 Found 331 hits with Last Name = 'surya' and Initial = 'a'
Found 331 hits with Last Name = 'surya' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103072
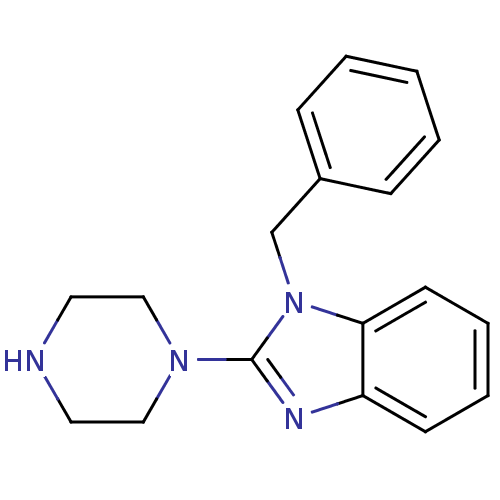
(1-Benzyl-2-piperazin-1-yl-1H-benzoimidazole | CHEM...)Show InChI InChI=1S/C18H20N4/c1-2-6-15(7-3-1)14-22-17-9-5-4-8-16(17)20-18(22)21-12-10-19-11-13-21/h1-9,19H,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103070

(1-(4-Methoxy-benzyl)-2-piperazin-1-yl-1H-benzoimid...)Show InChI InChI=1S/C19H22N4O/c1-24-16-8-6-15(7-9-16)14-23-18-5-3-2-4-17(18)21-19(23)22-12-10-20-11-13-22/h2-9,20H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103076
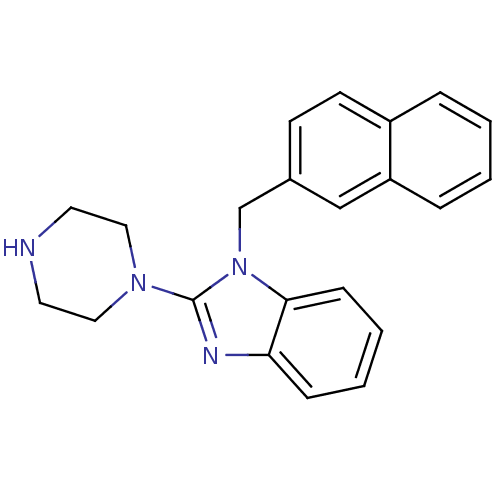
(1-Naphthalen-2-ylmethyl-2-piperazin-1-yl-1H-benzoi...)Show InChI InChI=1S/C22H22N4/c1-2-6-19-15-17(9-10-18(19)5-1)16-26-21-8-4-3-7-20(21)24-22(26)25-13-11-23-12-14-25/h1-10,15,23H,11-14,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103075
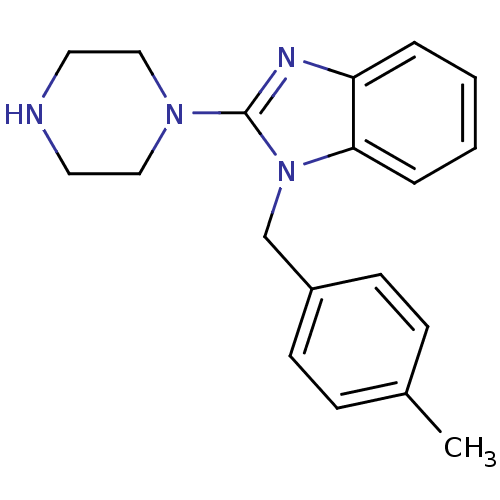
(1-(4-Methyl-benzyl)-2-piperazin-1-yl-1H-benzoimida...)Show InChI InChI=1S/C19H22N4/c1-15-6-8-16(9-7-15)14-23-18-5-3-2-4-17(18)21-19(23)22-12-10-20-11-13-22/h2-9,20H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103069
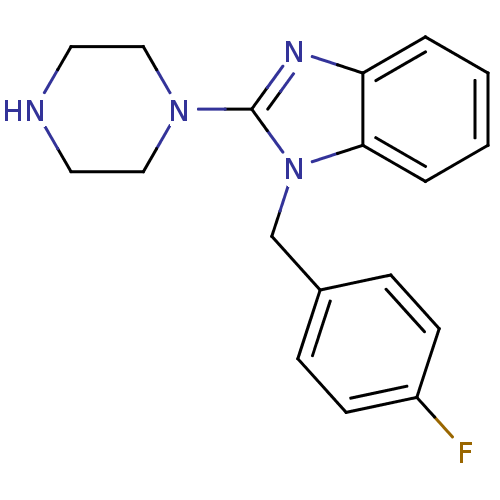
(1-(4-Fluoro-benzyl)-2-piperazin-1-yl-1H-benzoimida...)Show InChI InChI=1S/C18H19FN4/c19-15-7-5-14(6-8-15)13-23-17-4-2-1-3-16(17)21-18(23)22-11-9-20-10-12-22/h1-8,20H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103073
(1-Phenethyl-2-piperazin-1-yl-1H-benzoimidazole | C...)Show InChI InChI=1S/C19H22N4/c1-2-6-16(7-3-1)10-13-23-18-9-5-4-8-17(18)21-19(23)22-14-11-20-12-15-22/h1-9,20H,10-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103071
(1-(3-Phenyl-propyl)-2-piperazin-1-yl-1H-benzoimida...)Show InChI InChI=1S/C20H24N4/c1-2-7-17(8-3-1)9-6-14-24-19-11-5-4-10-18(19)22-20(24)23-15-12-21-13-16-23/h1-5,7-8,10-11,21H,6,9,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103077
(2-Piperazin-1-yl-1H-benzoimidazole | CHEMBL292066)Show InChI InChI=1S/C11H14N4/c1-2-4-10-9(3-1)13-11(14-10)15-7-5-12-6-8-15/h1-4,12H,5-8H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50323728
(2-((4-amino-3-(3-fluoro-5-hydroxyphenyl)-1H-pyrazo...)Show SMILES Cc1ccccc1-n1c(Cn2nc(-c3cc(O)cc(F)c3)c3c(N)ncnc23)nc2cccc(C)c2c1=O |(2.13,-14.38,;2.12,-12.84,;3.46,-12.06,;3.45,-10.52,;2.1,-9.75,;.77,-10.55,;.79,-12.08,;-.54,-12.85,;-.54,-14.41,;.81,-15.18,;.81,-16.72,;-.43,-17.63,;.05,-19.1,;-.74,-20.41,;-2.27,-20.38,;-3.06,-21.69,;-4.6,-21.66,;-2.32,-23.04,;-.78,-23.06,;-.03,-24.4,;.01,-21.74,;1.59,-19.09,;2.62,-20.22,;2.15,-21.69,;4.12,-19.9,;4.59,-18.43,;3.56,-17.3,;2.07,-17.63,;-1.87,-15.18,;-3.21,-14.41,;-4.55,-15.19,;-5.88,-14.42,;-5.87,-12.87,;-4.55,-12.1,;-4.56,-10.56,;-3.21,-12.87,;-1.88,-12.1,;-1.89,-10.56,)| Show InChI InChI=1S/C28H22FN7O2/c1-15-6-3-4-9-21(15)36-22(33-20-8-5-7-16(2)23(20)28(36)38)13-35-27-24(26(30)31-14-32-27)25(34-35)17-10-18(29)12-19(37)11-17/h3-12,14,37H,13H2,1-2H3,(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50323728

