 Found 20332 hits with Last Name = 'su' and Initial = 'n'
Found 20332 hits with Last Name = 'su' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Enoyl-acyl-carrier protein reductase
(Plasmodium falciparum) | BDBM8726
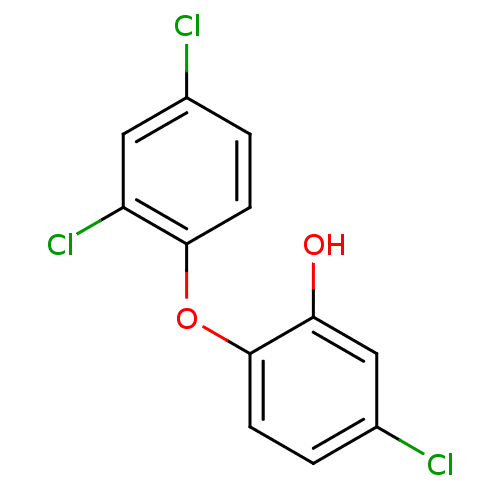
(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.00190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ENR in presence of EGCG by dilution assay |
J Med Chem 50: 765-75 (2007)
Article DOI: 10.1021/jm061154d
BindingDB Entry DOI: 10.7270/Q2QJ7J4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930
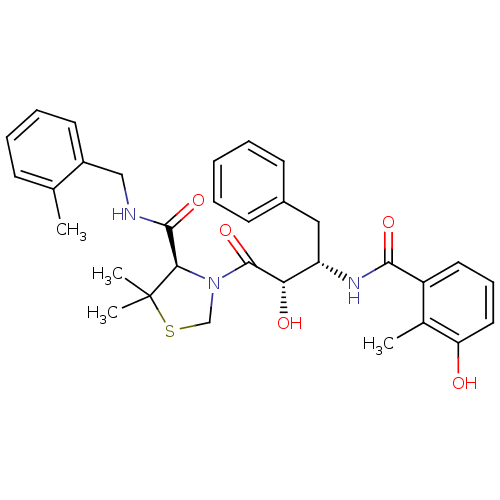
(CHEMBL584130 | KNI-814)Show SMILES Cc1cccc(C)c1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C33H39N3O5S/c1-20-11-9-12-21(2)25(20)18-34-31(40)29-33(4,5)42-19-36(29)32(41)28(38)26(17-23-13-7-6-8-14-23)35-30(39)24-15-10-16-27(37)22(24)3/h6-16,26,28-29,37-38H,17-19H2,1-5H3,(H,34,40)(H,35,39)/t26-,28-,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50325534
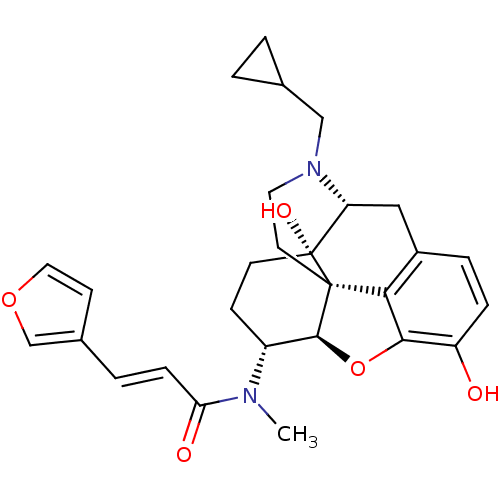
(CHEMBL267495 | nalfurafine)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50596292

(CHEMBL5185211)Show SMILES [H][C@]12Oc3c4c(C[C@]5([H])C(CC[C@H]1N(C)C(=O)\C=C\c1ccoc1)[C@]24CCN5CC1CC1)ccc3O |r,THB:28:27:9:4.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0272 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM580
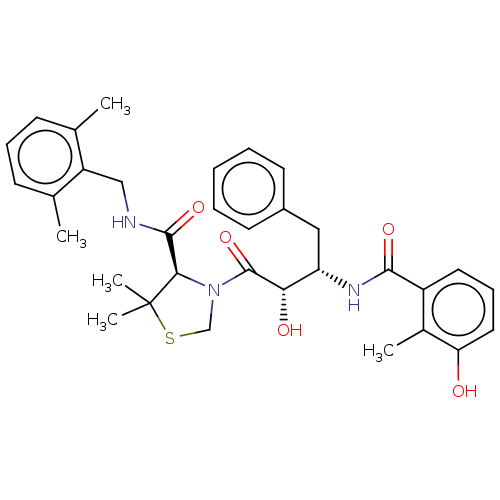
((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C32H37N3O5S/c1-20-11-8-9-14-23(20)18-33-30(39)28-32(3,4)41-19-35(28)31(40)27(37)25(17-22-12-6-5-7-13-22)34-29(38)24-15-10-16-26(36)21(24)2/h5-16,25,27-28,36-37H,17-19H2,1-4H3,(H,33,39)(H,34,38)/t25-,27-,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50596293
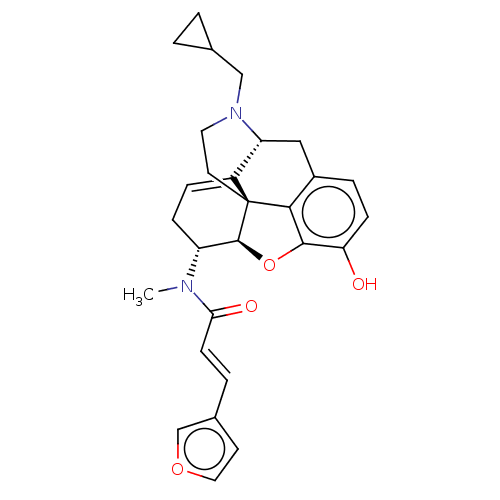
(CHEMBL5188658)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14C5=CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)ccc3O |r,t:21,THB:10:9:17:4.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50013388
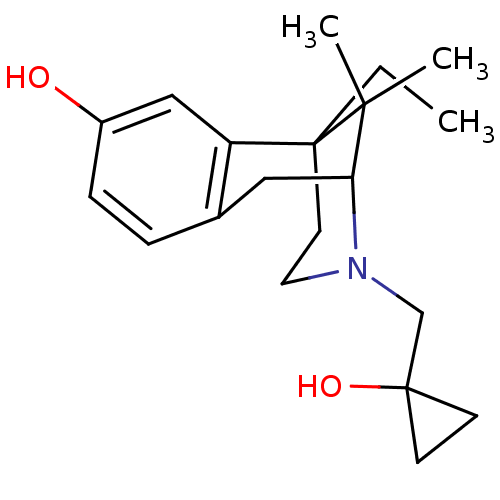
(6-Ethyl-3-(1-hydroxy-cyclopropylmethyl)-11,11-dime...)Show SMILES CCC12CCN(CC3(O)CC3)C(Cc3ccc(O)cc13)C2(C)C |TLB:6:5:20:19.13.12| Show InChI InChI=1S/C20H29NO2/c1-4-20-9-10-21(13-19(23)7-8-19)17(18(20,2)3)11-14-5-6-15(22)12-16(14)20/h5-6,12,17,22-23H,4,7-11,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem 18: 4446-52 (2010)
Article DOI: 10.1016/j.bmc.2010.04.069
BindingDB Entry DOI: 10.7270/Q23X87MV |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50071733
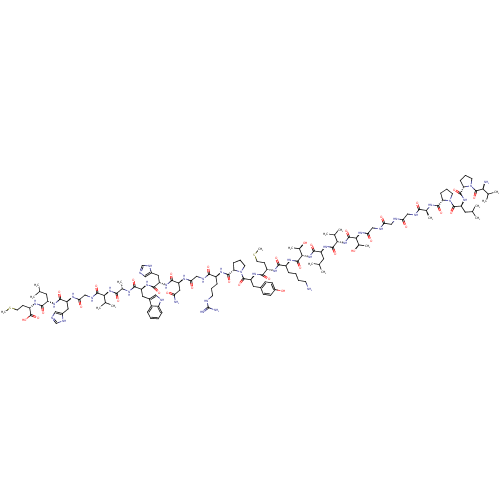
(CHEMBL413196 | Compound GRP)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C)[C@@H](C)O)C(C)C)[C@@H](C)O)C(C)C)C(O)=O Show InChI InChI=1S/C130H203N37O32S2/c1-65(2)47-86(114(183)154-85(129(198)199)39-46-201-18)155-115(184)89(52-77-56-136-63-145-77)149-101(175)62-144-122(191)104(69(9)10)162-109(178)72(14)147-113(182)88(51-76-55-139-81-28-20-19-27-80(76)81)156-116(185)90(53-78-57-137-64-146-78)157-117(186)91(54-97(132)171)150-100(174)61-143-110(179)82(30-23-41-138-130(134)135)152-120(189)95-32-25-43-166(95)127(196)93(50-75-34-36-79(170)37-35-75)159-112(181)84(38-45-200-17)151-111(180)83(29-21-22-40-131