 Found 61 hits with Last Name = 'summerfield' and Initial = 'j'
Found 61 hits with Last Name = 'summerfield' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159507
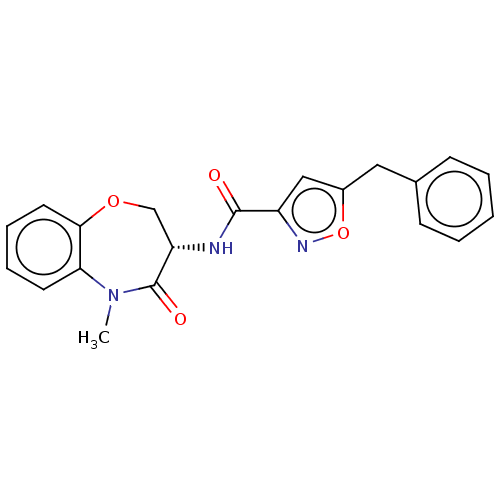
(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human RIP1 (1 to 375 residues) in presence of increasing ATP by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159696
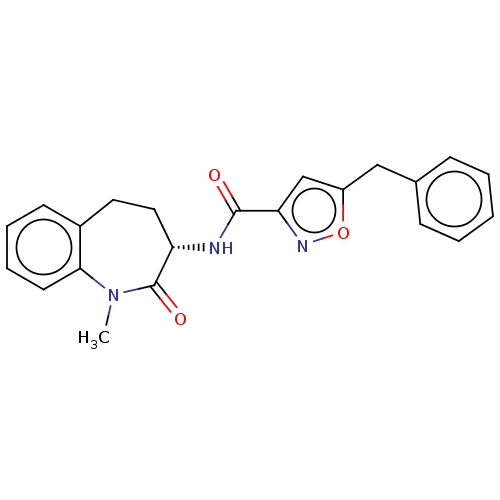
(CHEMBL3786997)Show SMILES CN1c2ccccc2CC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C22H21N3O3/c1-25-20-10-6-5-9-16(20)11-12-18(22(25)27)23-21(26)19-14-17(28-24-19)13-15-7-3-2-4-8-15/h2-10,14,18H,11-13H2,1H3,(H,23,26)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Flag-tagged human RIP1 (1 to 324 residues) after 30 mins by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159693

(CHEMBL3785482)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)[nH]n2)C1=O |r| Show InChI InChI=1S/C21H20N4O3/c1-25-18-9-5-6-10-19(18)28-13-17(21(25)27)22-20(26)16-12-15(23-24-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,26)(H,23,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Flag-tagged human RIP1 (1 to 324 residues) after 30 mins by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159508
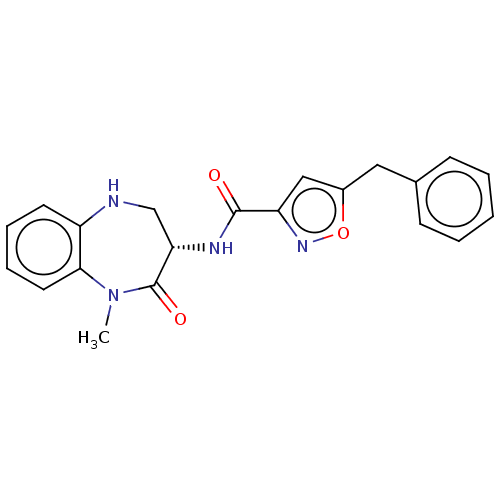
(CHEMBL3786078)Show SMILES CN1c2ccccc2NC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H20N4O3/c1-25-19-10-6-5-9-16(19)22-13-18(21(25)27)23-20(26)17-12-15(28-24-17)11-14-7-3-2-4-8-14/h2-10,12,18,22H,11,13H2,1H3,(H,23,26)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human RIP1 (1 to 375 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159697
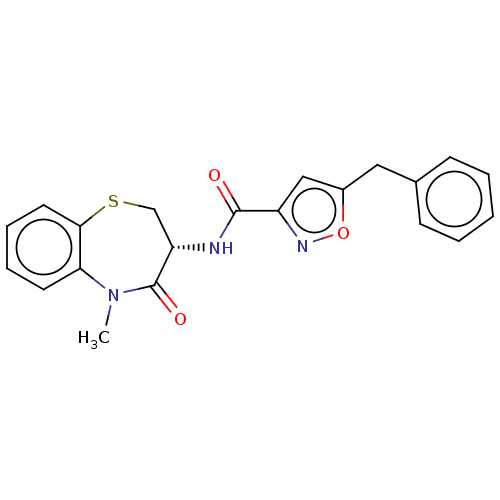
(CHEMBL3785745)Show SMILES CN1c2ccccc2SC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O3S/c1-24-18-9-5-6-10-19(18)28-13-17(21(24)26)22-20(25)16-12-15(27-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human RIP1 (1 to 375 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50573631
(CHEMBL4873767) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00156
BindingDB Entry DOI: 10.7270/Q21C21PV |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159507