(2-((4-amino-3-(3-fluoro-5-hydroxyphenyl)-1H-pyrazo...)Show SMILES Cc1ccccc1-n1c(Cn2nc(-c3cc(O)cc(F)c3)c3c(N)ncnc23)nc2cccc(C)c2c1=O |(2.13,-14.38,;2.12,-12.84,;3.46,-12.06,;3.45,-10.52,;2.1,-9.75,;.77,-10.55,;.79,-12.08,;-.54,-12.85,;-.54,-14.41,;.81,-15.18,;.81,-16.72,;-.43,-17.63,;.05,-19.1,;-.74,-20.41,;-2.27,-20.38,;-3.06,-21.69,;-4.6,-21.66,;-2.32,-23.04,;-.78,-23.06,;-.03,-24.4,;.01,-21.74,;1.59,-19.09,;2.62,-20.22,;2.15,-21.69,;4.12,-19.9,;4.59,-18.43,;3.56,-17.3,;2.07,-17.63,;-1.87,-15.18,;-3.21,-14.41,;-4.55,-15.19,;-5.88,-14.42,;-5.87,-12.87,;-4.55,-12.1,;-4.56,-10.56,;-3.21,-12.87,;-1.88,-12.1,;-1.89,-10.56,)| Show InChI InChI=1S/C28H22FN7O2/c1-15-6-3-4-9-21(15)36-22(33-20-8-5-7-16(2)23(20)28(36)38)13-35-27-24(26(30)31-14-32-27)25(34-35)17-10-18(29)12-19(37)11-17/h3-12,14,37H,13H2,1-2H3,(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) in presence of [gamma-32P]ATP by phosphorimaging assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201717
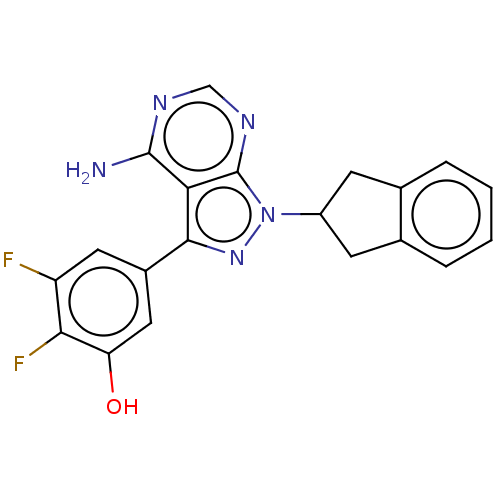
(CHEMBL3907591)Show SMILES Nc1ncnc2n(nc(-c3cc(O)c(F)c(F)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H15F2N5O/c21-14-7-12(8-15(28)17(14)22)18-16-19(23)24-9-25-20(16)27(26-18)13-5-10-3-1-2-4-11(10)6-13/h1-4,7-9,13,28H,5-6H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201728
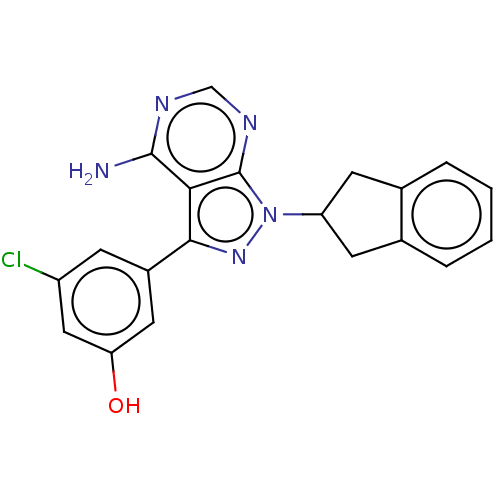
(CHEMBL3914552)Show SMILES Nc1ncnc2n(nc(-c3cc(O)cc(Cl)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16ClN5O/c21-14-5-13(8-16(27)9-14)18-17-19(22)23-10-24-20(17)26(25-18)15-6-11-3-1-2-4-12(11)7-15/h1-5,8-10,15,27H,6-7H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201716