)153-124(193)107(74(16)169)164-118(187)87(48-66(3)4)158-123(192)105(70(11)12)163-125(194)106(73(15)168)161-102(176)60-141-98(172)58-140-99(173)59-142-108(177)71(13)148-119(188)94-31-24-42-165(94)126(195)92(49-67(5)6)160-121(190)96-33-26-44-167(96)128(197)103(133)68(7)8/h19-20,27-28,34-37,55-57,63-74,82-96,103-107,139,168-170H,21-26,29-33,38-54,58-62,131,133H2,1-18H3,(H2,132,171)(H,136,145)(H,137,146)(H,140,173)(H,141,172)(H,142,177)(H,143,179)(H,144,191)(H,147,182)(H,148,188)(H,149,175)(H,150,174)(H,151,180)(H,152,189)(H,153,193)(H,154,183)(H,155,184)(H,156,185)(H,157,186)(H,158,192)(H,159,181)(H,160,190)(H,161,176)(H,162,178)(H,163,194)(H,164,187)(H,198,199)(H4,134,135,138)/t71-,72-,73+,74+,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,103-,104-,105-,106-,107-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Bombesin BB2 receptor in the presence of [125I]-[Tyr] bombesin. |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl-carrier protein reductase
(Plasmodium falciparum) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0521 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ENR in presence of ECG by dilution assay |
J Med Chem 50: 765-75 (2007)
Article DOI: 10.1021/jm061154d
BindingDB Entry DOI: 10.7270/Q2QJ7J4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071745
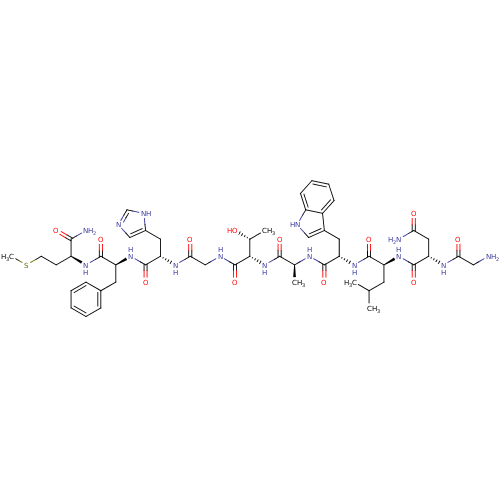
(CHEMBL403317 | Compound NMB | Gly-Asn-Leu-Trp-Ala-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)CN)[C@@H](C)O)C(N)=O Show InChI InChI=1S/C52H73N15O12S/c1-27(2)17-36(64-51(78)40(21-41(54)69)61-42(70)22-53)48(75)66-38(19-31-23-57-34-14-10-9-13-33(31)34)47(74)60-28(3)46(73)67-44(29(4)68)52(79)58-25-43(71)62-39(20-32-24-56-26-59-32)50(77)65-37(18-30-11-7-6-8-12-30)49(76)63-35(45(55)72)15-16-80-5/h6-14,23-24,26-29,35-40,44,57,68H,15-22,25,53H2,1-5H3,(H2,54,69)(H2,55,72)(H,56,59)(H,58,79)(H,60,74)(H,61,70)(H,62,71)(H,63,76)(H,64,78)(H,65,77)(H,66,75)(H,67,73)/t28-,29+,35-,36-,37-,38-,39-,40-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl-carrier protein reductase
(Plasmodium falciparum) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ENR in presence of EGC by dilution assay |
J Med Chem 50: 765-75 (2007)
Article DOI: 10.1021/jm061154d
BindingDB Entry DOI: 10.7270/Q2QJ7J4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391386
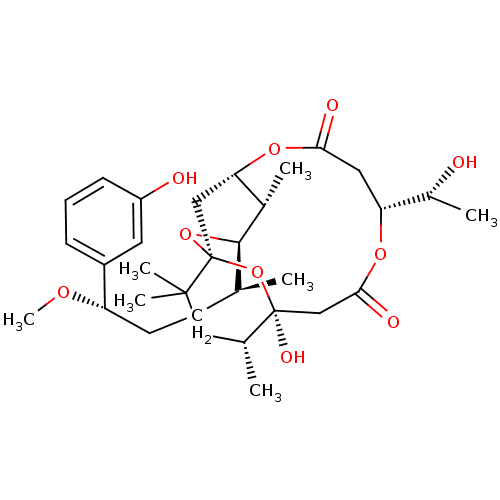
(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50321030
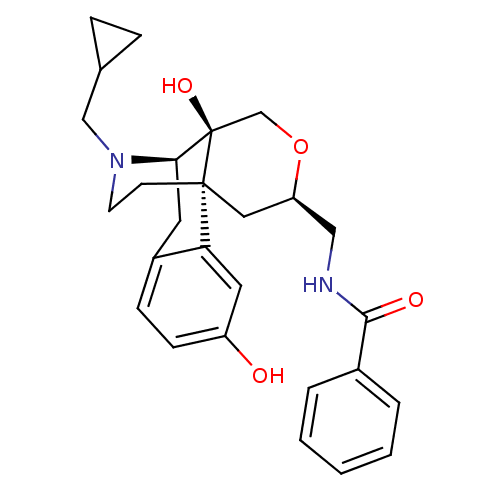
(CHEMBL1163924 | N-{[(1R,9R,10S,13R)-17-(cyclopropy...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]4(C[C@H](CNC(=O)c5ccccc5)OC[C@@]34O)c2c1 |r| Show InChI InChI=1S/C27H32N2O4/c30-21-9-8-20-12-24-27(32)17-33-22(15-28-25(31)19-4-2-1-3-5-19)14-26(27,23(20)13-21)10-11-29(24)16-18-6-7-18/h1-5,8-9,13,18,22,24,30,32H,6-7,10-12,14-17H2,(H,28,31)/t22-,24-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem 18: 4446-52 (2010)
Article DOI: 10.1016/j.bmc.2010.04.069
BindingDB Entry DOI: 10.7270/Q23X87MV |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM85484
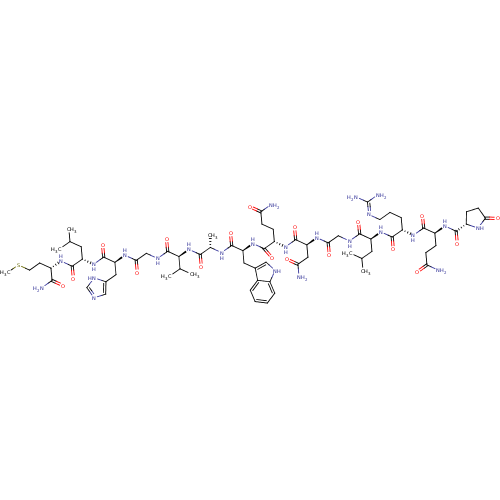