(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) after 4 hrs by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159507

(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human WT RIP1 infected in HEK293T cells assessed as reduction in S166 phosphorylation by ELISA |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159699
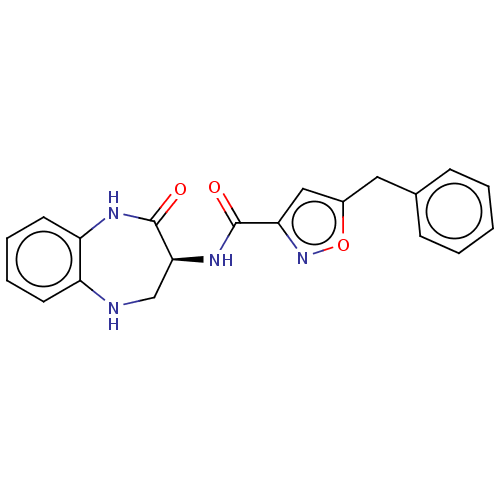
(CHEMBL3787689)Show SMILES O=C(N[C@H]1CNc2ccccc2NC1=O)c1cc(Cc2ccccc2)on1 |r| Show InChI InChI=1S/C20H18N4O3/c25-19(17-11-14(27-24-17)10-13-6-2-1-3-7-13)23-18-12-21-15-8-4-5-9-16(15)22-20(18)26/h1-9,11,18,21H,10,12H2,(H,22,26)(H,23,25)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human RIP1 (1 to 375 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50573631

(CHEMBL4873767) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RIP2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00156
BindingDB Entry DOI: 10.7270/Q21C21PV |
More data for this
Ligand-Target Pair | |
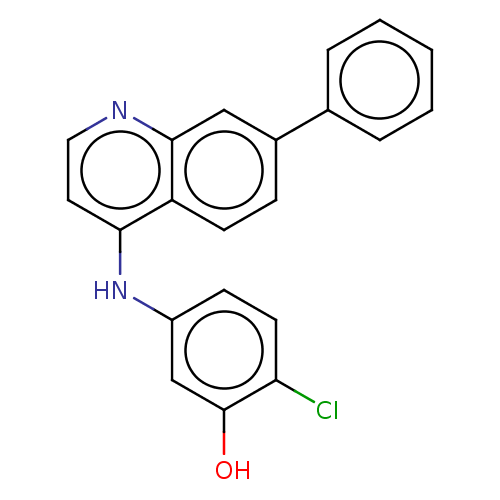
Receptor-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50573633

(CHEMBL4869572)Show SMILES COCCNC(=O)c1cc(CNc2ccc(Cl)c(O)c2)cc(c1)-c1ccc2c(Nc3ccc(Cl)c(O)c3)ccnc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RIP2 (unknown origin) by biochemical assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00156
BindingDB Entry DOI: 10.7270/Q21C21PV |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159511
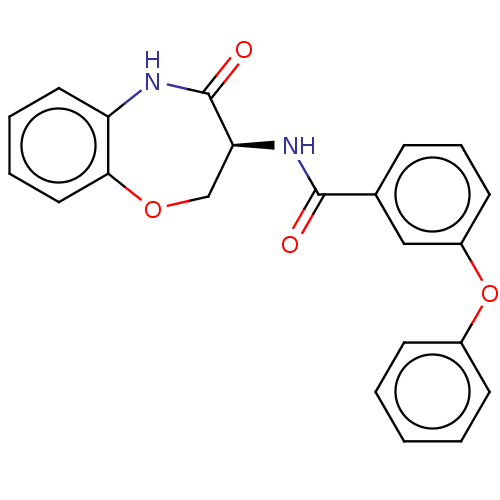
(CHEMBL3786162)Show SMILES O=C(N[C@H]1COc2ccccc2NC1=O)c1cccc(Oc2ccccc2)c1 |r| Show InChI InChI=1S/C22H18N2O4/c25-21(15-7-6-10-17(13-15)28-16-8-2-1-3-9-16)24-19-14-27-20-12-5-4-11-18(20)23-22(19)26/h1-13,19H,14H2,(H,23,26)(H,24,25)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) after 4 hrs by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159507

(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human RIP1 (1 to 375 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159507

