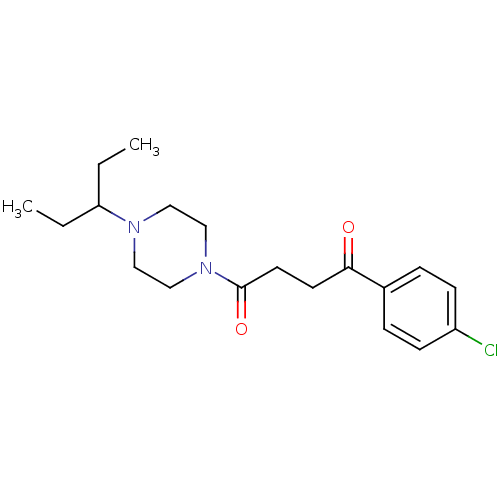 (1-(4-Chloro-phenyl)-4-[4-(1-ethyl-propyl)-piperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370331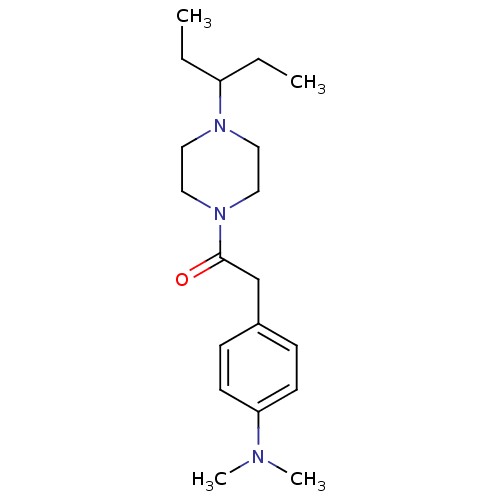 (CHEMBL1202896) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159113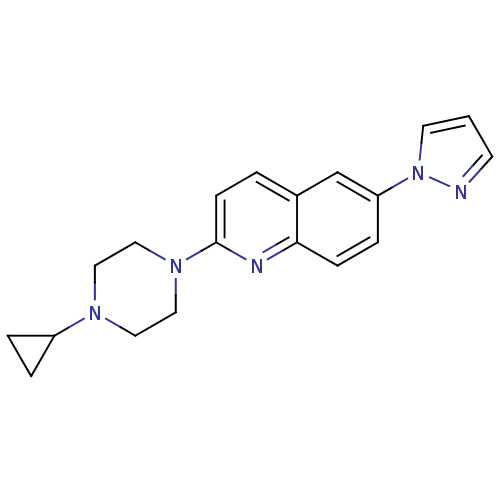 (2-(4-Cyclopropyl-piperazin-1-yl)-6-pyrazol-1-yl-qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146835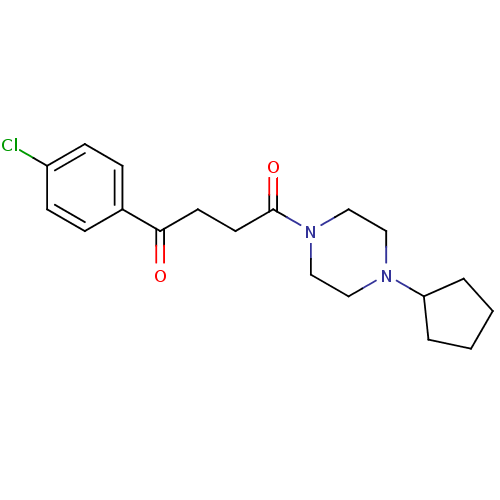 (1-(4-Chloro-phenyl)-4-(4-cyclopentyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146826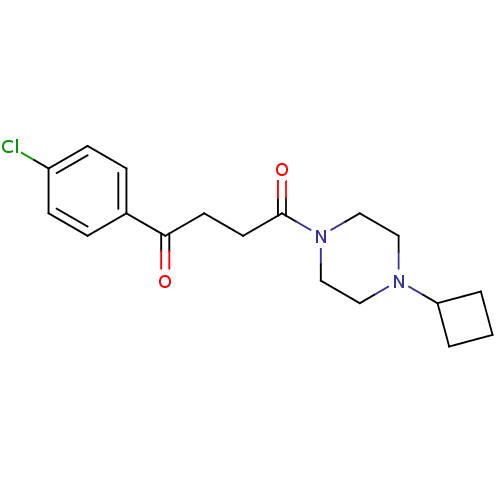 (1-(4-Chloro-phenyl)-4-(4-cyclobutyl-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146840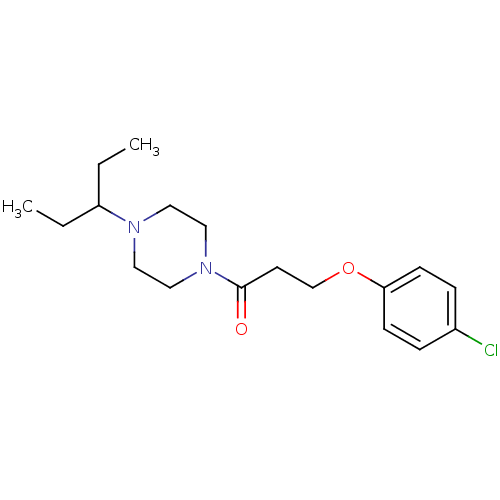 (3-(4-Chloro-phenoxy)-1-[4-(1-ethyl-propyl)-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370336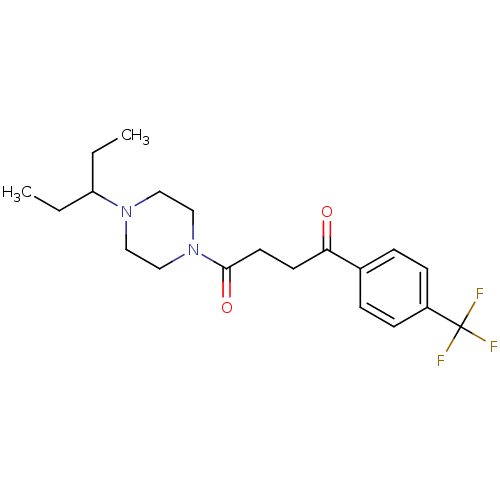 (CHEMBL1202903) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370338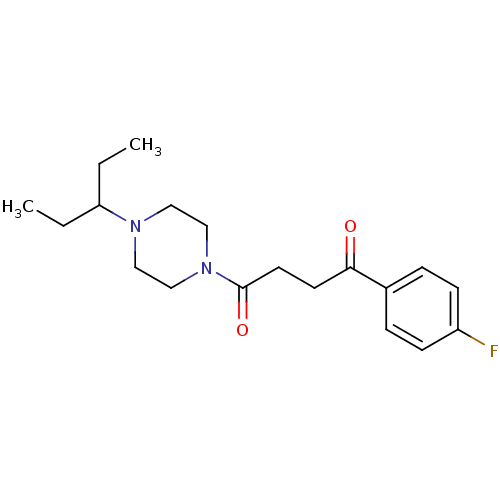 (CHEMBL1202904) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159114 (CHEMBL179234 | Cyclopropyl-[2-(4-cyclopropyl-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120543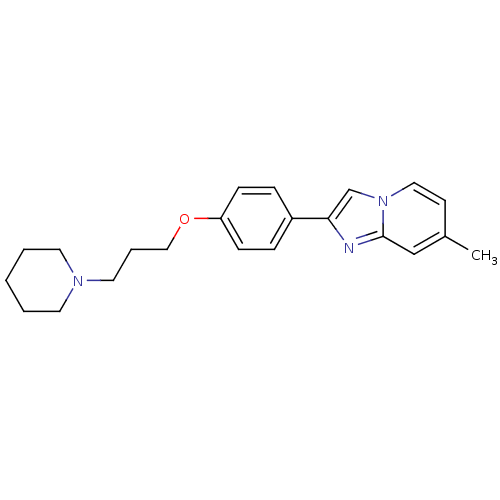 (7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370327 (CHEMBL1202901) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370326 (CHEMBL1202900) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370329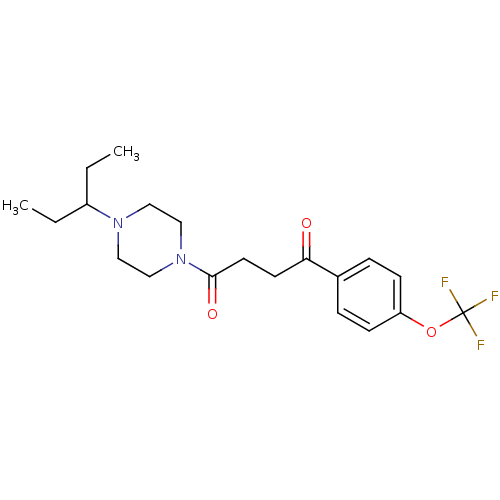 (CHEMBL1202894) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370339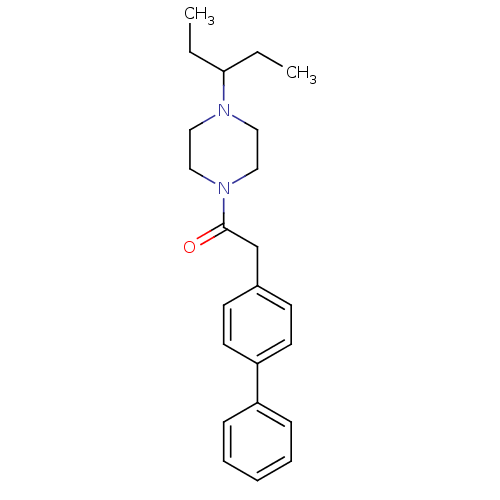 (CHEMBL1202899) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159116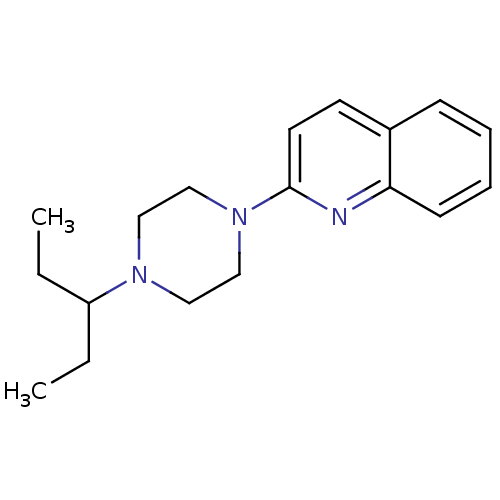 (2-[4-(1-Ethyl-propyl)-piperazin-1-yl]-quinoline | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159122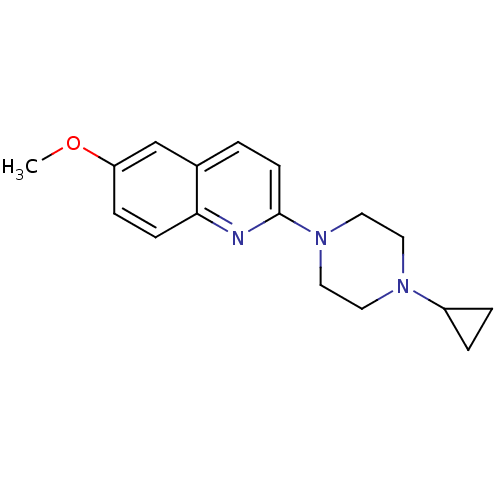 (2-(4-Cyclopropyl-piperazin-1-yl)-6-methoxy-quinoli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146843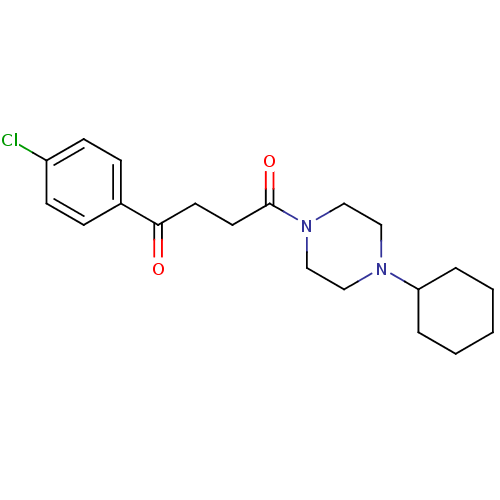 (1-(4-Chloro-phenyl)-4-(4-cyclohexyl-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146839 (1-(4-Chloro-phenyl)-4-(4-isopropyl-piperazin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159121 (2-(4-Cyclopropyl-piperazin-1-yl)-quinoline-6-carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159120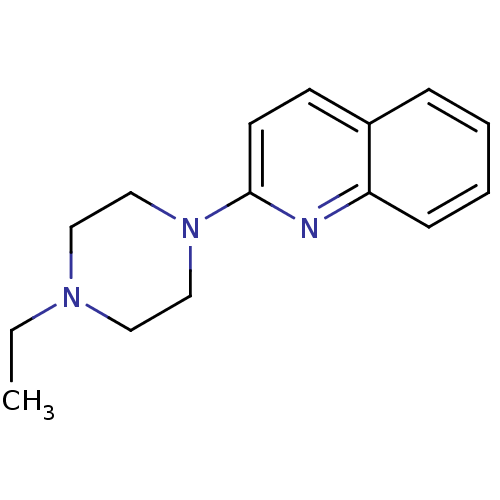 (2-(4-Propyl-piperazin-1-yl)-quinoline | CHEMBL1804...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159108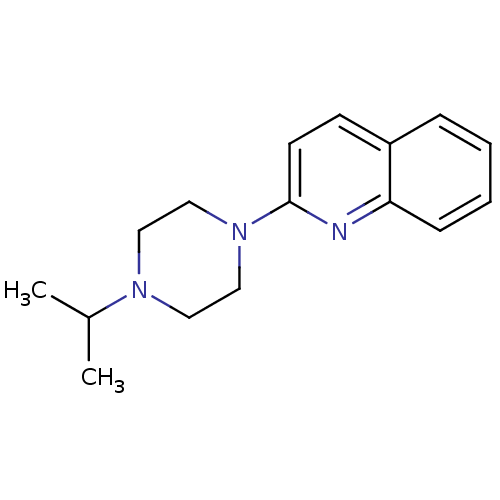 (2-(4-Isopropyl-piperazin-1-yl)-quinoline | CHEMBL3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146820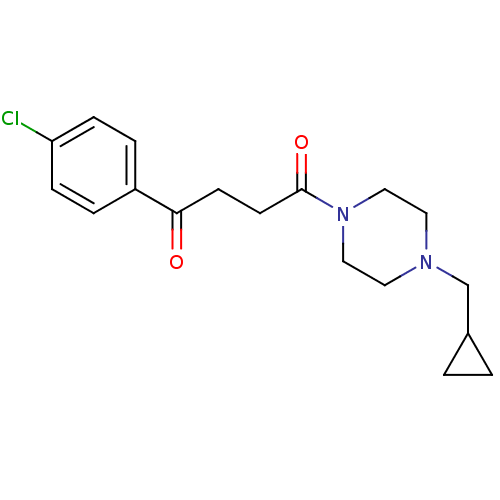 (1-(4-Chloro-phenyl)-4-(4-cyclopropylmethyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370337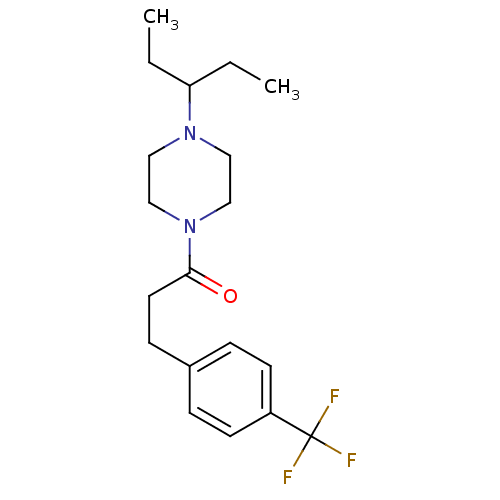 (CHEMBL1202902) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146818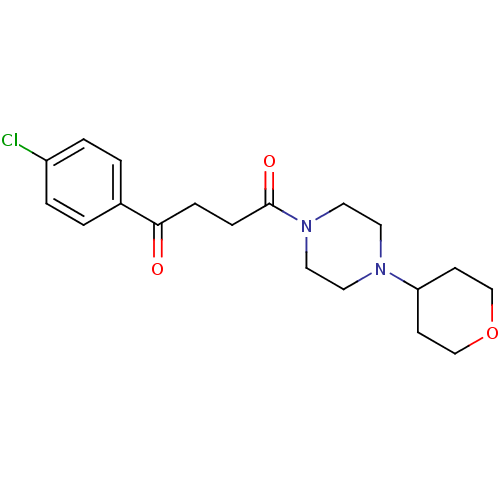 (1-(4-Chloro-phenyl)-4-[4-(tetrahydro-pyran-4-yl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50080367 ((R)-1-{2-[(Z)-2-(2-Chloro-phenyl)-2-phenyl-vinylox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes | J Med Chem 42: 3447-62 (1999) Article DOI: 10.1021/jm981027k BindingDB Entry DOI: 10.7270/Q2057GMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50080358 (1-{2-[2,2-Bis-(2-chloro-phenyl)-vinyloxy]-ethyl}-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes | J Med Chem 42: 3447-62 (1999) Article DOI: 10.1021/jm981027k BindingDB Entry DOI: 10.7270/Q2057GMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50080382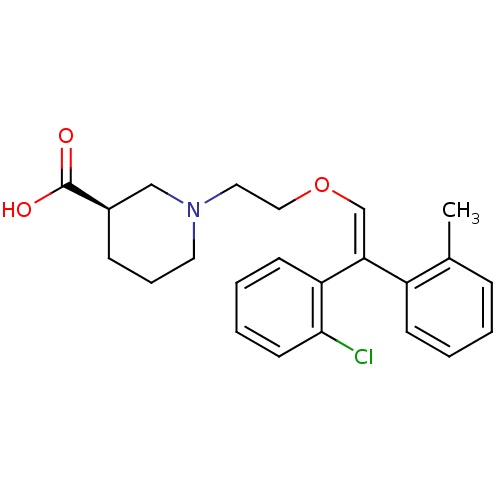 ((R)-1-{2-[(Z)-2-(2-Chloro-phenyl)-2-o-tolyl-vinylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes | J Med Chem 42: 3447-62 (1999) Article DOI: 10.1021/jm981027k BindingDB Entry DOI: 