(Bombesin)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |wU:82.92,74.81,8.12,4.4,30.31,53.61,16.23,wD:93.101,39.52,62.69,102.104,34.35,(24.84,-22.59,;25.32,-21.13,;24.29,-19.98,;24.77,-18.52,;23.74,-17.37,;24.22,-15.91,;25.72,-15.59,;26.75,-16.74,;26.2,-14.13,;27.71,-13.81,;28.74,-14.96,;30.24,-14.64,;28.26,-16.42,;25.17,-12.98,;25.65,-11.52,;27.16,-11.2,;24.62,-10.37,;25.1,-8.91,;26.61,-8.59,;27.75,-9.62,;29.09,-8.86,;28.77,-7.35,;27.24,-7.19,;23.12,-10.69,;22.09,-9.54,;22.57,-8.08,;20.58,-9.86,;19.55,-8.72,;18.04,-9.03,;17.57,-10.5,;17.02,-7.89,;15.51,-8.2,;14.48,-7.06,;14.96,-5.59,;12.97,-7.38,;12.5,-8.84,;11.95,-6.23,;10.44,-6.55,;9.96,-8.01,;9.41,-5.4,;7.9,-5.72,;7.42,-7.18,;8.32,-8.42,;7.42,-9.66,;5.97,-9.19,;4.63,-9.96,;3.3,-9.19,;3.3,-7.65,;4.63,-6.88,;5.97,-7.65,;9.89,-3.94,;8.86,-2.79,;7.35,-3.11,;9.34,-1.33,;10.85,-1.01,;11.32,.45,;12.83,.77,;13.31,2.24,;13.86,-.37,;8.31,-.18,;8.79,1.28,;10.3,1.6,;7.76,2.43,;6.25,2.11,;5.22,3.26,;3.72,2.94,;5.7,4.72,;8.24,3.89,;9.75,4.21,;10.77,3.06,;10.22,5.67,;11.73,5.99,;12.21,7.46,;11.18,8.6,;13.72,7.77,;14.74,6.63,;16.25,6.94,;17.28,5.8,;16.73,8.41,;14.19,9.24,;13.17,10.38,;11.66,10.07,;13.64,11.85,;15.15,12.17,;15.63,13.63,;17.14,13.95,;17.61,15.41,;19.12,15.73,;19.6,17.19,;20.15,14.58,;12.62,12.99,;13.09,14.46,;14.6,14.78,;12.07,15.6,;10.56,15.29,;9.53,16.43,;8.02,16.11,;7,17.26,;7.55,14.65,;12.54,17.07,;11.52,18.21,;10.01,17.9,;11.99,19.68,;11.09,20.93,;12,22.17,;13.46,21.69,;14.71,22.59,;13.46,20.15,;17.49,-6.42,;19,-6.11,;16.47,-5.28,;22.23,-17.69,;21.75,-19.16,;21.2,-16.55,)| Show InChI InChI=1S/C71H110N24O18S/c1-34(2)24-47(92-62(105)43(14-11-22-79-71(76)77)89-64(107)45(15-18-52(72)96)90-63(106)44-17-20-55(99)85-44)61(104)81-31-56(100)87-51(28-54(74)98)69(112)91-46(16-19-53(73)97)65(108)94-49(26-38-29-80-41-13-10-9-12-40(38)41)66(109)84-37(7)60(103)95-58(36(5)6)70(113)82-32-57(101)86-50(27-39-30-78-33-83-39)68(111)93-48(25-35(3)4)67(110)88-42(59(75)102)21-23-114-8/h9-10,12-13,29-30,33-37,42-51,58,80H,11,14-28,31-32H2,1-8H3,(H2,72,96)(H2,73,97)(H2,74,98)(H2,75,102)(H,78,83)(H,81,104)(H,82,113)(H,84,109)(H,85,99)(H,86,101)(H,87,100)(H,88,110)(H,89,107)(H,90,106)(H,91,112)(H,92,105)(H,93,111)(H,94,108)(H,95,103)(H4,76,77,79)/t37-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,58-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Bombesin BB2 receptor in the presence of [125I]-[Tyr] bombesin. |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071735
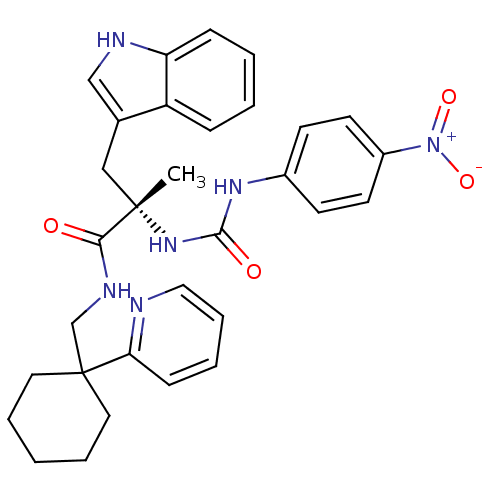
((S)-3-(1H-Indol-3-yl)-2-methyl-2-[3-(4-nitro-pheny...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)C(=O)NCC1(CCCCC1)c1ccccn1 Show InChI InChI=1S/C31H34N6O4/c1-30(19-22-20-33-26-10-4-3-9-25(22)26,36-29(39)35-23-12-14-24(15-13-23)37(40)41)28(38)34-21-31(16-6-2-7-17-31)27-11-5-8-18-32-27/h3-5,8-15,18,20,33H,2,6-7,16-17,19,21H2,1H3,(H,34,38)(H2,35,36,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM85484