(CHEMBL3942550)Show SMILES Nc1ncnc2n(nc(-c3ccc(F)c(O)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16FN5O/c21-15-6-5-13(9-16(15)27)18-17-19(22)23-10-24-20(17)26(25-18)14-7-11-3-1-2-4-12(11)8-14/h1-6,9-10,14,27H,7-8H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201720
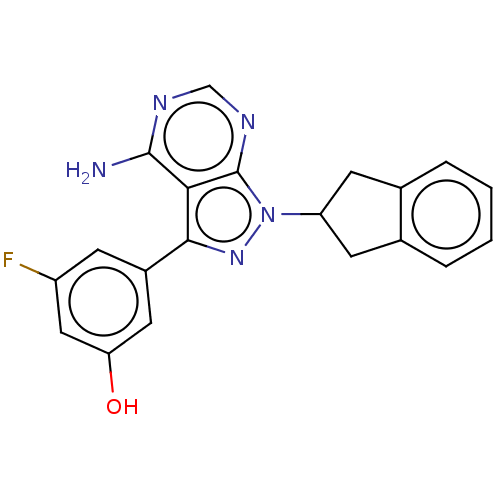
(CHEMBL3896635)Show SMILES Nc1ncnc2n(nc(-c3cc(O)cc(F)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16FN5O/c21-14-5-13(8-16(27)9-14)18-17-19(22)23-10-24-20(17)26(25-18)15-6-11-3-1-2-4-12(11)7-15/h1-5,8-10,15,27H,6-7H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201721
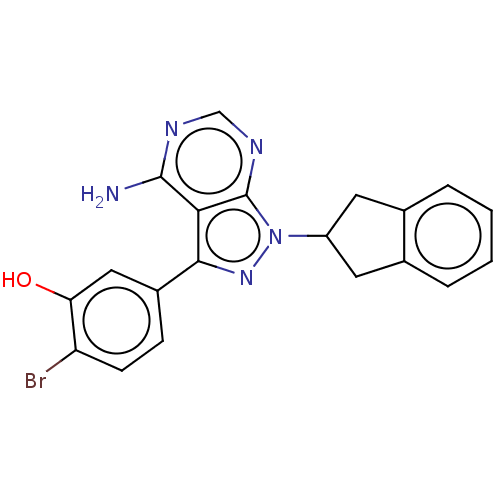
(CHEMBL3964073)Show SMILES Nc1ncnc2n(nc(-c3ccc(Br)c(O)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16BrN5O/c21-15-6-5-13(9-16(15)27)18-17-19(22)23-10-24-20(17)26(25-18)14-7-11-3-1-2-4-12(11)8-14/h1-6,9-10,14,27H,7-8H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50388180
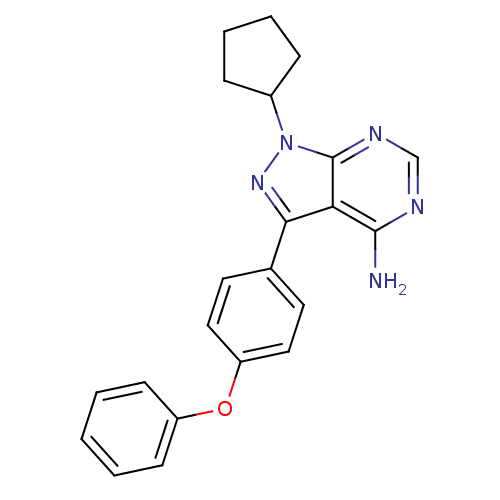
(CHEMBL2057912)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)C1CCCC1 Show InChI InChI=1S/C22H21N5O/c23-21-19-20(26-27(16-6-4-5-7-16)22(19)25-14-24-21)15-10-12-18(13-11-15)28-17-8-2-1-3-9-17/h1-3,8-14,16H,4-7H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201723
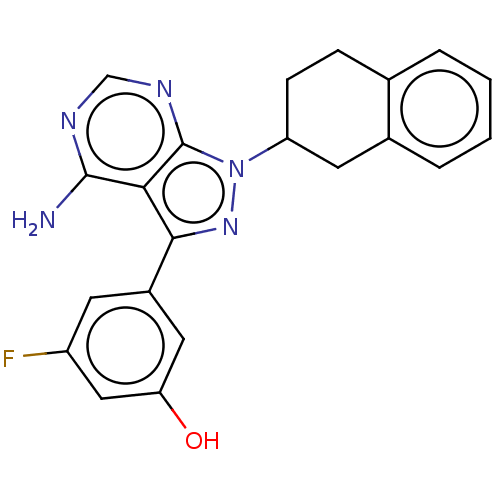
(CHEMBL3905578)Show SMILES Nc1ncnc2n(nc(-c3cc(O)cc(F)c3)c12)C1CCc2ccccc2C1 Show InChI InChI=1S/C21H18FN5O/c22-15-7-14(9-17(28)10-15)19-18-20(23)24-11-25-21(18)27(26-19)16-6-5-12-3-1-2-4-13(12)8-16/h1-4,7,9-11,16,28H,5-6,8H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201721