10.7270/Q2057GMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159109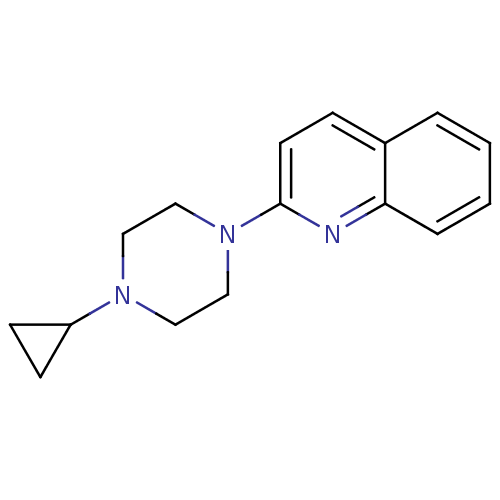 (2-(4-Cyclopropyl-piperazin-1-yl)-quinoline | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146829 (1-(4-Chloro-phenyl)-4-(4-cyclopropyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50080337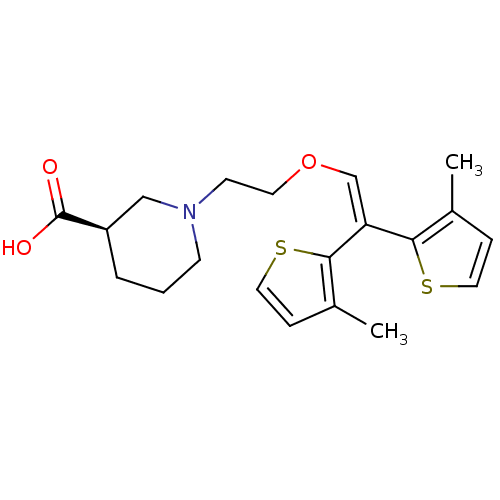 ((R)-1-{2-[2,2-Bis-(3-methyl-thiophen-2-yl)-vinylox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes | J Med Chem 42: 3447-62 (1999) Article DOI: 10.1021/jm981027k BindingDB Entry DOI: 10.7270/Q2057GMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50080376 ((R)-1-{2-[(E)-2-(3-Methyl-thiophen-2-yl)-2-o-tolyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes | J Med Chem 42: 3447-62 (1999) Article DOI: 10.1021/jm981027k BindingDB Entry DOI: 10.7270/Q2057GMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159111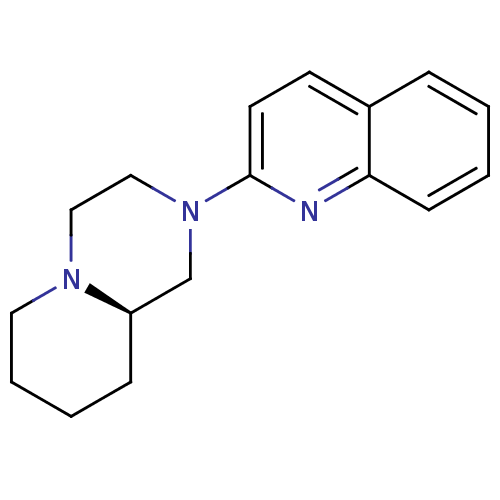 ((R)-2-Quinolin-2-yl-octahydro-pyrido[1,2-a]pyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50080327 (1-{2-[(Z)-2-(2-Fluoro-phenyl)-2-o-tolyl-vinyloxy]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes | J Med Chem 42: 3447-62 (1999) Article DOI: 10.1021/jm981027k BindingDB Entry DOI: 10.7270/Q2057GMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50080335 ((R)-1-[2-(2,2-Di-o-tolyl-vinyloxy)-ethyl]-piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes | J Med Chem 42: 3447-62 (1999) Article DOI: 10.1021/jm981027k BindingDB Entry DOI: 10.7270/Q2057GMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50080375 ((R)-1-[2-((E)-2-Phenyl-2-o-tolyl-vinyloxy)-ethyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes | J Med Chem 42: 3447-62 (1999) Article DOI: 10.1021/jm981027k BindingDB Entry DOI: 10.7270/Q2057GMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370334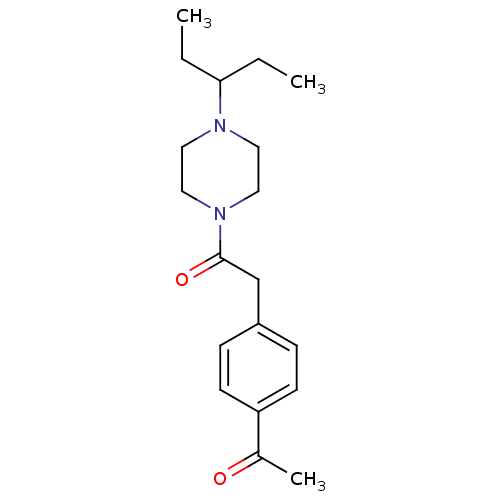 (CHEMBL1202897) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159118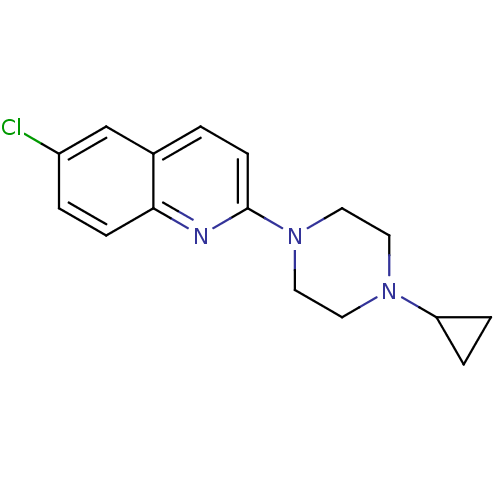 (6-Chloro-2-(4-cyclopropyl-piperazin-1-yl)-quinolin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human H3 receptor in GTPgamma[S]-Assay | J Med Chem 48: 306-11 (2005) Article DOI: 10.1021/jm040873u BindingDB Entry DOI: 10.7270/Q2N0161T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50080368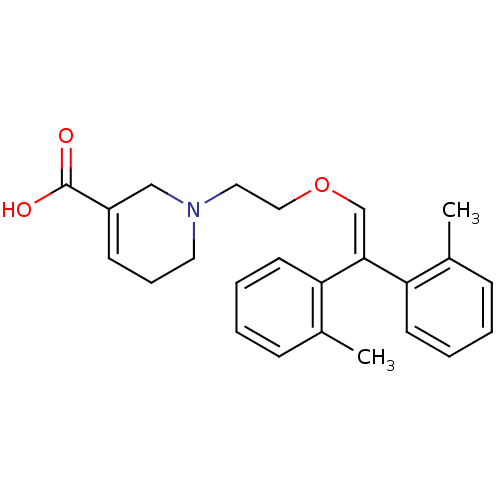 (1-[2-(2,2-Di-o-tolyl-vinyloxy)-ethyl]-1,2,5,6-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes | J Med Chem 42: 3447-62 (1999) Article DOI: 10.1021/jm981027k BindingDB Entry DOI: 10.7270/Q2057GMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50080388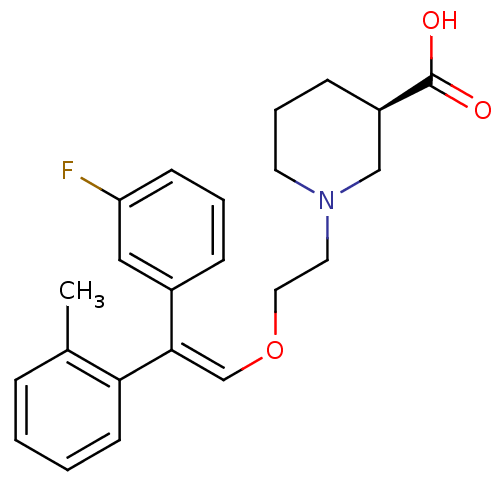 ((R)-1-{2-[(E)-2-(3-Fluoro-phenyl)-2-o-tolyl-vinylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes | J Med Chem 42: 3447-62 (1999) Article DOI: 10.1021/jm981027k BindingDB Entry DOI: 10.7270/Q2057GMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 132 total ) | Next | Last >> |