(Bombesin)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |wU:82.92,74.81,8.12,4.4,30.31,53.61,16.23,wD:93.101,39.52,62.69,102.104,34.35,(24.84,-22.59,;25.32,-21.13,;24.29,-19.98,;24.77,-18.52,;23.74,-17.37,;24.22,-15.91,;25.72,-15.59,;26.75,-16.74,;26.2,-14.13,;27.71,-13.81,;28.74,-14.96,;30.24,-14.64,;28.26,-16.42,;25.17,-12.98,;25.65,-11.52,;27.16,-11.2,;24.62,-10.37,;25.1,-8.91,;26.61,-8.59,;27.75,-9.62,;29.09,-8.86,;28.77,-7.35,;27.24,-7.19,;23.12,-10.69,;22.09,-9.54,;22.57,-8.08,;20.58,-9.86,;19.55,-8.72,;18.04,-9.03,;17.57,-10.5,;17.02,-7.89,;15.51,-8.2,;14.48,-7.06,;14.96,-5.59,;12.97,-7.38,;12.5,-8.84,;11.95,-6.23,;10.44,-6.55,;9.96,-8.01,;9.41,-5.4,;7.9,-5.72,;7.42,-7.18,;8.32,-8.42,;7.42,-9.66,;5.97,-9.19,;4.63,-9.96,;3.3,-9.19,;3.3,-7.65,;4.63,-6.88,;5.97,-7.65,;9.89,-3.94,;8.86,-2.79,;7.35,-3.11,;9.34,-1.33,;10.85,-1.01,;11.32,.45,;12.83,.77,;13.31,2.24,;13.86,-.37,;8.31,-.18,;8.79,1.28,;10.3,1.6,;7.76,2.43,;6.25,2.11,;5.22,3.26,;3.72,2.94,;5.7,4.72,;8.24,3.89,;9.75,4.21,;10.77,3.06,;10.22,5.67,;11.73,5.99,;12.21,7.46,;11.18,8.6,;13.72,7.77,;14.74,6.63,;16.25,6.94,;17.28,5.8,;16.73,8.41,;14.19,9.24,;13.17,10.38,;11.66,10.07,;13.64,11.85,;15.15,12.17,;15.63,13.63,;17.14,13.95,;17.61,15.41,;19.12,15.73,;19.6,17.19,;20.15,14.58,;12.62,12.99,;13.09,14.46,;14.6,14.78,;12.07,15.6,;10.56,15.29,;9.53,16.43,;8.02,16.11,;7,17.26,;7.55,14.65,;12.54,17.07,;11.52,18.21,;10.01,17.9,;11.99,19.68,;11.09,20.93,;12,22.17,;13.46,21.69,;14.71,22.59,;13.46,20.15,;17.49,-6.42,;19,-6.11,;16.47,-5.28,;22.23,-17.69,;21.75,-19.16,;21.2,-16.55,)| Show InChI InChI=1S/C71H110N24O18S/c1-34(2)24-47(92-62(105)43(14-11-22-79-71(76)77)89-64(107)45(15-18-52(72)96)90-63(106)44-17-20-55(99)85-44)61(104)81-31-56(100)87-51(28-54(74)98)69(112)91-46(16-19-53(73)97)65(108)94-49(26-38-29-80-41-13-10-9-12-40(38)41)66(109)84-37(7)60(103)95-58(36(5)6)70(113)82-32-57(101)86-50(27-39-30-78-33-83-39)68(111)93-48(25-35(3)4)67(110)88-42(59(75)102)21-23-114-8/h9-10,12-13,29-30,33-37,42-51,58,80H,11,14-28,31-32H2,1-8H3,(H2,72,96)(H2,73,97)(H2,74,98)(H2,75,102)(H,78,83)(H,81,104)(H,82,113)(H,84,109)(H,85,99)(H,86,101)(H,87,100)(H,88,110)(H,89,107)(H,90,106)(H,91,112)(H,92,105)(H,93,111)(H,94,108)(H,95,103)(H4,76,77,79)/t37-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,58-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against [125 I][4Tyr]-bombesin labeled cloned human GRP(gastrin releasing peptide) receptors stably expressed in CHO cells |
Bioorg Med Chem Lett 6: 2617-2622 (1996)
Article DOI: 10.1016/0960-894X(96)00481-7
BindingDB Entry DOI: 10.7270/Q2NC61QD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50321028

((1S,2R,6S,14R,15R,16R)-3-(cyclopropylmethyl)-11,15...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@]1(O)CC[C@@]35O[C@H]1C(=O)Nc1ccccc1 |r,THB:26:25:14.15:22.21| Show InChI InChI=1S/C28H30N2O5/c31-19-9-8-17-14-20-28-11-10-27(33,23(35-28)24(32)29-18-4-2-1-3-5-18)25-26(28,21(17)22(19)34-25)12-13-30(20)15-16-6-7-16/h1-5,8-9,16,20,23,25,31,33H,6-7,10-15H2,(H,29,32)/t20-,23+,25-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem 18: 4446-52 (2010)
Article DOI: 10.1016/j.bmc.2010.04.069
BindingDB Entry DOI: 10.7270/Q23X87MV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50596292

(CHEMBL5185211)Show SMILES [H][C@]12Oc3c4c(C[C@]5([H])C(CC[C@H]1N(C)C(=O)\C=C\c1ccoc1)[C@]24CCN5CC1CC1)ccc3O |r,THB:28:27:9:4.5.6| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCbeta C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50049805