(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 in human U937 cells assessed as inhibition of TNF/zVAD.fmk induced necroptosis after 24 hrs by Cell titer-Glo luminescence a... |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50573632
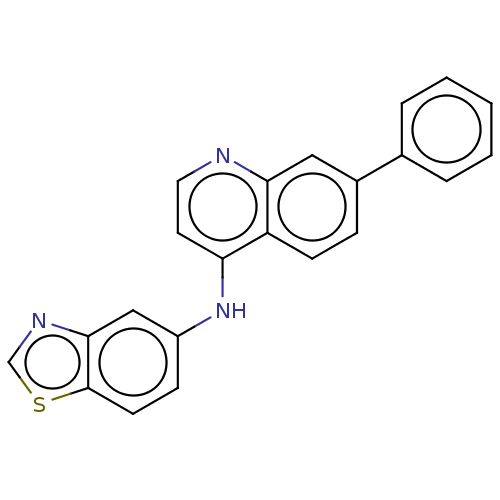
(CHEMBL4876135) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RIP2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00156
BindingDB Entry DOI: 10.7270/Q21C21PV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50573632

(CHEMBL4876135) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00156
BindingDB Entry DOI: 10.7270/Q21C21PV |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159512
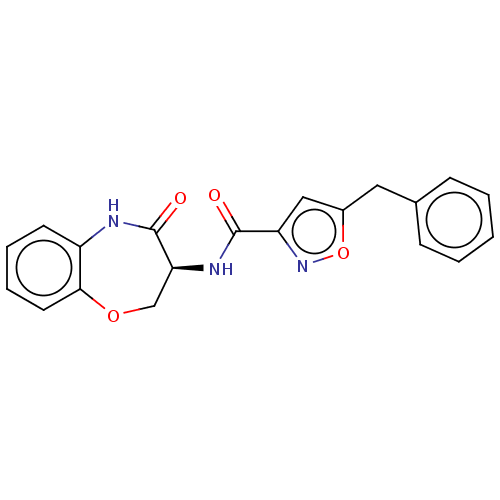
(CHEMBL3786293)Show SMILES O=C(N[C@H]1COc2ccccc2NC1=O)c1cc(Cc2ccccc2)on1 |r| Show InChI InChI=1S/C20H17N3O4/c24-19(16-11-14(27-23-16)10-13-6-2-1-3-7-13)22-17-12-26-18-9-5-4-8-15(18)21-20(17)25/h1-9,11,17H,10,12H2,(H,21,25)(H,22,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) after 4 hrs by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50446278
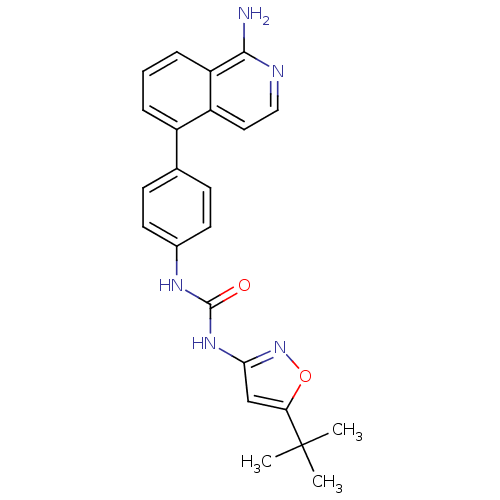
(CHEMBL3109202)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cccc3c(N)nccc23)no1 Show InChI InChI=1S/C23H23N5O2/c1-23(2,3)19-13-20(28-30-19)27-22(29)26-15-9-7-14(8-10-15)16-5-4-6-18-17(16)11-12-25-21(18)24/h4-13H,1-3H3,(H2,24,25)(H2,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human RIP1 (1 to 375 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159700
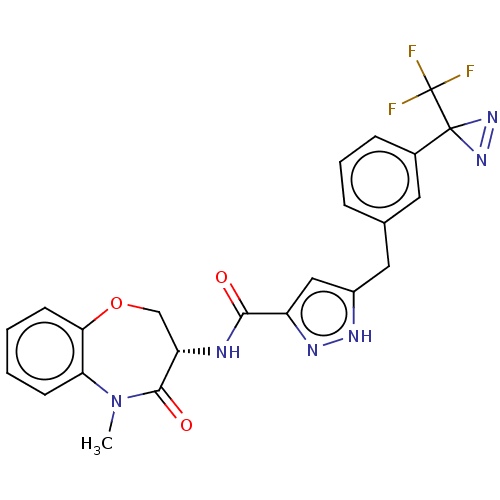
(CHEMBL3786660)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3cccc(c3)C3(N=N3)C(F)(F)F)[nH]n2)C1=O |r,c:27| Show InChI InChI=1S/C23H19F3N6O3/c1-32-18-7-2-3-8-19(18)35-12-17(21(32)34)27-20(33)16-11-15(28-29-16)10-13-5-4-6-14(9-13)22(30-31-22)23(24,25)26/h2-9,11,17H,10,12H2,1H3,(H,27,33)(H,28,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to Flag-tagged human RIP1 (1 to 324 residues) at 10 uM after 30 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Mus musculus) | BDBM50446278