(CHEMBL3964073)Show SMILES Nc1ncnc2n(nc(-c3ccc(Br)c(O)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16BrN5O/c21-15-6-5-13(9-16(15)27)18-17-19(22)23-10-24-20(17)26(25-18)14-7-11-3-1-2-4-12(11)8-14/h1-6,9-10,14,27H,7-8H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201727
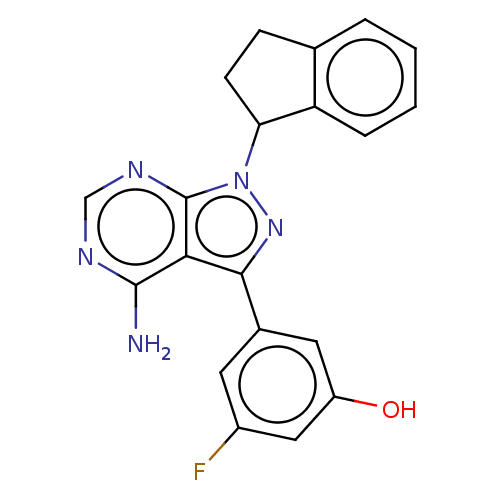
(CHEMBL3924678)Show SMILES Nc1ncnc2n(nc(-c3cc(O)cc(F)c3)c12)C1CCc2ccccc12 Show InChI InChI=1S/C20H16FN5O/c21-13-7-12(8-14(27)9-13)18-17-19(22)23-10-24-20(17)26(25-18)16-6-5-11-3-1-2-4-15(11)16/h1-4,7-10,16,27H,5-6H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201729
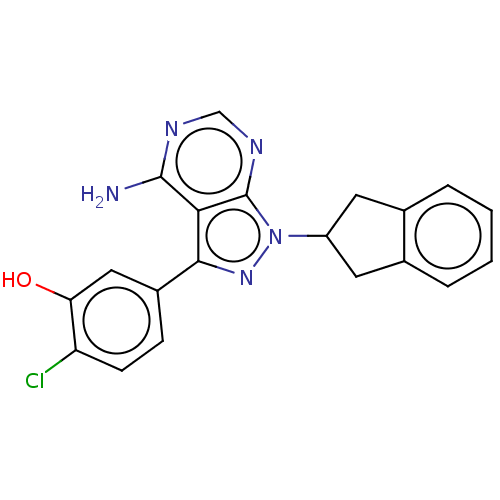
(CHEMBL3923573)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)c(O)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16ClN5O/c21-15-6-5-13(9-16(15)27)18-17-19(22)23-10-24-20(17)26(25-18)14-7-11-3-1-2-4-12(11)8-14/h1-6,9-10,14,27H,7-8H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201726
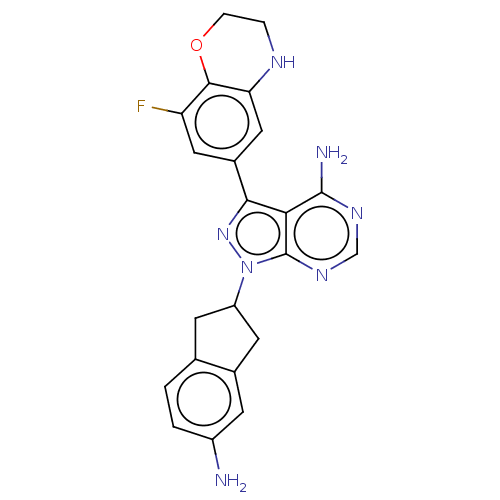
(CHEMBL3897842)Show SMILES Nc1ccc2CC(Cc2c1)n1nc(-c2cc(F)c3OCCNc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C22H20FN7O/c23-16-8-13(9-17-20(16)31-4-3-26-17)19-18-21(25)27-10-28-22(18)30(29-19)15-6-11-1-2-14(24)5-12(11)7-15/h1-2,5,8-10,15,26H,3-4,6-7,24H2,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201726

(CHEMBL3897842)Show SMILES Nc1ccc2CC(Cc2c1)n1nc(-c2cc(F)c3OCCNc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C22H20FN7O/c23-16-8-13(9-17-20(16)31-4-3-26-17)19-18-21(25)27-10-28-22(18)30(29-19)15-6-11-1-2-14(24)5-12(11)7-15/h1-2,5,8-10,15,26H,3-4,6-7,24H2,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201717