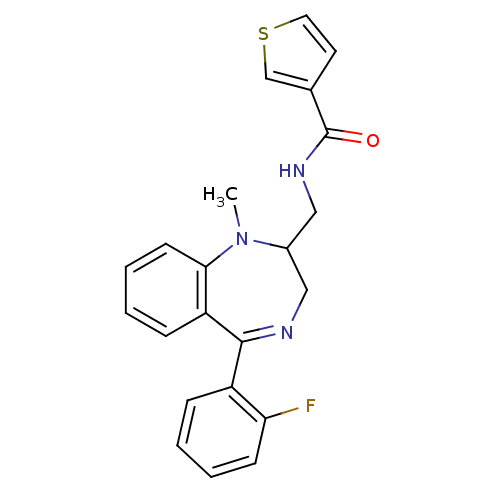
(CHEMBL169703 | Thiophene-3-carboxylic acid [5-(2-f...)Show SMILES CN1C(CNC(=O)c2ccsc2)CN=C(c2ccccc2F)c2ccccc12 |t:14| Show InChI InChI=1S/C22H20FN3OS/c1-26-16(13-25-22(27)15-10-11-28-14-15)12-24-21(17-6-2-4-8-19(17)23)18-7-3-5-9-20(18)26/h2-11,14,16H,12-13H2,1H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem 18: 4446-52 (2010)
Article DOI: 10.1016/j.bmc.2010.04.069
BindingDB Entry DOI: 10.7270/Q23X87MV |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071739
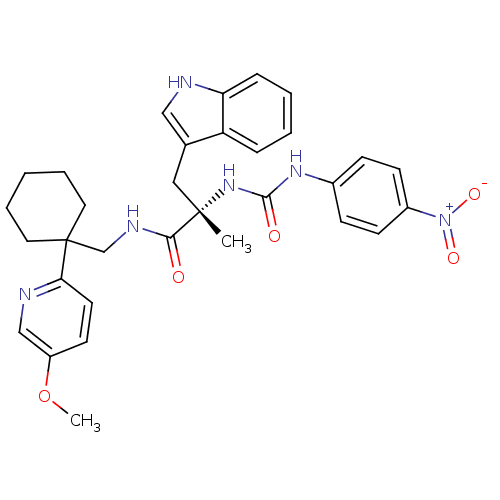
((S)-3-(1H-Indol-3-yl)-N-[1-(5-methoxy-pyridin-2-yl...)Show SMILES COc1ccc(nc1)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C32H36N6O5/c1-31(18-22-19-33-27-9-5-4-8-26(22)27,37-30(40)36-23-10-12-24(13-11-23)38(41)42)29(39)35-21-32(16-6-3-7-17-32)28-15-14-25(43-2)20-34-28/h4-5,8-15,19-20,33H,3,6-7,16-18,21H2,1-2H3,(H,35,39)(H2,36,37,40)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50493217
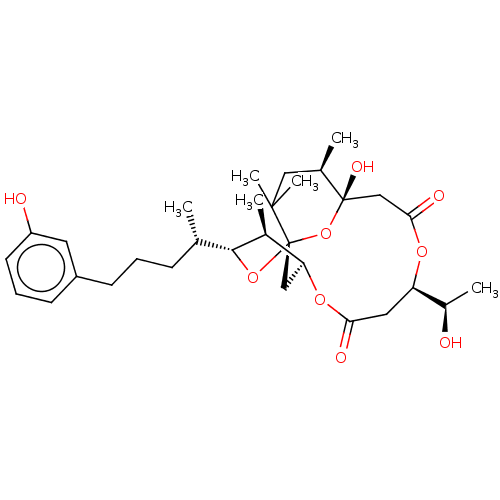
(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCeta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50480931
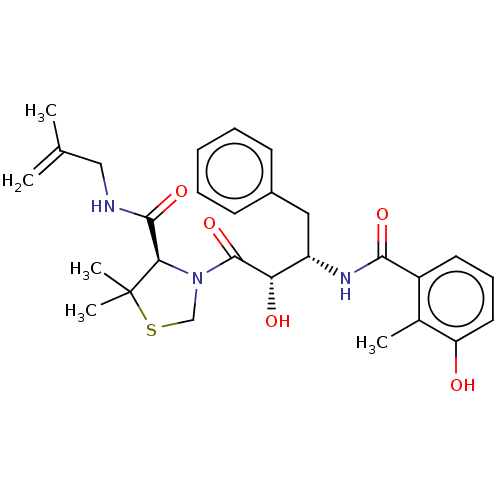
(CHEMBL575512 | KNI-1614)Show SMILES CC(=C)CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C28H35N3O5S/c1-17(2)15-29-26(35)24-28(4,5)37-16-31(24)27(36)23(33)21(14-19-10-7-6-8-11-19)30-25(34)20-12-9-13-22(32)18(20)3/h6-13,21,23-24,32-33H,1,14-16H2,2-5H3,(H,29,35)(H,30,34)/t21-,23-,24+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50274257
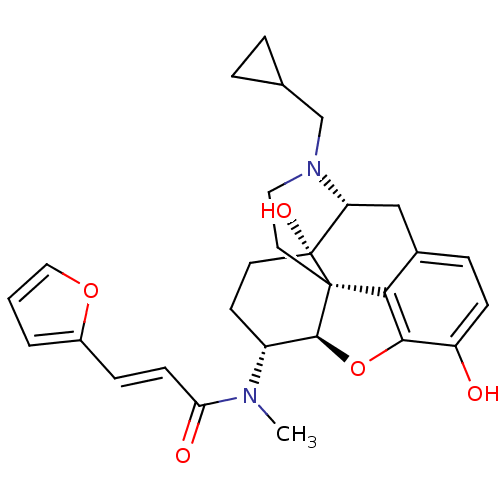
((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccco1 |r,TLB:4:5:9.25.8:18.20.19,THB:6:5:9.25.8:18.20.19| Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)9-7-19-3-2-14-34-19)20-10-11-28(33)22-15-18-6-8-21(31)25-24(18)27(28,26(20)35-25)12-13-30(22)16-17-4-5-17/h2-3,6-9,14,17,20,22,26,31,33H,4-5,10-13,15-16H2,1H3/b9-7+/t20-,22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem 18: 4446-52 (2010)
Article DOI: 10.1016/j.bmc.2010.04.069
BindingDB Entry DOI: 10.7270/Q23X87MV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281737
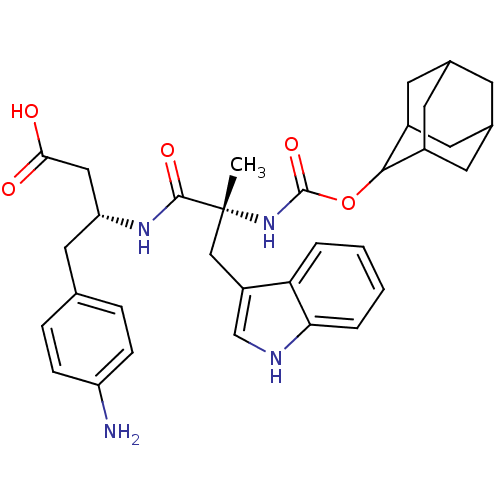
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N)cc1 |wU:1.0,wD:1.13,29.33,TLB:22:17:25:21.23.20,22:21:16.17.18:25,15:16:21.23.22:19.18.25,THB:20:21:16:19.18.25,20:19:16:21.23.22,15:16:25:21.23.20,(3.41,-9.92,;4.74,-10.71,;6.07,-9.95,;6.09,-8.41,;5.19,-7.17,;6.1,-5.93,;7.57,-6.4,;8.89,-5.63,;10.23,-6.4,;10.23,-7.94,;8.89,-8.71,;7.56,-7.94,;3.39,-11.46,;2.06,-10.69,;2.08,-9.15,;.73,-11.44,;-.81,-11.44,;-.62,-13,;-2,-13.53,;-3.42,-13.21,;-4.47,-14.66,;-3,-14.03,;-1.49,-14.43,;-3.21,-12.42,;-2.3,-11.04,;-3.61,-11.69,;6.07,-11.48,;6.05,-13.02,;7.4,-10.71,;8.73,-11.48,;8.73,-13.02,;10.06,-13.79,;10.06,-15.33,;8.73,-14.54,;10.06,-10.71,;11.39,-11.48,;11.39,-13.02,;12.72,-13.79,;14.05,-13.02,;15.38,-13.79,;14.05,-11.46,;12.72,-10.71,)| Show InChI InChI=1S/C33H40N4O5/c1-33(17-24-18-35-28-5-3-2-4-27(24)28,31(40)36-26(16-29(38)39)15-19-6-8-25(34)9-7-19)37-32(41)42-30-22-11-20-10-21(13-22)14-23(30)12-20/h2-9,18,20-23,26,30,35H,10-17,34H2,1H3,(H,36,40)(H,37,41)(H,38,39)/t20?,21?,22?,23?,26-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of binding of [3H]-PD 140376 to Cholecystokinin type B receptor in mouse cortex. |