(CHEMBL3109202)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cccc3c(N)nccc23)no1 Show InChI InChI=1S/C23H23N5O2/c1-23(2,3)19-13-20(28-30-19)27-22(29)26-15-9-7-14(8-10-15)16-5-4-6-18-17(16)11-12-25-21(18)24/h4-13H,1-3H3,(H2,24,25)(H2,26,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to mouse RIP1 (1 to 378 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Mus musculus) | BDBM50446278

(CHEMBL3109202)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cccc3c(N)nccc23)no1 Show InChI InChI=1S/C23H23N5O2/c1-23(2,3)19-13-20(28-30-19)27-22(29)26-15-9-7-14(8-10-15)16-5-4-6-18-17(16)11-12-25-21(18)24/h4-13H,1-3H3,(H2,24,25)(H2,26,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of mouse WT RIP1 transfected in HEK293T cells assessed as reduction in S166 phosphorylation by ELISA |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50573631

(CHEMBL4873767) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ALK5 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00156
BindingDB Entry DOI: 10.7270/Q21C21PV |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159698
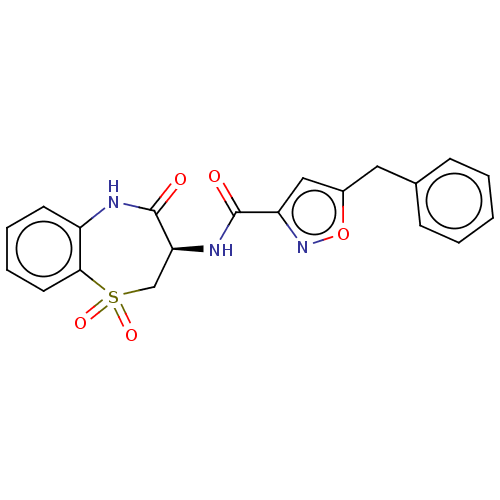
(CHEMBL3785838)Show SMILES O=C(N[C@H]1CS(=O)(=O)c2ccccc2NC1=O)c1cc(Cc2ccccc2)on1 |r| Show InChI InChI=1S/C20H17N3O5S/c24-19(16-11-14(28-23-16)10-13-6-2-1-3-7-13)22-17-12-29(26,27)18-9-5-4-8-15(18)21-20(17)25/h1-9,11,17H,10,12H2,(H,21,25)(H,22,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human RIP1 (1 to 375 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50573631

(CHEMBL4873767) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00156
BindingDB Entry DOI: 10.7270/Q21C21PV |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159512

(CHEMBL3786293)Show SMILES O=C(N[C@H]1COc2ccccc2NC1=O)c1cc(Cc2ccccc2)on1 |r| Show InChI InChI=1S/C20H17N3O4/c24-19(16-11-14(27-23-16)10-13-6-2-1-3-7-13)22-17-12-26-18-9-5-4-8-15(18)21-20(17)25/h1-9,11,17H,10,12H2,(H,21,25)(H,22,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 in human U937 cells assessed as inhibition of TNF/zVAD.fmk induced necroptosis after 24 hrs by Cell titer-Glo luminescence a... |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM36372
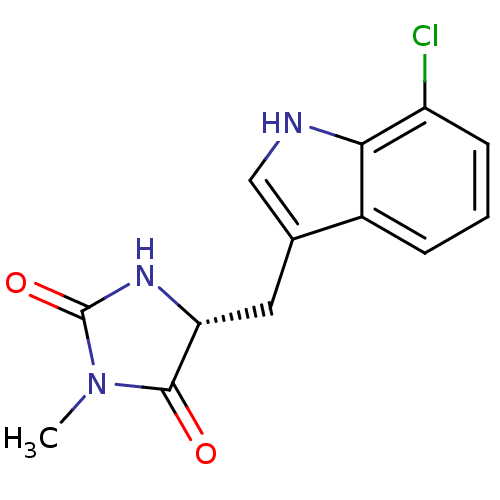
((5R)-5-[(7-chloro-1H-indol-3-yl)methyl]-3-methyl-2...)Show InChI InChI=1S/C13H12ClN3O2/c1-17-12(18)10(16-13(17)19)5-7-6-15-11-8(7)3-2-4-9(11)14/h2-4,6,10,15H,5H2,1H3,(H,16,19)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) after 4 hrs by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159512