(CHEMBL3907591)Show SMILES Nc1ncnc2n(nc(-c3cc(O)c(F)c(F)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H15F2N5O/c21-14-7-12(8-15(28)17(14)22)18-16-19(23)24-9-25-20(16)27(26-18)13-5-10-3-1-2-4-11(10)6-13/h1-4,7-9,13,28H,5-6H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201724
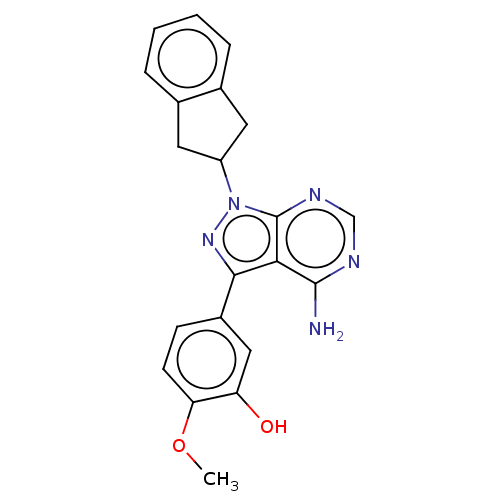
(CHEMBL3983045)Show SMILES COc1ccc(cc1O)-c1nn(C2Cc3ccccc3C2)c2ncnc(N)c12 Show InChI InChI=1S/C21H19N5O2/c1-28-17-7-6-14(10-16(17)27)19-18-20(22)23-11-24-21(18)26(25-19)15-8-12-4-2-3-5-13(12)9-15/h2-7,10-11,15,27H,8-9H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201729

(CHEMBL3923573)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)c(O)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16ClN5O/c21-15-6-5-13(9-16(15)27)18-17-19(22)23-10-24-20(17)26(25-18)14-7-11-3-1-2-4-12(11)8-14/h1-6,9-10,14,27H,7-8H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50323728

(2-((4-amino-3-(3-fluoro-5-hydroxyphenyl)-1H-pyrazo...)Show SMILES Cc1ccccc1-n1c(Cn2nc(-c3cc(O)cc(F)c3)c3c(N)ncnc23)nc2cccc(C)c2c1=O |(2.13,-14.38,;2.12,-12.84,;3.46,-12.06,;3.45,-10.52,;2.1,-9.75,;.77,-10.55,;.79,-12.08,;-.54,-12.85,;-.54,-14.41,;.81,-15.18,;.81,-16.72,;-.43,-17.63,;.05,-19.1,;-.74,-20.41,;-2.27,-20.38,;-3.06,-21.69,;-4.6,-21.66,;-2.32,-23.04,;-.78,-23.06,;-.03,-24.4,;.01,-21.74,;1.59,-19.09,;2.62,-20.22,;2.15,-21.69,;4.12,-19.9,;4.59,-18.43,;3.56,-17.3,;2.07,-17.63,;-1.87,-15.18,;-3.21,-14.41,;-4.55,-15.19,;-5.88,-14.42,;-5.87,-12.87,;-4.55,-12.1,;-4.56,-10.56,;-3.21,-12.87,;-1.88,-12.1,;-1.89,-10.56,)| Show InChI InChI=1S/C28H22FN7O2/c1-15-6-3-4-9-21(15)36-22(33-20-8-5-7-16(2)23(20)28(36)38)13-35-27-24(26(30)31-14-32-27)25(34-35)17-10-18(29)12-19(37)11-17/h3-12,14,37H,13H2,1-2H3,(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) in presence of [gamma-32P]ATP by phosphorimaging assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201725
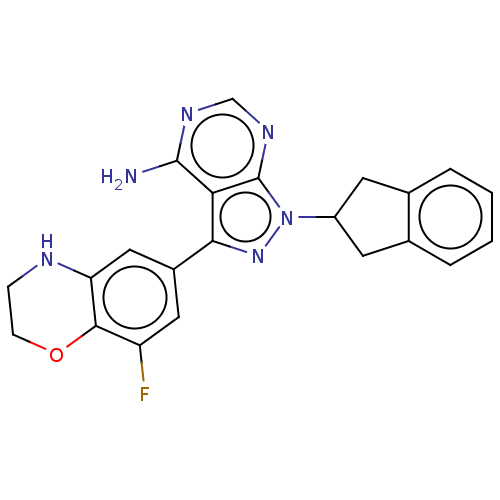
(CHEMBL3948373)Show SMILES Nc1ncnc2n(nc(-c3cc(F)c4OCCNc4c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C22H19FN6O/c23-16-9-14(10-17-20(16)30-6-5-25-17)19-18-21(24)26-11-27-22(18)29(28-19)15-7-12-3-1-2-4-13(12)8-15/h1-4,9-11,15,25H,5-8H2,(H2,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201719

(CHEMBL3957821)Show SMILES Cc1ccc(cc1O)-c1nn(C2Cc3ccccc3C2)c2ncnc(N)c12 Show InChI InChI=1S/C21H19N5O/c1-12-6-7-15(10-17(12)27)19-18-20(22)23-11-24-21(18)26(25-19)16-8-13-4-2-3-5-14(13)9-16/h2-7,10-11,16,27H,8-9H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201726

(CHEMBL3897842)Show SMILES Nc1ccc2CC(Cc2c1)n1nc(-c2cc(F)c3OCCNc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C22H20FN7O/c23-16-8-13(9-17-20(16)31-4-3-26-17)19-18-21(25)27-10-28-22(18)30(29-19)15-6-11-1-2-14(24)5-12(11)7-15/h1-2,5,8-10,15,26H,3-4,6-7,24H2,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201726

(CHEMBL3897842)Show SMILES Nc1ccc2CC(Cc2c1)n1nc(-c2cc(F)c3OCCNc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C22H20FN7O/c23-16-8-13(9-17-20(16)31-4-3-26-17)19-18-21(25)27-10-28-22(18)30(29-19)15-6-11-1-2-14(24)5-12(11)7-15/h1-2,5,8-10,15,26H,3-4,6-7,24H2,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201718