Bioorg Med Chem Lett 3: 885-888 (1993)
Article DOI: 10.1016/S0960-894X(00)80686-1
BindingDB Entry DOI: 10.7270/Q2D79BWZ |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCtheta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Opioid growth factor receptor-like protein 1
(Homo sapiens (Human)) | BDBM220380
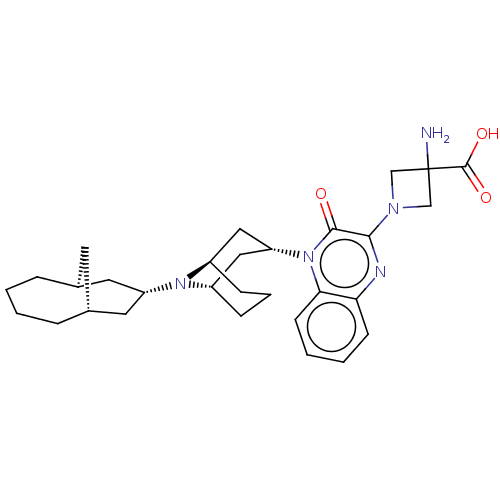
(US9290488, ZA01)Show SMILES NC1(CN(C1)c1nc2ccccc2n([C@@H]2C[C@@H]3CCC[C@H](C2)N3[C@H]2C[C@@H]3C[C@H](C2)CCCC3)c1=O)C(O)=O |r,THB:23:22:14.15.21:17.19.18| Show InChI InChI=1S/C30H41N5O3/c31-30(29(37)38)17-33(18-30)27-28(36)35(26-11-4-3-10-25(26)32-27)24-15-21-8-5-9-22(16-24)34(21)23-13-19-6-1-2-7-20(12-19)14-23/h3-4,10-11,19-24H,1-2,5-9,12-18,31H2,(H,37,38)/t19-,20+,21-,22+,23-,24+ | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... |
US Patent US9290488 (2016)
BindingDB Entry DOI: 10.7270/Q2GF0SCW |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50274257

((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccco1 |r,TLB:4:5:9.25.8:18.20.19,THB:6:5:9.25.8:18.20.19| Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)9-7-19-3-2-14-34-19)20-10-11-28(33)22-15-18-6-8-21(31)25-24(18)27(28,26(20)35-25)12-13-30(22)16-17-4-5-17/h2-3,6-9,14,17,20,22,26,31,33H,4-5,10-13,15-16H2,1H3/b9-7+/t20-,22-,26+,27+,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from guinea pig cerebellum kappa opioid receptor |
Bioorg Med Chem Lett 20: 121-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.027
BindingDB Entry DOI: 10.7270/Q2GX4CJH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50274347
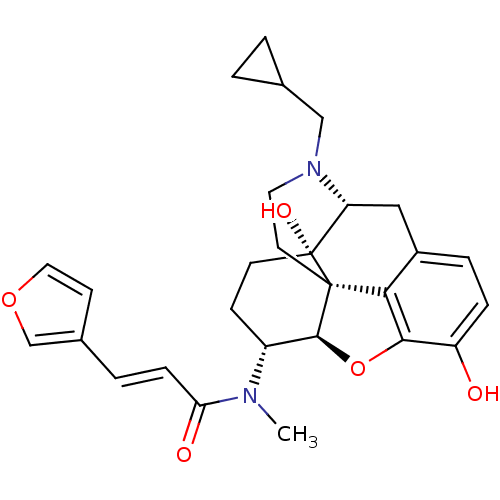
((2E)-N-[(5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-3...)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 |r,TLB:4:5:9.25.8:18.20.19,THB:6:5:9.25.8:18.20.19| Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes by micro-beta scintillation counting method |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCalpha C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50321029

((1R,2R,6S,14R,15R,16S)-3-(cyclopropylmethyl)-11,15...)Show SMILES CN(CCc1ccccc1)C(=O)[C@H]1C[C@]23C=C[C@]1(O)[C@@H]1Oc4c5c(C[C@H]2N(CC2CC2)CC[C@@]315)ccc4O |r,wU:25.28,14.14,wD:33.39,19.21,17.19,12.12,c:16,TLB:10:12:33.19:15.16,(.95,-49.83,;1.01,-48.3,;2.37,-47.58,;3.67,-48.4,;5.03,-47.67,;6.32,-48.5,;7.68,-47.78,;7.74,-46.24,;6.42,-45.42,;5.07,-46.14,;-.3,-47.48,;-.24,-45.94,;-1.66,-48.21,;-2.96,-47.37,;-4.32,-48.08,;-2.8,-48.08,;-3.57,-49.41,;-1.69,-49.72,;-.39,-50.53,;-3.06,-50.43,;-3.88,-52.04,;-5.74,-51.88,;-5.71,-50.34,;-7,-49.54,;-6.98,-48.01,;-5.62,-47.28,;-6.35,-45.85,;-6.89,-44.41,;-8.44,-44.43,;-9.79,-43.67,;-9.78,-45.22,;-6.82,-47.35,;-6,-49.12,;-4.35,-49.63,;-8.35,-50.3,;-8.4,-51.82,;-7.08,-52.62,;-7.12,-54.15,)| Show InChI InChI=1S/C32H36N2O4/c1-33(15-11-20-5-3-2-4-6-20)28(36)23-18-30-12-13-32(23,37)29-31(30)14-16-34(19-21-7-8-21)25(30)17-22-9-10-24(35)27(38-29)26(22)31/h2-6,9-10,12-13,21,23,25,29,35,37H,7-8,11,14-19H2,1H3/t23-,25-,29-,30-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.279 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem 18: 4446-52 (2010)