(CHEMBL3786293)Show SMILES O=C(N[C@H]1COc2ccccc2NC1=O)c1cc(Cc2ccccc2)on1 |r| Show InChI InChI=1S/C20H17N3O4/c24-19(16-11-14(27-23-16)10-13-6-2-1-3-7-13)22-17-12-26-18-9-5-4-8-15(18)21-20(17)25/h1-9,11,17H,10,12H2,(H,21,25)(H,22,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 in human U937 cells assessed as inhibition of TNF/zVAD.fmk induced necroptosis after 24 hrs by Cell titer-Glo luminescence a... |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159695
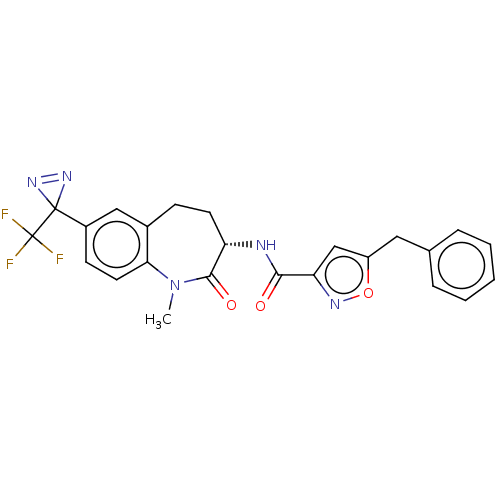
(CHEMBL3787617)Show SMILES CN1c2ccc(cc2CC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O)C1(N=N1)C(F)(F)F |r,c:33| Show InChI InChI=1S/C24H20F3N5O3/c1-32-20-10-8-16(23(30-31-23)24(25,26)27)12-15(20)7-9-18(22(32)34)28-21(33)19-13-17(35-29-19)11-14-5-3-2-4-6-14/h2-6,8,10,12-13,18H,7,9,11H2,1H3,(H,28,33)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to Flag-tagged human RIP1 (1 to 324 residues) at 10 uM after 30 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM36372

((5R)-5-[(7-chloro-1H-indol-3-yl)methyl]-3-methyl-2...)Show InChI InChI=1S/C13H12ClN3O2/c1-17-12(18)10(16-13(17)19)5-7-6-15-11-8(7)3-2-4-9(11)14/h2-4,6,10,15H,5H2,1H3,(H,16,19)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 in human U937 cells assessed as inhibition of TNF/zVAD.fmk induced necroptosis after 24 hrs by Cell titer-Glo luminescence a... |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159511

(CHEMBL3786162)Show SMILES O=C(N[C@H]1COc2ccccc2NC1=O)c1cccc(Oc2ccccc2)c1 |r| Show InChI InChI=1S/C22H18N2O4/c25-21(15-7-6-10-17(13-15)28-16-8-2-1-3-9-16)24-19-14-27-20-12-5-4-11-18(20)23-22(19)26/h1-13,19H,14H2,(H,23,26)(H,24,25)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 in human U937 cells assessed as inhibition of TNF/zVAD.fmk induced necroptosis after 24 hrs by Cell titer-Glo luminescence a... |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159511

(CHEMBL3786162)Show SMILES O=C(N[C@H]1COc2ccccc2NC1=O)c1cccc(Oc2ccccc2)c1 |r| Show InChI InChI=1S/C22H18N2O4/c25-21(15-7-6-10-17(13-15)28-16-8-2-1-3-9-16)24-19-14-27-20-12-5-4-11-18(20)23-22(19)26/h1-13,19H,14H2,(H,23,26)(H,24,25)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 in human U937 cells assessed as inhibition of TNF/zVAD.fmk induced necroptosis after 24 hrs by Cell titer-Glo luminescence a... |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50446278

(CHEMBL3109202)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cccc3c(N)nccc23)no1 Show InChI InChI=1S/C23H23N5O2/c1-23(2,3)19-13-20(28-30-19)27-22(29)26-15-9-7-14(8-10-15)16-5-4-6-18-17(16)11-12-25-21(18)24/h4-13H,1-3H3,(H2,24,25)(H2,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human RIP1 (1 to 375 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM36372

((5R)-5-[(7-chloro-1H-indol-3-yl)methyl]-3-methyl-2...)Show InChI InChI=1S/C13H12ClN3O2/c1-17-12(18)10(16-13(17)19)5-7-6-15-11-8(7)3-2-4-9(11)14/h2-4,6,10,15H,5H2,1H3,(H,16,19)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Flag-tagged human RIP1 (1 to 324 residues) after 30 mins by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Mus musculus) | BDBM50159508