(CHEMBL3925929)Show SMILES Nc1ccc2CCC(Cc2c1)n1nc(-c2cc(F)c3OCCNc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C23H22FN7O/c24-17-9-14(10-18-21(17)32-6-5-27-18)20-19-22(26)28-11-29-23(19)31(30-20)16-4-2-12-1-3-15(25)7-13(12)8-16/h1,3,7,9-11,16,27H,2,4-6,8,25H2,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201718

(CHEMBL3925929)Show SMILES Nc1ccc2CCC(Cc2c1)n1nc(-c2cc(F)c3OCCNc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C23H22FN7O/c24-17-9-14(10-18-21(17)32-6-5-27-18)20-19-22(26)28-11-29-23(19)31(30-20)16-4-2-12-1-3-15(25)7-13(12)8-16/h1,3,7,9-11,16,27H,2,4-6,8,25H2,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201716

(CHEMBL3942550)Show SMILES Nc1ncnc2n(nc(-c3ccc(F)c(O)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16FN5O/c21-15-6-5-13(9-16(15)27)18-17-19(22)23-10-24-20(17)26(25-18)14-7-11-3-1-2-4-12(11)8-14/h1-6,9-10,14,27H,7-8H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201720

(CHEMBL3896635)Show SMILES Nc1ncnc2n(nc(-c3cc(O)cc(F)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16FN5O/c21-14-5-13(8-16(27)9-14)18-17-19(22)23-10-24-20(17)26(25-18)15-6-11-3-1-2-4-12(11)7-15/h1-5,8-10,15,27H,6-7H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201724

(CHEMBL3983045)Show SMILES COc1ccc(cc1O)-c1nn(C2Cc3ccccc3C2)c2ncnc(N)c12 Show InChI InChI=1S/C21H19N5O2/c1-28-17-7-6-14(10-16(17)27)19-18-20(22)23-11-24-21(18)26(25-19)15-8-12-4-2-3-5-13(12)9-15/h2-7,10-11,15,27H,8-9H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201723

(CHEMBL3905578)Show SMILES Nc1ncnc2n(nc(-c3cc(O)cc(F)c3)c12)C1CCc2ccccc2C1 Show InChI InChI=1S/C21H18FN5O/c22-15-7-14(9-17(28)10-15)19-18-20(23)24-11-25-21(18)27(26-19)16-6-5-12-3-1-2-4-13(12)8-16/h1-4,7,9-11,16,28H,5-6,8H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201725

(CHEMBL3948373)Show SMILES Nc1ncnc2n(nc(-c3cc(F)c4OCCNc4c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C22H19FN6O/c23-16-9-14(10-17-20(16)30-6-5-25-17)19-18-21(24)26-11-27-22(18)29(28-19)15-7-12-3-1-2-4-13(12)8-15/h1-4,9-11,15,25H,5-8H2,(H2,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201728

(CHEMBL3914552)Show SMILES Nc1ncnc2n(nc(-c3cc(O)cc(Cl)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16ClN5O/c21-14-5-13(8-16(27)9-14)18-17-19(22)23-10-24-20(17)26(25-18)15-6-11-3-1-2-4-12(11)7-15/h1-5,8-10,15,27H,6-7H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201719

(CHEMBL3957821)Show SMILES Cc1ccc(cc1O)-c1nn(C2Cc3ccccc3C2)c2ncnc(N)c12 Show InChI InChI=1S/C21H19N5O/c1-12-6-7-15(10-17(12)27)19-18-20(22)23-11-24-21(18)26(25-19)16-8-13-4-2-3-5-14(13)9-16/h2-7,10-11,16,27H,8-9H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201718

(CHEMBL3925929)Show SMILES Nc1ccc2CCC(Cc2c1)n1nc(-c2cc(F)c3OCCNc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C23H22FN7O/c24-17-9-14(10-18-21(17)32-6-5-27-18)20-19-22(26)28-11-29-23(19)31(30-20)16-4-2-12-1-3-15(25)7-13(12)8-16/h1,3,7,9-11,16,27H,2,4-6,8,25H2,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201718

(CHEMBL3925929)Show SMILES Nc1ccc2CCC(Cc2c1)n1nc(-c2cc(F)c3OCCNc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C23H22FN7O/c24-17-9-14(10-18-21(17)32-6-5-27-18)20-19-22(26)28-11-29-23(19)31(30-20)16-4-2-12-1-3-15(25)7-13(12)8-16/h1,3,7,9-11,16,27H,2,4-6,8,25H2,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM387452