Article DOI: 10.1016/j.bmc.2010.04.069
BindingDB Entry DOI: 10.7270/Q23X87MV |
More data for this
Ligand-Target Pair | |
Enoyl-acyl-carrier protein reductase
(Plasmodium falciparum) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ENR in presence of quercetin by dilution assay |
J Med Chem 50: 765-75 (2007)
Article DOI: 10.1021/jm061154d
BindingDB Entry DOI: 10.7270/Q2QJ7J4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071750
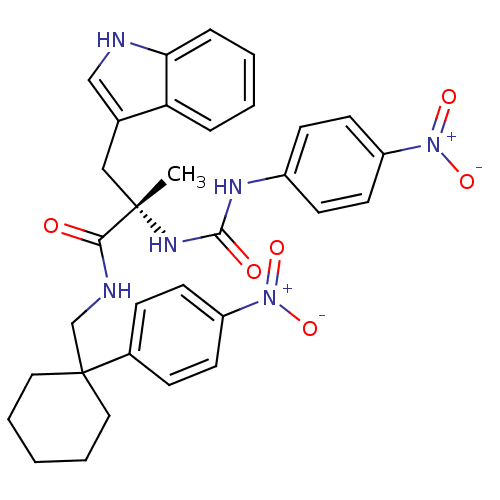
((S)-3-(1H-Indol-3-yl)-2-methyl-N-[1-(4-nitro-pheny...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)C(=O)NCC1(CCCCC1)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C32H34N6O6/c1-31(19-22-20-33-28-8-4-3-7-27(22)28,36-30(40)35-24-11-15-26(16-12-24)38(43)44)29(39)34-21-32(17-5-2-6-18-32)23-9-13-25(14-10-23)37(41)42/h3-4,7-16,20,33H,2,5-6,17-19,21H2,1H3,(H,34,39)(H2,35,36,40)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071746
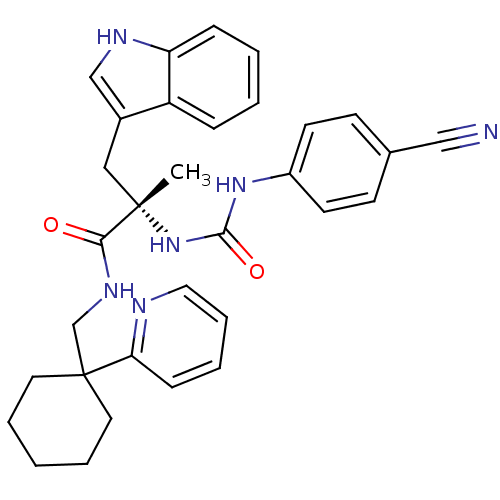
((S)-2-[3-(4-Cyano-phenyl)-ureido]-3-(1H-indol-3-yl...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)C#N)C(=O)NCC1(CCCCC1)c1ccccn1 Show InChI InChI=1S/C32H34N6O2/c1-31(19-24-21-35-27-10-4-3-9-26(24)27,38-30(40)37-25-14-12-23(20-33)13-15-25)29(39)36-22-32(16-6-2-7-17-32)28-11-5-8-18-34-28/h3-5,8-15,18,21,35H,2,6-7,16-17,19,22H2,1H3,(H,36,39)(H2,37,38,40)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCdelta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1
(Homo sapiens (Human)) | BDBM50601067
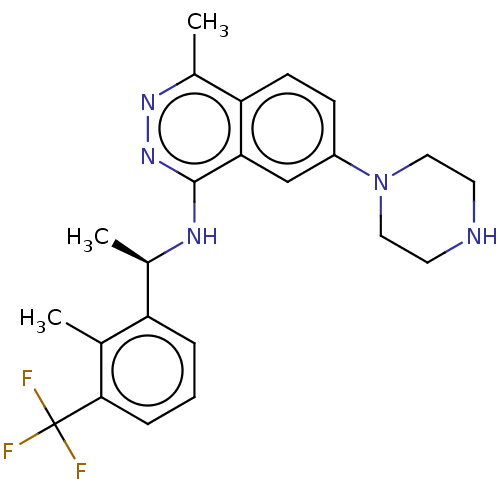
(CHEMBL5208088 | US11702418, Example 6-4)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCNCC1)c1cccc(c1C)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00741
BindingDB Entry DOI: 10.7270/Q2XD15Q0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Opioid growth factor receptor-like protein 1
(Homo sapiens (Human)) | BDBM220382
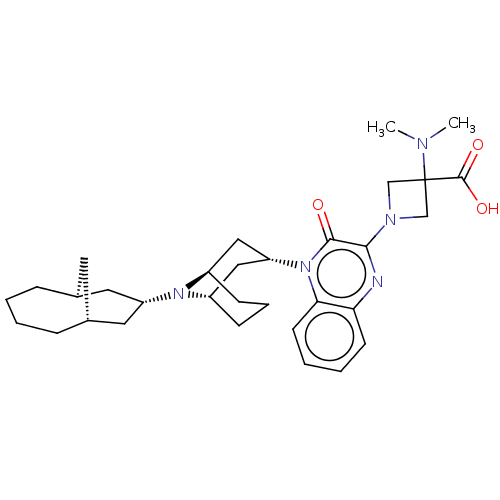
(US9290488, ZA03)Show SMILES CN(C)C1(CN(C1)c1nc2ccccc2n([C@@H]2C[C@@H]3CCC[C@H](C2)N3[C@H]2C[C@@H]3C[C@H](C2)CCCC3)c1=O)C(O)=O |r,THB:25:24:16.17.23:19.21.20| Show InChI InChI=1S/C32H45N5O3/c1-34(2)32(31(39)40)19-35(20-32)29-30(38)37(28-13-6-5-12-27(28)33-29)26-17-23-10-7-11-24(18-26)36(23)25-15-21-8-3-4-9-22(14-21)16-25/h5-6,12-13,21-26H,3-4,7-11,14-20H2,1-2H3,(H,39,40)/t21-,22+,23-,24+,25-,26+ | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.340 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... |
US Patent US9290488 (2016)
BindingDB Entry DOI: 10.7270/Q2GF0SCW |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1
(Homo sapiens (Human)) | BDBM50601075
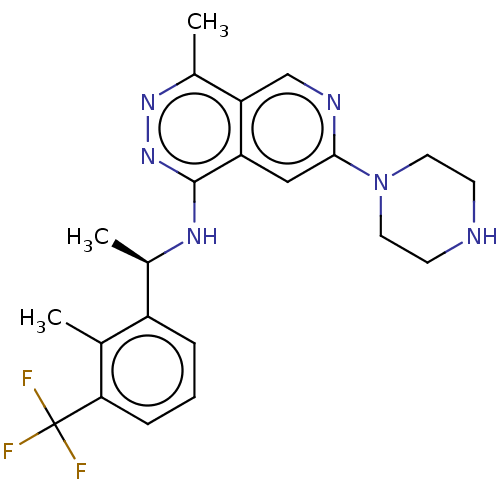
(CHEMBL5202472 | US11702418, Example 6-9)Show SMILES C[C@@H](Nc1nnc(C)c2cnc(cc12)N1CCNCC1)c1cccc(c1C)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00741
BindingDB Entry DOI: 10.7270/Q2XD15Q0 |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCgamma C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071748
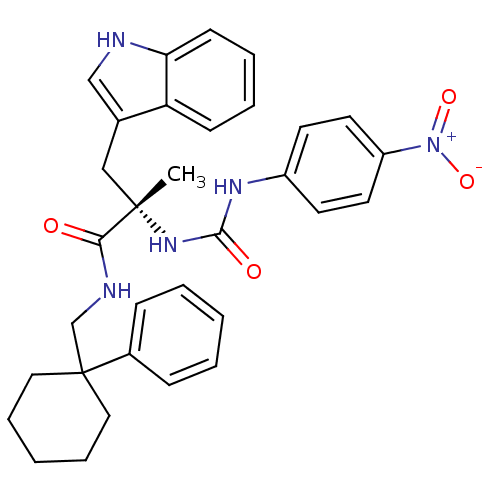