(CHEMBL3786078)Show SMILES CN1c2ccccc2NC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H20N4O3/c1-25-19-10-6-5-9-16(19)22-13-18(21(25)27)23-20(26)17-12-15(28-24-17)11-14-7-3-2-4-8-14/h2-10,12,18,22H,11,13H2,1H3,(H,23,26)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to mouse RIP1 (1 to 378 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159510
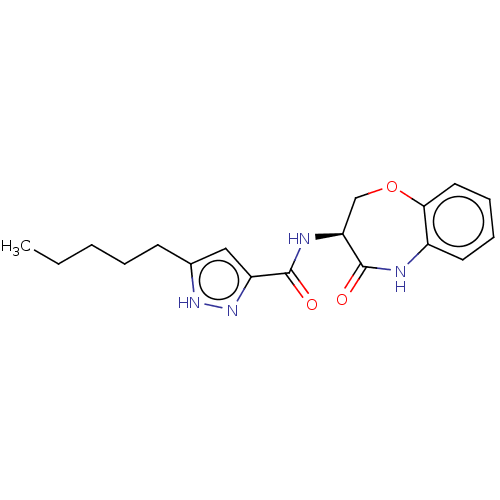
(CHEMBL3786778)Show SMILES CCCCCc1cc(n[nH]1)C(=O)N[C@H]1COc2ccccc2NC1=O |r| Show InChI InChI=1S/C18H22N4O3/c1-2-3-4-7-12-10-14(22-21-12)17(23)20-15-11-25-16-9-6-5-8-13(16)19-18(15)24/h5-6,8-10,15H,2-4,7,11H2,1H3,(H,19,24)(H,20,23)(H,21,22)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 in human U937 cells assessed as inhibition of TNF/zVAD.fmk induced necroptosis after 24 hrs by Cell titer-Glo luminescence a... |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159510

(CHEMBL3786778)Show SMILES CCCCCc1cc(n[nH]1)C(=O)N[C@H]1COc2ccccc2NC1=O |r| Show InChI InChI=1S/C18H22N4O3/c1-2-3-4-7-12-10-14(22-21-12)17(23)20-15-11-25-16-9-6-5-8-13(16)19-18(15)24/h5-6,8-10,15H,2-4,7,11H2,1H3,(H,19,24)(H,20,23)(H,21,22)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 in human U937 cells assessed as inhibition of TNF/zVAD.fmk induced necroptosis after 24 hrs by Cell titer-Glo luminescence a... |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50122749
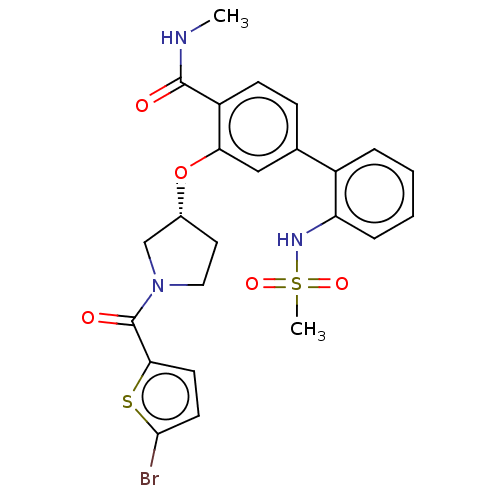
(CHEMBL3623144)Show SMILES CNC(=O)c1ccc(cc1O[C@@H]1CCN(C1)C(=O)c1ccc(Br)s1)-c1ccccc1NS(C)(=O)=O |r| Show InChI InChI=1S/C24H24BrN3O5S2/c1-26-23(29)18-8-7-15(17-5-3-4-6-19(17)27-35(2,31)32)13-20(18)33-16-11-12-28(14-16)24(30)21-9-10-22(25)34-21/h3-10,13,16,27H,11-12,14H2,1-2H3,(H,26,29)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human BCATm after 10 mins by fluorescent assay |
ACS Med Chem Lett 6: 919-24 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00179
BindingDB Entry DOI: 10.7270/Q2GB25T0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50573632

(CHEMBL4876135) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ALK5 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00156
BindingDB Entry DOI: 10.7270/Q21C21PV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50573632