(N3-(3-Chloro-4- fluorophenyl)- 5-methoxy-7- methyl...)Show InChI InChI=1S/C15H13ClFN3O2/c1-7-14-9(6-12(19-7)21-2)13(15(18)22-14)20-8-3-4-11(17)10(16)5-8/h3-6,20H,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
| Assay Description
Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... |
J Med Chem 52: 74-86 (2009)
BindingDB Entry DOI: 10.7270/Q24M96V5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM387576
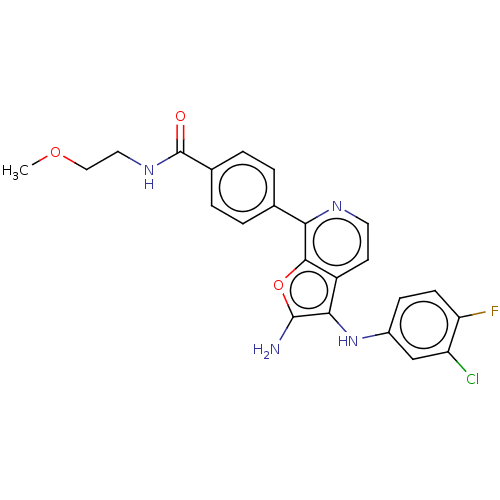
(4-(2-Amino-3- ((3-chloro-4- fluorophenyl) amino)fu...)Show SMILES COCCNC(=O)c1ccc(cc1)-c1nccc2c(Nc3ccc(F)c(Cl)c3)c(N)oc12 Show InChI InChI=1S/C23H20ClFN4O3/c1-31-11-10-28-23(30)14-4-2-13(3-5-14)19-21-16(8-9-27-19)20(22(26)32-21)29-15-6-7-18(25)17(24)12-15/h2-9,12,29H,10-11,26H2,1H3,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
| Assay Description
Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... |
J Med Chem 52: 74-86 (2009)
BindingDB Entry DOI: 10.7270/Q24M96V5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM387577
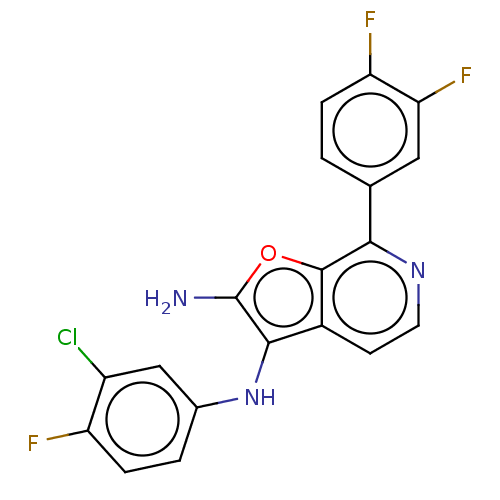
(N3-(3-chloro-4- fluorophenyl)- 7-(3,4- difluorophe...)Show SMILES Nc1oc2c(nccc2c1Nc1ccc(F)c(Cl)c1)-c1ccc(F)c(F)c1 Show InChI InChI=1S/C19H11ClF3N3O/c20-12-8-10(2-4-13(12)21)26-17-11-5-6-25-16(18(11)27-19(17)24)9-1-3-14(22)15(23)7-9/h1-8,26H,24H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
| Assay Description
Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... |
J Med Chem 52: 74-86 (2009)
BindingDB Entry DOI: 10.7270/Q24M96V5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM387578
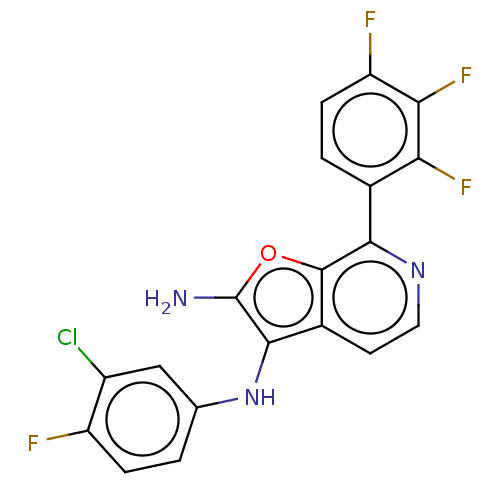
(N3-(3-Chloro-4- fluorophenyl)- 7-(2,3,4- trifluoro...)Show SMILES Nc1oc2c(nccc2c1Nc1ccc(F)c(Cl)c1)-c1ccc(F)c(F)c1F Show InChI InChI=1S/C19H10ClF4N3O/c20-11-7-8(1-3-12(11)21)27-17-10-5-6-26-16(18(10)28-19(17)25)9-2-4-13(22)15(24)14(9)23/h1-7,27H,25H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
| Assay Description
Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... |
J Med Chem 52: 74-86 (2009)
BindingDB Entry DOI: 10.7270/Q24M96V5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM387579
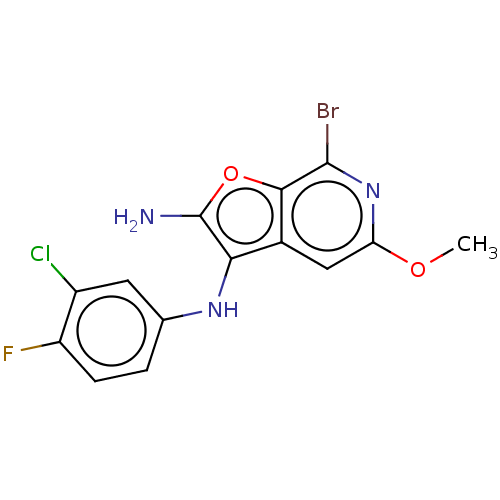
(7-Bromo-N3-(3- chloro-4- fluorophenyl)- 5-methoxy-...)Show InChI InChI=1S/C14H10BrClFN3O2/c1-21-10-5-7-11(14(18)22-12(7)13(15)20-10)19-6-2-3-9(17)8(16)4-6/h2-5,19H,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