((S)-3-(1H-Indol-3-yl)-2-methyl-2-[3-(4-nitro-pheny...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)C(=O)NCC1(CCCCC1)c1ccccc1 Show InChI InChI=1S/C32H35N5O4/c1-31(20-23-21-33-28-13-7-6-12-27(23)28,36-30(39)35-25-14-16-26(17-15-25)37(40)41)29(38)34-22-32(18-8-3-9-19-32)24-10-4-2-5-11-24/h2,4-7,10-17,21,33H,3,8-9,18-20,22H2,1H3,(H,34,38)(H2,35,36,39)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50360626
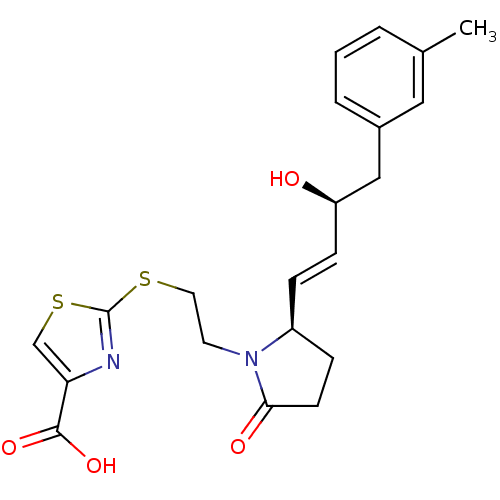
(CHEMBL1933722)Show SMILES Cc1cccc(C[C@H](O)\C=C\[C@H]2CCC(=O)N2CCSc2nc(cs2)C(O)=O)c1 |r| Show InChI InChI=1S/C21H24N2O4S2/c1-14-3-2-4-15(11-14)12-17(24)7-5-16-6-8-19(25)23(16)9-10-28-21-22-18(13-29-21)20(26)27/h2-5,7,11,13,16-17,24H,6,8-10,12H2,1H3,(H,26,27)/b7-5+/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 396-401 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.109
BindingDB Entry DOI: 10.7270/Q27P8ZT2 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1
(Homo sapiens (Human)) | BDBM50601069
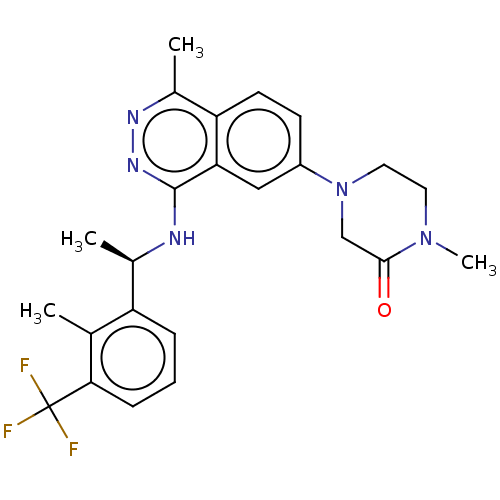
(CHEMBL5191446 | US11702418, Example 6-7)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(C)C(=O)C1)c1cccc(c1C)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00741
BindingDB Entry DOI: 10.7270/Q2XD15Q0 |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071736
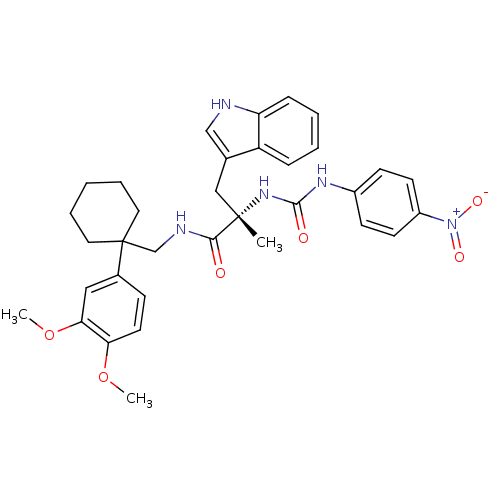
((S)-N-[1-(3,4-Dimethoxy-phenyl)-cyclohexylmethyl]-...)Show SMILES COc1ccc(cc1OC)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C34H39N5O6/c1-33(20-23-21-35-28-10-6-5-9-27(23)28,38-32(41)37-25-12-14-26(15-13-25)39(42)43)31(40)36-22-34(17-7-4-8-18-34)24-11-16-29(44-2)30(19-24)45-3/h5-6,9-16,19,21,35H,4,7-8,17-18,20,22H2,1-3H3,(H,36,40)(H2,37,38,41)/t33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391388
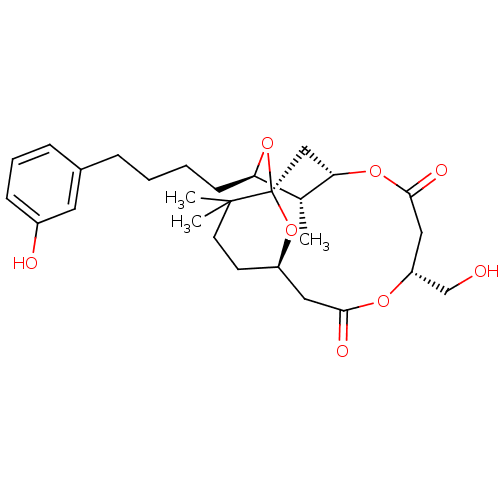
(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071743
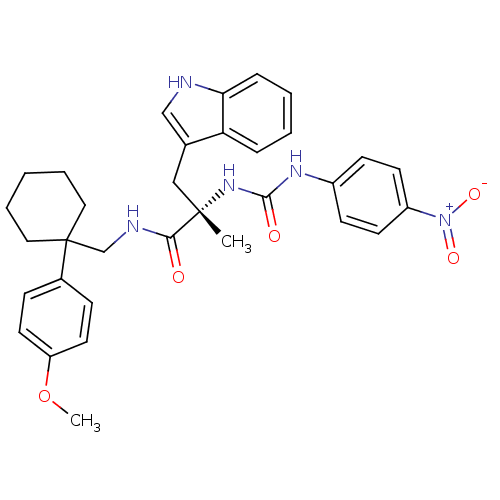
((S)-3-(1H-Indol-3-yl)-N-[1-(4-methoxy-phenyl)-cycl...)Show SMILES COc1ccc(cc1)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C33H37N5O5/c1-32(20-23-21-34-29-9-5-4-8-28(23)29,37-31(40)36-25-12-14-26(15-13-25)38(41)42)30(39)35-22-33(18-6-3-7-19-33)24-10-16-27(43-2)17-11-24/h4-5,8-17,21,34H,3,6-7,18-20,22H2,1-2H3,(H,35,39)(H2,36,37,40)/t32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1
(Homo sapiens (Human)) | BDBM50601065
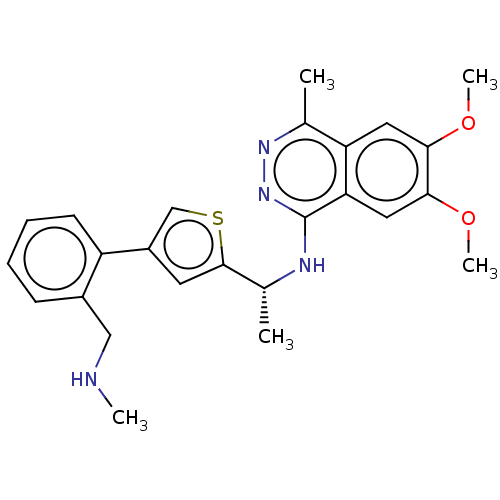
(CHEMBL5206295 | US11702418, Example 4-4)Show SMILES CNCc1ccccc1-c1csc(c1)[C@@H](C)Nc1nnc(C)c2cc(OC)c(OC)cc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00741
BindingDB Entry DOI: 10.7270/Q2XD15Q0 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50360628
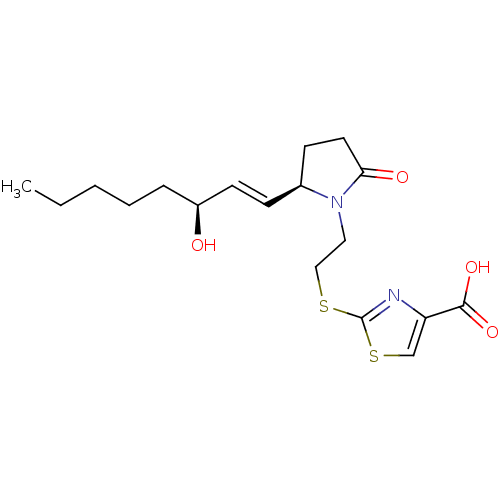
(CHEMBL1933724)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCSc1nc(cs1)C(O)=O |r| Show InChI InChI=1S/C18H26N2O4S2/c1-2-3-4-5-14(21)8-6-13-7-9-16(22)20(13)10-11-25-18-19-15(12-26-18)17(23)24/h6,8,12-14,21H,2-5,7,9-11H2,1H3,(H,23,24)/b8-6+/t13-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 396-401 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.109
BindingDB Entry DOI: 10.7270/Q27P8ZT2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data