(CHEMBL4876135) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00156
BindingDB Entry DOI: 10.7270/Q21C21PV |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50122748
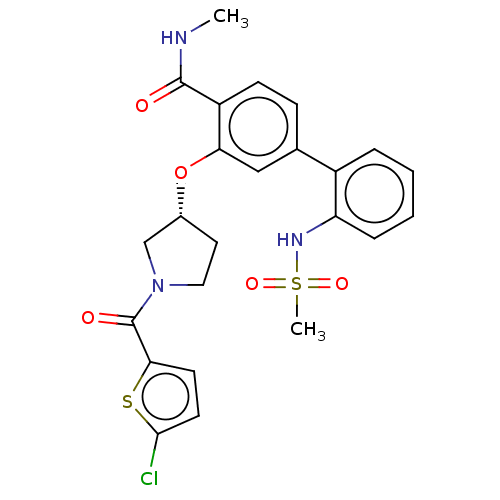
(CHEMBL3623145)Show SMILES CNC(=O)c1ccc(cc1O[C@@H]1CCN(C1)C(=O)c1ccc(Cl)s1)-c1ccccc1NS(C)(=O)=O |r| Show InChI InChI=1S/C24H24ClN3O5S2/c1-26-23(29)18-8-7-15(17-5-3-4-6-19(17)27-35(2,31)32)13-20(18)33-16-11-12-28(14-16)24(30)21-9-10-22(25)34-21/h3-10,13,16,27H,11-12,14H2,1-2H3,(H,26,29)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human BCATm after 10 mins by fluorescent assay |
ACS Med Chem Lett 6: 919-24 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00179
BindingDB Entry DOI: 10.7270/Q2GB25T0 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Mus musculus) | BDBM50159507

(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of mouse RIP1 in mouse L929 cells assessed as inhibition of TNF/zVAD.fmk induced necroptosis by Cell titer-Glo luminescence assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Mus musculus) | BDBM50159508

(CHEMBL3786078)Show SMILES CN1c2ccccc2NC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H20N4O3/c1-25-19-10-6-5-9-16(19)22-13-18(21(25)27)23-20(26)17-12-15(28-24-17)11-14-7-3-2-4-8-14/h2-10,12,18,22H,11,13H2,1H3,(H,23,26)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of mouse WT RIP1 transfected in HEK293T cells assessed as reduction in S166 phosphorylation by ELISA |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Mus musculus) | BDBM50159507

(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to mouse RIP1 (1 to 378 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50122750
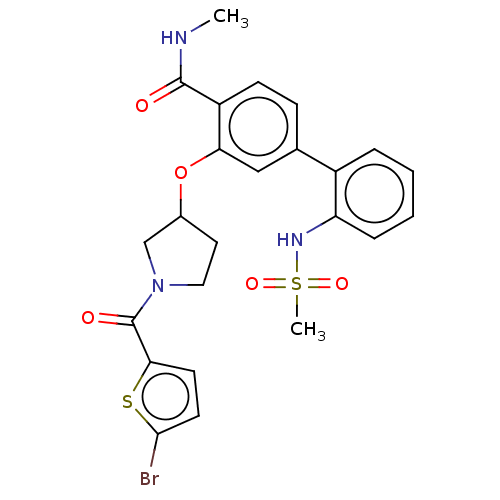
(CHEMBL3623143)Show SMILES CNC(=O)c1ccc(cc1OC1CCN(C1)C(=O)c1ccc(Br)s1)-c1ccccc1NS(C)(=O)=O Show InChI InChI=1S/C24H24BrN3O5S2/c1-26-23(29)18-8-7-15(17-5-3-4-6-19(17)27-35(2,31)32)13-20(18)33-16-11-12-28(14-16)24(30)21-9-10-22(25)34-21/h3-10,13,16,27H,11-12,14H2,1-2H3,(H,26,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human BCATm after 10 mins by fluorescent assay |
ACS Med Chem Lett 6: 919-24 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00179
BindingDB Entry DOI: 10.7270/Q2GB25T0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159509
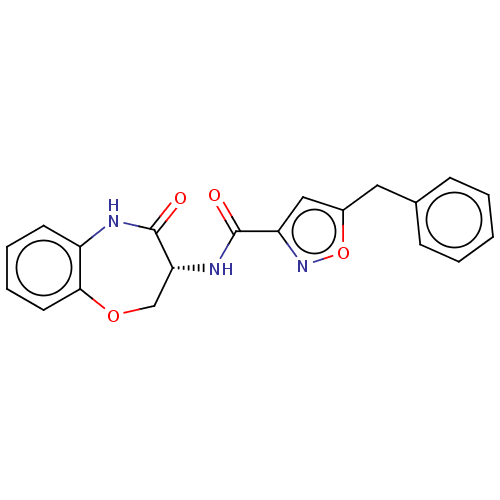
(CHEMBL3787216)Show SMILES O=C(N[C@@H]1COc2ccccc2NC1=O)c1cc(Cc2ccccc2)on1 |r| Show InChI InChI=1S/C20H17N3O4/c24-19(16-11-14(27-23-16)10-13-6-2-1-3-7-13)22-17-12-26-18-9-5-4-8-15(18)21-20(17)25/h1-9,11,17H,10,12H2,(H,21,25)(H,22,24)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human RIP1 (1 to 375 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Mus musculus) | BDBM50159507