| Assay Description
Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... |
J Med Chem 52: 74-86 (2009)
BindingDB Entry DOI: 10.7270/Q24M96V5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM387580
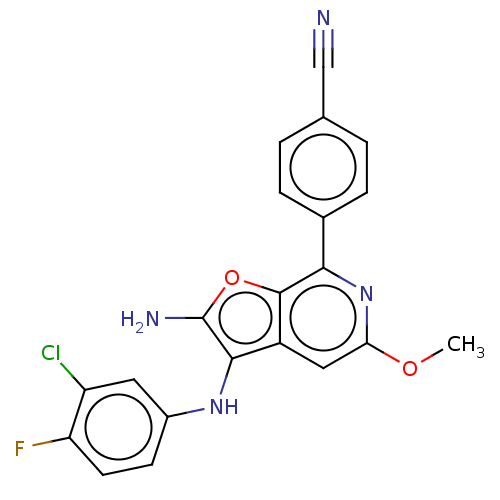
(4-(2-Amino-3- ((3-chloro-4- fluorophenyl) amino)-5...)Show SMILES COc1cc2c(Nc3ccc(F)c(Cl)c3)c(N)oc2c(n1)-c1ccc(cc1)C#N Show InChI InChI=1S/C21H14ClFN4O2/c1-28-17-9-14-19(26-13-6-7-16(23)15(22)8-13)21(25)29-20(14)18(27-17)12-4-2-11(10-24)3-5-12/h2-9,26H,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
| Assay Description
Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... |
J Med Chem 52: 74-86 (2009)
BindingDB Entry DOI: 10.7270/Q24M96V5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM387582
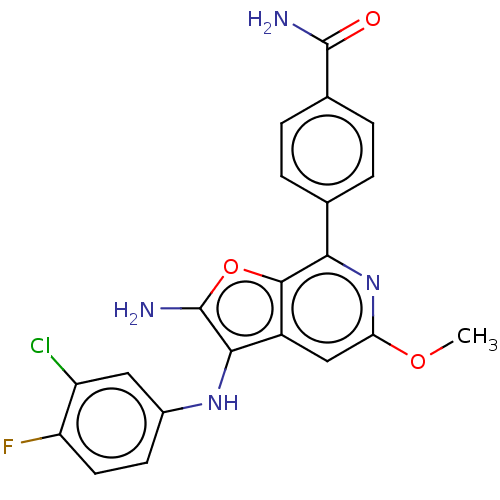
(4-(2-amino-3- ((3-chloro-4- fluorophenyl) amino)-5...)Show SMILES COc1cc2c(Nc3ccc(F)c(Cl)c3)c(N)oc2c(n1)-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C21H16ClFN4O3/c1-29-16-9-13-18(26-12-6-7-15(23)14(22)8-12)21(25)30-19(13)17(27-16)10-2-4-11(5-3-10)20(24)28/h2-9,26H,25H2,1H3,(H2,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
| Assay Description
Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... |
J Med Chem 52: 74-86 (2009)
BindingDB Entry DOI: 10.7270/Q24M96V5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM387585
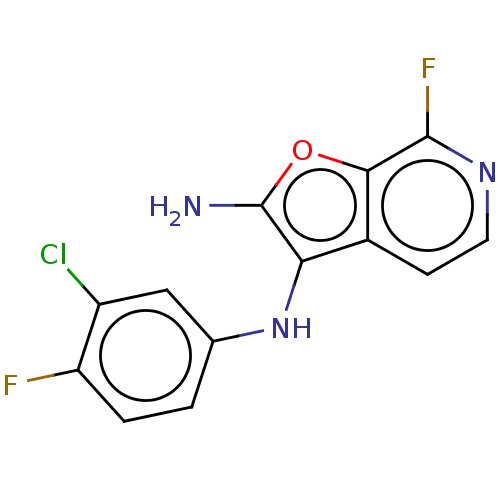
(N3-(3-chloro-4- fluorophenyl)- 7-fluorofuro [2,3-c...)Show InChI InChI=1S/C13H8ClF2N3O/c14-8-5-6(1-2-9(8)15)19-10-7-3-4-18-12(16)11(7)20-13(10)17/h1-5,19H,17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
| Assay Description
Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... |
J Med Chem 52: 74-86 (2009)
BindingDB Entry DOI: 10.7270/Q24M96V5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM387586
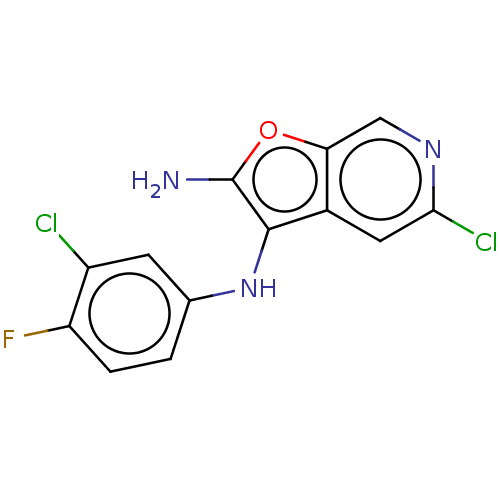
(5-Chloro-N3- (3-chloro-4- fluorophenyl) furo[2,3-c...)Show InChI InChI=1S/C13H8Cl2FN3O/c14-8-3-6(1-2-9(8)16)19-12-7-4-11(15)18-5-10(7)20-13(12)17/h1-5,19H,17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
| Assay Description
Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... |
J Med Chem 52: 74-86 (2009)
BindingDB Entry DOI: 10.7270/Q24M96V5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data