(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of mouse WT RIP1 transfected in HEK293T cells assessed as reduction in S166 phosphorylation by ELISA |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159509

(CHEMBL3787216)Show SMILES O=C(N[C@@H]1COc2ccccc2NC1=O)c1cc(Cc2ccccc2)on1 |r| Show InChI InChI=1S/C20H17N3O4/c24-19(16-11-14(27-23-16)10-13-6-2-1-3-7-13)22-17-12-26-18-9-5-4-8-15(18)21-20(17)25/h1-9,11,17H,10,12H2,(H,21,25)(H,22,24)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human RIP1 (1 to 375 residues) preincubated for 10 mins measured after 20 mins by fluorescence polarization assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50122747
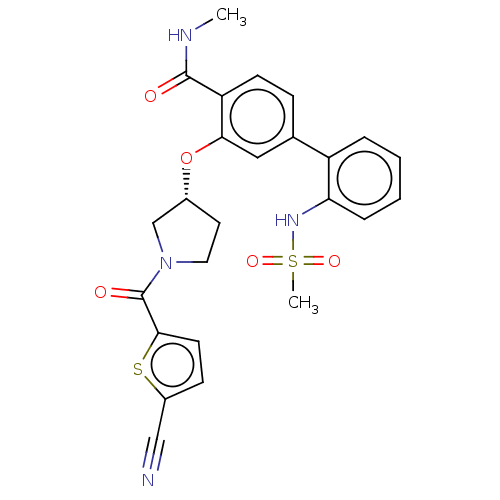
(CHEMBL3623146)Show SMILES CNC(=O)c1ccc(cc1O[C@@H]1CCN(C1)C(=O)c1ccc(s1)C#N)-c1ccccc1NS(C)(=O)=O |r| Show InChI InChI=1S/C25H24N4O5S2/c1-27-24(30)20-9-7-16(19-5-3-4-6-21(19)28-36(2,32)33)13-22(20)34-17-11-12-29(15-17)25(31)23-10-8-18(14-26)35-23/h3-10,13,17,28H,11-12,15H2,1-2H3,(H,27,30)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human BCATm after 10 mins by fluorescent assay |
ACS Med Chem Lett 6: 919-24 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00179
BindingDB Entry DOI: 10.7270/Q2GB25T0 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50573630

(CHEMBL4869360)Show SMILES COCCNC(=O)c1cc(CNc2ccc(Cl)c(O)c2)cc(c1)-c1ccc2c(Cl)ccnc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RIP2 (unknown origin) by fluorescence polarization based binding assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00156
BindingDB Entry DOI: 10.7270/Q21C21PV |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50122746
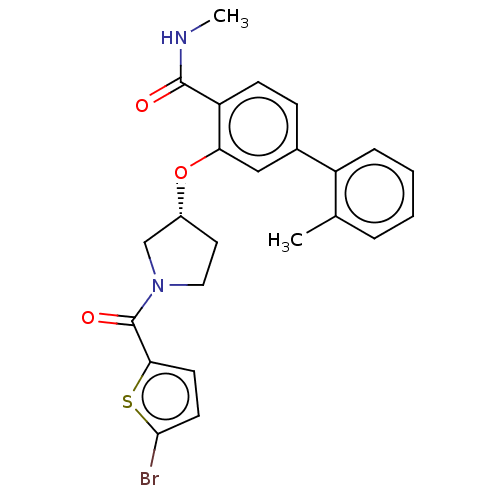
(CHEMBL3623147)Show SMILES CNC(=O)c1ccc(cc1O[C@@H]1CCN(C1)C(=O)c1ccc(Br)s1)-c1ccccc1C |r| Show InChI InChI=1S/C24H23BrN2O3S/c1-15-5-3-4-6-18(15)16-7-8-19(23(28)26-2)20(13-16)30-17-11-12-27(14-17)24(29)21-9-10-22(25)31-21/h3-10,13,17H,11-12,14H2,1-2H3,(H,26,28)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human BCATm after 10 mins by fluorescent assay |
ACS Med Chem Lett 6: 919-24 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00179
BindingDB Entry DOI: 10.7270/Q2GB25T0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data